Abstract
Stachyurus macrocarpus is a narrowly endemic endangered shrub of which only one population remains on Chichi-jima Island (Ogasawara). We surveyed its population dynamics and reproductive states for 4 years and analyzed the population viability. In a survey of its entire distribution range, a total of 87 S. macrocarpus individuals have been found and 68 individuals have been confirmed in 2007. Thus, the actual population size is estimated to be less than 100 individuals. Environmental conditions and the results of a hand-pollination experiment suggest that low fruit setting in both sexes (female: 4.9–11.2%; hermaphrodite: 0.0%) may be caused by resource limitation. In addition, fruit predation by alien Rattus rattus was observed despite the rare fruit setting. The small effective population size (23.1–24.6 individuals) and hermaphrodite-biased sex ratio (46 hermaphrodites:12 females) would increase the risk of extinction. The habitat of S. macrocarpus was limited to dense scrubland and forest understory, and seedling regeneration was very scarce. During the survey period, 19 individuals (21.8%) were found dead, there were only two seedling recruitments, and the annual population growth rate was 0.979. The lack of occurrence on the south and west slopes and the shortness of shrubs in open spaces suggests that S. macrocarpus suffers stress from both dryness and frequent typhoons. However, S. macrocarpus also exhibited high mortality of shoot in forest understories. These findings suggest that the suitable habitat of S. macrocarpus is likely to be narrow in Ogasawara. While the recent increase of goat grazing has not affected individual mortality, 58.6% of shoots that had been grazed by goats were dead within 2 years. As elasticity analysis had shown that larger individuals make a greater contribution to the population growth rate, repeated goat grazing would impact the S. macrocarpus population in the near future by decreasing the vitality of larger individuals. Emergency measures for protecting the shrub from goat grazing and reinforcing the population through nursery cultivation were proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small populations such as narrowly endemic endangered species have a high extinction risk by various threats (Lande 1993; Oostermeijer 2003). For example, more than 8,000 angiosperm species are endangered in the world, specially, in oceanic islands (Baillie et al. 2004). Biota of oceanic islands usually consists of many narrowly endemic species. To maintain global biodiversity, such biodiversity hotspots have high priority for conservation. It is essential for the evaluation of the extinction risk of narrowly endemic or endangered species to study ecological aspects such as population dynamics and biological threats as well as genetic aspects such as decreasing genetic diversity and inbreeding depression (Schemske et al. 1994; Frankham et al. 2002). Population viability analysis using a matrix population model is useful for formulating strategies for conserving such endangered species (Brook et al. 2000; Menges 2000; Coulson et al. 2001).
In addition to small population size, alien species pose serious threats for endangered endemic species in oceanic islands (Loope et al. 1988; Loope and Mueller-Dombois 1989; Simberloff 1995; Lonsdale 1999; O’Dowd et al. 2003). The decline of pollination efficiency and the loss of genetic variation are also serious problems for endangered species (Oostermeijer 2003). To evaluate genetic degradation, it is necessary to understand the effective population size of small population species (Lande and Barrowclough 1987; Nunney and Campbell 1993). Thus, it is important to understand the impact of alien species, the state of reproduction, and the effective population size in order to evaluate the population viability of insular endangered species.
The endemic rate of vascular plants (44.6%) in the Ogasawara (Bonin) Islands is as high as that of the Galapagos Islands (Abe 2006). Many of these species on the islands have been highly threatened by development and biological invasions (Shimizu 2003a). One reason for this crisis situation is the small area of the Ogasawara Islands; the largest island (Chichi-jima), for example, occupies only 24 km2, which is less than 1/13 the area of Hawaii Island and 1/14 that of Isabela Island in the Galapágos. In such small areas, native flora account for 37.4% of endangered species that are designated by the Japanese Red Data Book (Environmental Agency of Japan 2000; Abe 2006). Several of these species have extremely small populations. For example, Cirsium toyoshimae has not been found since 1936, there is only one remaining individual of Rhododendron boninense, and only six surviving individuals of Pittosporum parvifolium including two females are known (Wada, unpublished data). Despite such critical situations, the population and reproductive traits of the species have not been studied yet in the Ogasawaras.
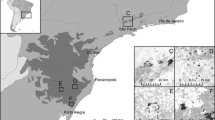
Unlike the widespread pioneer shrub, Stachyurus praecox (Li 1943; Ohi and Murata 1999; Ohi et al. 2003), Stachyurus macrocarpus is a narrowly endemic shrub, and the approximately 4 km2 Higashidaira dry forest is its only habitat on Chichi-jima Island (Ohi et al. 1998; Ohi and Murata 1999; Toyoda 2003) (Fig. 1). One population of S. macrocarpus had been found on Ani-jima (Kasaki 1991; Shimizu 1991), but after these reports, the lack of information on the species existence despite the efforts of many researchers on Ani-jima to find it suggest that the Ani-jima population is either critically endangered or already extinct. Thus, S. macrocarpus is thought to be declining and has been classified as “critical” in the Japanese Red Data Book (Environmental Agency of Japan 2000). However, further ecological information is needed to evaluate the risk of extinction (Schemske et al. 1994; Sutherland et al. 2004).
The aim of this study is to develop means to conserve S. macrocarpus and address the following questions: (1) How many individuals are remaining? (2) Is the population declining? (3) How many fruits are produced and is fruit production affected by pollen limitations? (4) How do alien species affect the population viability? There is also a discussion of approaches to the conservation of S. macrocarpus.
Materials and methods
The species
Stachyurus macrocarpus is a gynodioecious shrub usually less than 4 m in height. It is closely related to S. praecox, especially to the populations on Kyushu Island (Ohi et al. 2003). The flowering season is from January to early February. Fruits usually contain about 40 seeds and mature in late autumn. Individuals maintain themselves with coppice shoots, which replace the primary shoots within several years. Coppice shoots occasionally grow along the ground, but vegetative propagation by layered shoots is very rare. Thus, it is easy to identify individuals. S. macrocarpus typically inhabits shrub land that has a relatively sunny understory. In contrast, S. praecox is a pioneer shrub inhabiting forest edges and gaps. Thus, S. macrocarpus may have characteristics of sun shrubs, but there is still very little that is known about its regeneration sites in the Ogasawara Islands.
Study site
The Ogasawara Islands are volcanic islands that were formed millions of years ago (Kaizuka 1977). Located about 1,000 km south of the Japanese mainland (Fig. 1), the islands are in the subtropical climate zone and are hit by typhoons frequently. The mean annual precipitation (1,292 mm) is somewhat lower than that of the Okinawa Islands (2,037 mm) which are at approximately the same latitude (Toyoda 2003), and has gradually been decreasing (Oka et al. 2000; Yoshida et al. 2006). Thus, with the exception of some high elevation areas, dry forests and scrublands dominate in the Ogasawaras.
Major human settlement started in the 1880s, and industrial development including forestry and agriculture had progressed until World War II. As a result, most primary forests were disturbed intensely and various alien species were introduced (Shimizu 2003a; Toyoda 2003).
Population survey
We looked for S. macrocarpus in the Higashidaira area (alt. 200–300 m) during 2004–2007 (Fig. 1c). When S. macrocarpus was found, its survival at shoot level, stem diameter at 15-cm-height, H (height), crown condition, and reproductive state (sex, number of inflorescences, and fruit set) were recorded. The survival, size, number of inflorescences, and fruit set of all individuals were reassessed in the following 2 years. Individuals that had identifiable reproductive organs during the 4-year-survey were classified as adults, while those without identifiable reproductive organs were classified as juveniles. Individuals of less than 25 cm in height without sprouts were classified as seedlings.
Population dynamics were analyzed using a transition matrix. Stages of the transition matrix were classified by stem diameter rather than by shrub height. This was because shrub height is strongly affected by the frequent occurrence of typhoons and other disturbances, while stem diameter growth is less influenced by them. The analysis of the matrix model was performed using R (for the calculation of eigenvectors). Although several unknown individuals were found in each year’s survey, they were omitted to evaluate the transition probability in each year. The transition probabilities in the matrix of the entire survey period were calculated by averaging probabilities in three annual matrices (2004–2005, 2005–2006, and 2006–2007). Regarding the seed stage, it appears that S. macrocarpus would not form a viable seed bank in the subtropical Ogasawaras because S. praecox seeds perfectly germinate when the temperature becomes consistently warm (Abe and Matsunaga 2007). Thus, we assumed there is almost zero probability that S. macrocarpus seeds would not germinate within 1 year. Although some parameters of establishment stage such as germination rate and mortality of current seedlings could not be obtained, the transition probability from fruiting size class to minimum size class was calculated by the proportion of the number of new seedlings to the number of seeds (a fruit was assumed to contain 40 seeds) produced in each year. To identify the important life stages for population growth, elasticity analysis was conducted (Caswell 2001). Because hermaphrodite plants produce almost no fruits even if they are hand-pollinated (see Results), the approximate functional gender of the hermaphrodite can be assumed to be male. Thus, effective population size was calculated by N e = 4N h N f/(N h + N f), where N h is the number of hermaphrodite plants and N f is the number of female plants (Falconer 1989). The effective population size of the entire survey period (N e-total) was the harmonic average of all annual N e (N e-total = 4/[(1/N e-2004) + (1/N e-2005) + (1/N e-2006) + (1/N e-2007)]) (Frankham et al. 2002).
Damage by goat grazing was recorded for all shoots in every yearly population census. Grazing marks by goats could be distinguished by shoot ends crushed by chewing in contrast to the grazing marks of ship rats (Rattus rattus, another potential feeder in the Ogasawaras), which are either clean-cut or show teeth marks on cross sections. If individuals or shoot died within 2 years after goat grazing, the cause of death was determined to be goat grazing. Although other factors such as poor light conditions and typhoons could also lead to death, we concluded that goat grazing had the greatest impact on survival because of the relatively short duration between grazing and death. A “young shoot” was defined as a shoot of less than 2 years old. Current shoots can be identified easily by their fresh green stems and small sizes. Shoots that were 2 years old were also identified by the greenish color of their subdued stems, while shoots that were more than 3 years old gradually turned brown. In addition, 2-year-old shoots could be easily distinguished after the 2004 season because they were recorded as current shoots in previous year survey. Although it was difficult to identify the 2-year-old shoots in the first survey in 2003, it did not become a problem later because there was no grazing damage on any shoots in 2003. The effect of goat grazing on individual mortality was analyzed by G-test (Sokal and Rohlf 1995) using 2 × 2 contingency tables (dead vs. alive × grazed vs. not grazed). The effect of goat grazing on shoot mortality was tested using the Wilcoxon rank-sum test. These analyses were performed using JMP software (Sall et al. 2004).
Reproductive success
The sex and number of inflorescences per shoot were surveyed during the flowering season of all years. The number of flowers per inflorescence was recorded for all inflorescences when the number of inflorescences per shoot was less than 20. For the shoots that produced 20 or more inflorescences, the number of flowers was recorded for 20 randomly selected inflorescences, and then the number of inflorescences per individual was counted. Flower visitors were observed for 13 h 20 min (14 days) in the daytime and 4 h (3 days) at night. At the initiation stage of fruit development after flowering, fruits were covered with wire netting to prevent rat predation and fruit set was surveyed in April.
Sexual differences in stem diameter, H, the number of shoots, and the degree of crown suppression were compared at the individual level. Stem diameter and H were tested using ANOVA, the number of shoots was tested using the Wilcoxon rank-sum test, and the degree of crown suppression was analyzed by G-test using a 2 × 3 contingency table (hermaphrodite vs. female × shaded vs. open vs. intermediate). The growth rates of stem diameter and H were averaged annual growth of all years and were compared using ANOVA at the shoot level.
To test the effect of pollen limitation for fruit set, we selected one female and three hermaphrodites in 2003, five females and three hermaphrodites in 2004, five females and four hermaphrodites in 2005, three females in 2006, and three females in 2007 for hand-pollination. Pollen donors for the hand-pollination were selected from two or three nearby hermaphrodite plants. As the controls of hand-pollination, natural fruit sets were measured within the same individual. Because of the limited number of females and flowers, we tested only large females having more than 30 inflorescences. Since the amount of data was insufficient, all data from the 5 years were pooled for the significance test. The effect of hand-pollination on fruit set was analyzed by G-test using a 2 × 2 contingency table (fruited vs. not fruited × hand-pollination vs. natural).
Habitat characteristics
To describe the habitat of each S. macrocarpus individual, vegetation was surveyed using the phytosociological method. A 15 × 15 m quadrate “habitat” was set up with a focus on S. macrocarpus individuals, and the species composition of each forest layer, coverage of each species, community height, and slope (direction and degree) of the habitat were recorded. The height of each layer was defined by the maximum height of aggregated individual crowns belonging to each layer and was estimated by eye to the nearest 1 m. Coverage of each species was evaluated for six classes, as follows: 5, 75–100%; 4, 50–75%; 3, 25–50%; 2, 10–25%; 1, 1–10%; +, <1%. Definitions of both endemic and alien species followed Toyoda (2003). Categories of endangered species were according to the Environmental Agency of Japan (2000), which uses the same category divisions as the 1994 IUCN Red Data Book (IUCN 1994), except that the former does not have “Conservation Dependant”, “Least Concern” in “Lower Risk”, and “Not Evaluated”.
To estimate the light environment, hemispheric photographs were taken using a fisheye lens (Nikon, FC-E9) at 1 m above ground level at five points (center and 5 m distant looking toward N, S, W, E) for each S. macrocarpus individual. Then, crown openness was measured using the CanopOn 2 free software (http://www.takenaka-akio.cool.ne.jp/etc/canopon2/). Mean openness used for analysis was the average of these five measurements.
The degree of crown suppression was judged by eye according to the following classifications: “shaded” (individual crown of S. macrocarpus is completely covered by other tall trees), “open” (individual crown of S. macrocarpus reaches the community crown level and can receive full sunlight), and “intermediate” (individual crown is partly shaded by other shrubs or trees).
The effect of crown suppression on individual mortality was analyzed by G-test using a 2 × 3 contingency tables (dead vs. alive × shaded vs. open vs. intermediate), and its effects on the log-transformed mortality rate of shoots within an individual was analyzed by ANOVA. The relationship between the openness and relative growth rate of shrub height was analyzed by linear regression. The relative growth rate of shrub height was the ratio of the height before the growth to the amount of growth that occurred during the observation period. These analyses were performed using JMP software (Sall et al. 2004).
Results
Status of population
A total of 87 individuals of S. macrocarpus were found in Chichi-jima Island during the 4 years of the survey of which 19 individuals (21.8%) died. Mortality rates per year were 4.5% in 2004, 3.2% in 2005, 10.5% in 2006, and 15.5% in 2007. At the same time, only two seedlings were found to have germinated from seeds during the survey period.
The size of adult S. macrocarpus ranged from 23 to 330 cm in height and from 9.1 to 41.3 mm in stem diameter (Fig. 2), and 44.4% of adults (24 individuals) flowered in the forest understory. There were few individuals of the smallest size class (Fig. 2a and Table 1). The mean number of shoots per individual was 4.4 ± 0.6 (SE, n = 22) in 2003, 3.3 ± 0.3 (n = 62) in 2004, 3.0 ± 0.2 (n = 76) in 2005, 2.8 ± 0.3 (n = 71) in 2006, and 3.1 ± 0.3 (n = 68) in 2007.
The total number of shoots observed during the 4 years was positively correlated with the stem diameter of the largest shoot of an individual (F 1,65 = 9.9, P = 0.003). The annual transition probability for the 4 years was low for the smallest size class (Table 2a) and the population growth rate was 0.979. Elasticity analysis showed that survival of the largest individuals was the most important factor in the population growth rate (Table 2b). The effective population size (N e) of the survey years varied from 23.1 to 24.6 individuals and the ratio of effective population size (N e/N) ranged from 0.31 to 0.38 (Table 3).
Although S. macrocarpus persisted by basal rooting of coppice shoots, grazing by wild goats (Fig. 4a) was gradually increasing and its impact was more intense in young shoots (Fig. 3 and Table 4). Goat grazing caused the death of up to 45 shoots during the survey period, accounting for up to 25.3% of the 178 dead shoots and 54.9% of the 82 grazed shoots. The occurrence of goat grazing was not related with individual mortality (G = 2.0, P = 0.153) but it was significantly related with shoot mortality (Z = 3.3, P = 0.001).
Reproductive success
In total, 58 of 87 individuals (66.7%) flowered at some time during the 4 years. Among these 58 adults, there were only 12 females (20.7%) and the others were hermaphrodites. Changes in sex expression either from hermaphrodite to female or female to hermaphrodite were not observed during the survey. Sexual differences were not observed in stem diameter (F 1, 54 = 0.6, P = 0.449), H (F 1, 54 = 0.0, P = 0.972), number of shoots (Z = 0.7, P = 0.467), and crown condition (G = 4.4, P = 0.136). However, the growth rate of females was significantly higher in H (female = 32.5 ± 4.5 cm/year, hermaphrodite = 14.7 ± 2.4; F 1, 180 = 12.4, P < 0.001) and marginally significantly higher in stem diameter (female = 0.59 ± 0.09 mm/year, hermaphrodite = 0.41 ± 0.05; F 1, 168 = 3.2, P = 0.077) in the shoot level analysis. On average, 2.8 individuals produced fruits naturally each year (Table 5). The amount of flowering and fruiting exhibited annual fluctuations (Tables 3 and 5).
Flower visitors in daytime were very rare. Only two crane flies (Limonia sp.: Tipulidae) were observed while introduced honeybees, which are the dominant pollinators on the island (Abe 2006) did not visit. Four nocturnal moths (Noctuidae) and one crane fly were observed to have visited at night.
Fruit set during the 4 years was 0.0% in hermaphrodites and 7.4% in females. However, hermaphrodite flowers were not completely sterile because they produced a few fruits (Table 3). Outcrossing treatment by hand-pollination did not significantly increase the fruit set in either hermaphrodites (from 0.0 to 0.0%) or females (from 5.8 to 7.5%) (G = 0.0, P = 0.981).
Habitat characteristics
The vegetation of S. macrocarpus habitat consists of dry forest trees of 4–8 m in height and dry scrub of 1–3 m in height. The dominant species in the community are endemic trees such as Schima mertensiana, Distylium leidotum, and Boninia glabra (Table 6). This community is categorized as primary Distylium-Schima dry forest and secondary Pinus-Schima mesic forest (Shimizu and Tabata 1991) where the natural vegetation has not been affected by human disturbance (Shimizu 1992). Species richness within the 15 m square S. macrocarpus habitat was 30.0 ± 4.3 species in which 70.9 ± 6.2% of species were endemic (58 species in total), 3.0 ± 1.5% were alien (four species), 26.1 ± 6.1% were widespread (23 species), and 37.1 ± 5.9% were endangered species listed in the Red Data Book (38 species) (Table 6).
The “shaded” habitat (Fig. 4b) was characterized by 5 to 7-m-high crown trees such as Schima mertensiana and by a few emergent (8–20 m high) Pinus lutchuensis. The “open” habitat was typically formed by tree fall or die-back of large P. lutchuensis. The “intermediate” habitat was mainly located on the sloping crests where community height growth was restricted by intense winds or dry soil conditions.
(a) Young shoot damaged by goat grazing. The brown thick stems in the foreground are primary shoots and the shoot on the far right was damaged by goat trampling. (b) Typical habitat of S. macrocarpus (c) Hermaphrodite inflorescences in forest understory. (d) Leaves sunburned in open space. (e) Fruits predated by R. rattus. (f) Protective net against goat grazing. (g) Fruits covered by a protection wire bag. (h) Nursery plants to reduce the extinction risk of S. macrocarpus (Chichi-jima)
Regarding the crown condition, “shaded” individuals accounted for 42.5% while “intermediate” and “open” accounted for 21.8% and 35.6%, respectively. “Shaded” individuals had a significantly higher shoot mortality per individual (ANOVA, F 2, 86 = 7.1, P = 0.001), while their individual mortality (29.7%) was not significantly higher than “intermediate” (26.3%) and “open” (9.7%) (G = 4.7, P = 0.096).
Mean openness was 10.3 ± 2.1% (5.1–19.5%) and openness was mostly within the range of 8–14% (Fig. 5a). Distribution of slope direction of habitat aggregated toward the northern and eastern slopes (Fig. 5c). The relationship between openness and individual height was negatively correlated (height (cm) = 219.5 – 6.7 × openness (%), ANOVA, F 1, 86 = 4.4, P = 0.039, Fig. 6). Openness seems to have had a negative relationship with reproductive activity, except for the number of fruits in 2004, but this was significant only with the number of female inflorescences in 2007 (Table 7).
Discussion
Population and reproductive status of S. macrocarpus
In January 2007, the population of S. macrocarpus on Chichi-jima Island consisted of 68 individuals. Since the distribution is limited to the Higashidaira region, there might be less than 100 extant individuals. A previous study had noted that the population of S. macrocarpus was decreasing (Ohi et al. 1998), and our analysis of population growth rate provided quantitative evidence to support this. The high elasticity in the adult size class of S. macrocarpus is a common character of long-lived woody species (Kwit et al. 2004) and implies that an increase in mortality in reproductive adults would result in a rapid decrease of the population. In addition, the population of S. macrocarpus consisted of an extremely low number of females. Such a biased sex ratio would decrease the ratio of effective population size to actual population size (N e/N) (Franham 1995). Moreover, the small N e/N ratio of S. macrocarpus is even smaller than that of other endangered species (0.40–0.44 in Smith and McDougal (1991) and 0.44–1.41 in Nunney and Elam (1994)). These evidences collected during the 4 years of the survey implies that S. macrocarpus has a significantly increased chance of extinction in the near future.
Two extinction threats for S. macrocarpus are very low seed production (fruit set in only 7.4% of female) and the low annual recruitment rate of seedlings (2.4%) versus the 13.6% annual mortality. Since hermaphrodites produce almost no fruit, the hermaphrodite-biased sex ratio results in an average of only 2.8 individuals in the population naturally producing fruit per year. In addition, individual female performance of fruit set (approximately 7.4%) is at a much lower level compared with the closely related S. praecox (approximately 35% fruit set) (Abe 2007a). One cause of the low fruit set may be resource limitation in the stressful environment as mentioned below, and another may be inbreeding within the small population (Ellstrand and Elam 1993; Frankham 1996).
In general, female plants have high mortality and low reproductive frequency because of higher reproductive cost (Delph 1999). The hermaphrodite-biased sex ratio and annually fluctuating fruit set of females suggest a higher reproductive cost for females of S. macrocarpus. However, females tended to grow faster, perhaps because of their low fruit production (Gross and Soule 1981; Sakai and Burris 1985). Another possible reason may be that the trade-off between the number of flowers and the sink-regulated photosynthesis can lead to a higher growth rate in females (Delph and Meagher 1995). However, these potential factors could not be tested rigorously in this study. Unlike the pioneer shrub S. praecox, the main habitat of S. macrocarpus is forest understory. The result of hand-pollination slightly enhanced the fruit set but not significantly. The findings of the present study suggest that the main factor restricting fruit set may be poor resources rather than lack of pollen. The low fruit production and the hermaphrodite-biased sex ratio may be caused by shading in the habitat because females tend to be more affected for reproduction and have higher mortality than males in stressful environments (Delph 1999).
In addition, the pollination system has been disturbed in Chichi-jima Island (Abe 2006). The bell-shaped flowers of S. macrocarpus would be suitable for small bees or long-proboscid pollinators. Indeed, the closely related S. praecox typically inhabits sunny forest edges in temperate areas on the Japanese mainland and diverse flower visitors such as hover flies and flower bees help it to produce rich fruit sets (Abe 2007a). However, there are very few flower visitors of S. macrocarpus. The dominant pollinators on the island, introduced honeybees, do not visit shaded individuals in the forest understory (Fig. 4c). The primary pollinators of S. macrocarpus are probably nocturnal moths.
Habitat characteristics
Typical habitats of S. macrocarpus are in dry forest communities of 4–8 m in height, which is in stark contrast with the forest edge habitat of S. praecox (Abe 2007a). The Distylium dry forest in Higashidaira has the highest angiosperm species diversity in the Ogasawaras (Shimizu 1992) and includes many endangered species. These facts suggest that S. macrocarpus can survive in places that have not been influenced by human disturbances. Communities are characterized by a high occurrence of Pinus lutchuensis of more than 10 m in tree height but low coverage, and a forest crown that mostly consists of species of less than 10 m tree height such as Schima mertensiana, Planchonella obovata, and Distylium leidotum. Stachyurus macrocarpus individuals in open habitat or sunny crown conditions have no advantage in growth or the number of shoots. As the genus Stachyurus originally occurred in temperate East Asia (Li 1943; Iwatsuki et al. 1999), it is possible that the species is stressed by the intense sunlight and dry conditions of the subtropical Ogasawaras. The scarce distribution on southern and western slopes also supports this explanation. Indeed, leaf sunburn disease was observed in S. macrocarpus located in open spaces (Fig. 4d).
The population decline of endangered species, including S. macrocarpus, observed in Higashidaira can be explained by long-term aridification (Shimizu 1999). Annual precipitation in the Ogasawaras has been decreasing during the last few decades (Oka et al. 2000; Yoshida et al. 2006). Aridification would increase stress for plants because originally the Ogasawaras had a subtropical climate with a little annual precipitation. In addition to aridification, frequent typhoons bringing salt breezes (Shimizu 2003b) can damage S. macrocarpus in open habitat. The withering of current shoots of S. macrocarpus emerging from crowns has often been observed after a typhoon. Indeed, the height growth and reproductive activity tend to decrease as openness increases. These environmental restrictions allow S. macrocarpus to survive only in dense scrubland and forest understory.
At the same time, the suppressive environment increased the mortality of S. macrocarpus. Approximately 10% of openness is unlikely to provide a sufficient light environment for seedlings of this pioneer shrub. In addition, Shimizu (2005) showed that the community height of dry forests gradually increases in Higashidaira. If so, further S. macrocarpus would become affected by vegetative suppression.
These findings suggest that the suitable habitat of S. macrocarpus is narrow in the subtropical environment of the Ogasawaras. Narrowly endemic species usually adapt to a particular stressful habitat and are unable to compete in low-stress habitats (Lavergne et al. 2004). If this is the case with S. macrocarpus, it would be difficult to maintain or restore the population of the species in an artificial new habitat. The Ogasawara Islands have been settled since the 1880s and natural forests have decreased since then (Shimizu 2003a; Toyoda 2003). The most favorable habitats of S. macrocarpus were probably destroyed in this era.
Effects of alien species
Introduced goats have destroyed natural vegetation in islands around the world (Schofield 1989; Stone et al. 1992; Courchamp et al. 2003). Although control activity of goat population had been conducted in Chichi-jima, recent population increase has exceeded the degree of control activity. This would account for the recent increase in grazing damage of S. macrocarpus. Goat grazing of sprouts was not associated with the mortality of S. macrocarpus individuals probably because sprouts tend to occur in larger individuals. However, the mortality of grazed shoots was high.
Replacing the constituent stems of a shrub is more beneficial to its fitness (e.g., carbon gain and sexual reproduction) than growing or maintaining a single-stem in a high-stress environment such as forest understory (Midgley 1996; Kruger et al. 1997). Since S. macrocarpus maintains itself by sprouting under a suppressed environment, frequent grazing of young shoots would become lethal for individuals. It is possible that repeated grazing of sprouts damages the S. macrocarpus population because elasticity analysis showed that large individuals play a particularly significant role in the population growth rate. In addition, rarely produced fruits suffer predation threats from introduced black rats, Rattus rattus (Fig. 4e), which are intensive seed predators in the Ogasawaras (Yamashita et al. 2003; Abe 2007b) as well as in other invaded habitats (Courchamp et al. 2003; Shaw et al. 2005).
Implications for conservation management
The effective population size calculated from the number of effective hermaphrodites and females was much smaller than the 500 individuals required for the minimum effective population size for the long-term (Franklin 1980; Soulé 1980). Further study of population genetics is required to evaluate various genetic risks (Frankham et al. 2002).
There are two conservation measures that should be taken immediately. The first is to eliminate the threats posed by introduced herbivore mammals. Because of the difficulty to create new populations, the maintenance of natural habitat should be prioritized for the conservation of endangered species (Drayton and Primack 2000). Total eradication of goats from Chichi-jima Island will take a long time hence the protection of S. macrocarpus individuals from grazing would be an effective emergency interim measure. For example, we are using nets to protect against goat grazing (Fig. 4f) and covering fruits with wire net bags to prevent rat predation (Fig. 4g).
Second, the population should be reinforced by growing S. macrocarpus in nurseries. There is only one reliable population of S. macrocarpus and it is very small in size. Once the population becomes too small, it is difficult to restore it naturally because of its narrow niche, the allee effect, and bottleneck effects such as a decrease in genetic diversity and inbreeding depression (Schemske et al. 1994; Frankham et al. 2002). The seedling recruitment of S. macrocarpus is very low probably because of low seed production and goat grazing of seedlings. It would be better to produce stock in a nursery on Chichi-jima Island because it could avoid the risk of accidental introduction of alien species in the soil.
Nurseries actually did produce some stock from seeds in Chichi-jima Island in 2005 (Fig. 4h). If nursery-cultivation techniques can be established for S. macrocarpus, they may also be applied for the conservation of the endemic S. macrocarpus var. prunifolius, of which only one hermaphrodite individual remains in Haha-jima Island, today.
In summary, it became clear by our analyses that S. macrocarpus is in danger of extinction. The remaining population is estimated to be less than 100 individuals and declined 21.8% during the 4 years of the study. The high mortality in shaded habitats and low growth rate in open habitats suggest that there is little suitable habitat in the Ogasawaras. Moreover, fruit set was not restricted by pollen limitation but it was still very low. These results also suggest that the environmental conditions of the subtropical Ogasawaras are not so suitable for S. macrocarpus. In addition, grazing impact by goats has seriously increased the extinction risk. Since goat grazing may threaten other species besides S. macrocarpus, scientific research on population states and anti-goat measures are immediately needed for other endangered plants of the Ogasawaras.
References
Abe T (2006) Threatened pollination systems in native flora of the Ogasawara (Bonin) Islands. Ann Bot 98:317–334
Abe T (2007a) Sex expression and reproductive biology of Stachyurus praecox (Stachyuraceae). Bull For For Prod Res Inst 6:151–156
Abe T (2007b) Predator or disperser? A test of indigenous fruit preference of alien rats (Rattus rattus) on Nishi-jima (Ogasawara Islands). Pacific Conserv Biol 13:213–218
Abe T, Matsunaga M (2007) Germination characteristics of a pioneer shrub, Stachyurus praecox (Stachyuraceae). Bull For For Prod Res Inst 6:145–149
Baillie JEM, Hilton-Taylor C, Stuart SN (2004) 2004 IUCN red list of threatened species: a global species assessment. IUCN Publication Services Unit and The IUCN Species Programme, Cambridge
Brook BW, O’Grady JJ, Chapman AP, Burgman MA, Akçakaya HR, Frankham R (2000) Predictive accuracy of population viability analysis in conservation biology. Nature 404:385–387
Caswell H (2001) Matrix population models, 2nd edn. Sinauer, Sunderland
Coulson T, Mace GM, Hudson E, Possingham H (2001) The use and abuse of population viability analysis. Trends Ecol Evol 16:219–221
Courchamp F, Chapuis JL, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383
Delph LF (1999) Sexual dimorphism in life history. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 149–173
Delph LF, Meagher TR (1995) Sexual dimorphism masks life history trade-offs in the dioecious plant Silene latifolia. Ecology 76:775–785
Drayton B, Primack RB (2000) Rates of success in the reintroduction by four methods of several perennial plant species in eastern Massachusetts. Rhodora 102:299–331
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Ann Rev Ecol Syst 24:217–242
Environmental Agency of Japan (2000) Threatened wildlife of Japan–red data book, 2nd edn., vol 8. Vascular plants. Japan Wildlife Research Center, Tokyo (in Japanese)
Falconer DS (1989) Introduction of quantitative genetics. Longman, Harlow
Frankham R (1995) Effective population size/adult population size ratios in wildlife: a review. Genet Res 66:95–107
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Franklin IR (1980) Evolutionary change in small populations. In: Soulé ME, Wilcox BA (eds) Conservation biology: an evolutionary–ecological perspective. Sinauer, Sunderland, pp 135–150
Gross KL, Soule JD (1981) Differences in biomass allocation to reproductive and vegetative structures of male and female plants of a dioecious, perennial herb, Silene alba (Miller) Krause. Am J Bot 68:801–807
IUCN (1994) IUCN red list categories. IUCN-Species Survival Commission, Gland
Iwatsuki K, Boufford DE, Ohba H (1999) Floral of Japan, Volume IIc Angiospermae, Dicotyledoneae, Archichlamydeae (c). Kodansha Ltd., Tokyo
Kaizuka S (1977) The geology and geography of the Ogasawaras. Ogasawara Kenkyu Nenpo 1:29–34 (in Japanese)
Kasaki H (1991) Endangered plants on Ani-jima Island. Ogasawara Kenkyu Nenpo 15:1–19 (in Japanese)
Kruger LM, Midgley JJ, Cowling RM (1997) Resprouters vs reseeders in South African forest trees: a model based on forest canopy height. Func Ecol 11:101–105
Kwit C, Horvitz CC, Platt WJ (2004) Conserving slow-growing, long-lived tree species: input from the demography of a rare understory conifer, Taxus floridana. Conserv Biol 18:432–443
Lande R (1993) Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am Nat 142:911–927
Lande R, Barrowclough GF (1987) Effective population size, genetic variation and their use in population management. In: Soulé ME (ed) Viable populations for conservation. Cambridge University Press, New York, pp 87–123
Lavergne S, Thompson JD, Garnier E, Debussche M (2004) The biology and ecology of narrow endemic and widespread plants: a comparative study of trait variation in 20 congeneric pairs. Oikos 107:505–518
Li HL (1943) The genus Stachyurus. Bull Torrey Bot Club 70:615–628
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invisibility. Ecology 80:1522–1536
Loope LL, Hamann O, Stone CP (1988) Comparative conservation biology of oceanic archipelagoes Hawaii and the Galapagos. BioScience 38:272–282
Loope LL, Mueller-Dombois D (1989) Characteristics of invaded islands, with special reference to Hawaii. In: Drake JA, Mooney HA, Di Castri F, Groves RH, Kruger FJ, Rejmánek M, Williamson M (eds) Biological invasions: a global perspective. Wiley, West Sussex, pp 257–280
Menges ES (2000) Population viability analyses in plants: challenges and opportunities. Trends Ecol Evol 15:51–56
Midgley JJ (1996) Why the world’s vegetation is not totally dominated by resprouting plants; because resprouters are shooter than reseeders. Ecography 19:92–95
Nunney L, Campbell KA (1993) Assessing minimum viable population size: demography meets population genetics. Trends Ecol Evol 8:234–239
Nunney L, Elam DR (1994) Estimating the effective population size of conserved populations. Conserv Biol 8:175–184
O’Dowd DJ, Green PT, Lake PS (2003) Invasional ‘meltdown’ on an oceanic island. Ecol Let 6:812–817
Ohi T, Kato H, Murata J (1998) Distribution of endangered plants (Stachyurus macrocarpus, S. macrocarpus var. prunifolius, and Eurya boninensis) in the Ogasawara Islands. Ogasawara Kenkyu Nenpo 22:51–55 (in Japanese)
Ohi T, Murata J (1999) Geographical variations in chloroplast DNA of Stachyurus macrocarpus Koidz. and S. praecox Sieb. et Zucc. (Stachyuraceae). Ogasawara Res 25:1–25
Ohi T, Wakabayashi M, Wu S, Murata J (2003) Phylogeography of Stachyurus praecox (Stachyuraceae) in the Japanese Archipelago based on chloroplast DNA haplotypes. J Jpn Bot 78:1–14
Oka S, Yoshida K, Iwashita H, Iijima Y, Satoh T (2000) Interannual variability of the hydroclimatic environment, based on the water balance at Chichi-jima Island in the Bonin (Ogasawara) Islands. Ogasawara Res 26:15–33
Oostermeijer JGB (2003) Threats to rare plant persistence. In: Brigham CA, Schwartz MW (eds) Population viability in plants: conservation, management and modeling of rare plants. Springer, Berlin, pp 17–58
Sakai AK, Burris TA (1985) Growth in male and female aspen clones: a twenty-five-year longitudinal study. Ecology 66:1921–1927
Sall J, Creighton L, Lehman A (2004) JMP start statistics, 3rd edn. SAS Instutite, Cary
Schemske DW, Husband BC, Ruckelshaus MH, Goodwillie C, Parker IM, Bishop JG (1994) Evaluating approaches to the conservation of rare and endangered plants. Ecology 75:584–606
Schofield EK (1989) Effects of introduced plants and animals on island vegetation: examples from the Galapagos Archipelago. Conserv Biol 3:227–238
Shaw JD, Hovenden MJ, Bergstrom DM (2005) The impact of introduced ship rats (Rattus rattus) on seedling recruitment and distribution of a subantarctic megaherb (Pleurophyllum hookeri). Anim Ecol 30:118–125
Shimizu Y (1991) Vegetation of Ani-jima Is. in the Bonin (Ogasawara) Islands: distribution, composition, structure of dry forests. Komazawa Geography 27:7–130 (in Japanese)
Shimizu Y (1992) Origin of Distylium dry forest and occurrence of endangered species in the Bonin Islands. Pacific Sci 46:179–196
Shimizu Y (1999) A 21-year population dynamics in a dry forest at Chichijima in the Bonin Islands. Jpn J Conserv Ecol 4:175–197 (in Japanese)
Shimizu Y (2003a) The nature of Ogasawara and its conservation. Glob Environ Res 7:3–14
Shimizu Y (2003b) Typhoon damage to vegetation on the Ogasawara Islands–the case of Typhoon No. 9 (July 2002). Komazawa Geography 39:1–15 (in Japanese)
Shimizu Y (2005) A vegetative change during a 20-year period following two continuous disturbances (mass-dieback of pine trees and typhoon damage) in the Pinus-Schima secondary forest on Chichijima in the Ogasawara (Bonin) Islands: which won, advanced saplings or new seedlings? Ecol Res 20:708–725
Shimizu Y, Tabata H (1991) Forest structures, composition, and distribution on a Pacific island, with reference to ecological release and speciation. Pacific Sci 45:28–49
Simberloff D (1995) Why do introduced species appear to devastate islands more than mainland areas? Pacific Sci 49:87–97
Smith JLD, McDougal CW (1991) The contribution of variance in lifetime reproduction to effective population size in tigers. Conserv Biol 5:484–490
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Soulé ME (1980) Thresholds for survival: maintaining fitness and evolutionary potential. In: Soulé ME, Wilcox BA (eds) Conservation biology: an evolutionary-ecological perspective. Sinauer, Sunderland, pp 151–169
Stone CP, Cuddihy LW, Tunison JT (1992) Responses of Hawaiian ecosystems to removal of feral pigs and goats. In: Stone CP, Smith CW, Tunison JT (eds) Alien plant invasions in native ecosystems of Hawaii: management and research. University of Hawaii Press, Honolulu, pp 666–704
Sutherland WJ Pullin AS, Dolman PM, Knight TM (2004) The need for evidence-based conservation. Trends Ecol Evol 19:305–308
Toyoda T (2003) Flora of Bonin Islands. Aboc-sha, Kamakura (in Japanese)
Yamashita N, Tanaka N, Hoshi Y, Kushima H, Kamo K (2003) Seed and seedling demography of invasive and native trees of subtropical Pacific islands. J Veg Sci 14:15–24
Yoshida K, Iwashita H, Iijima Y, Oka S (2006) Long-term change in the hydroclimatic environment during the 20th century on Chichi-jima in the Ogasawara (Bonin) Islands. Geograph Rev Jpn 79:516–526 (in Japanese)
Acknowledgments
The authors would like to thank Mr. Takaya Yasui and Ms. Midori Yokoya (Ogasawara Wildlife Research Society) for their field assistance. This study was partly supported by funding from the Ministry of the Environment (Global Environment Research Fund F-051).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abe, T., Wada, K. & Nakagoshi, N. Extinction threats of a narrowly endemic shrub, Stachyurus macrocarpus (Stachyuraceae) in the Ogasawara Islands. Plant Ecol 198, 169–183 (2008). https://doi.org/10.1007/s11258-007-9393-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9393-7