Abstract
Despite of the widespread co-occurrence of multiple invaders, little is known on their combined ecological impacts and on their effects on different life stages of native species. We assessed the joint impacts of four non-native mammals (cattle, horse, European hare Lepus europaeus, and wild boar Sus scrofa) on seed surplus and seedling abundance of the Paraná pine (Araucaria angustifolia), a critically-endangered species of the Atlantic Forest. We found that its seeds constitute an autumn food resource for a native community richer than previously thought, with 70 bird and mammal species as confirmed or potential seed consumers, of which 40 were not previously recognized as such. We also recorded the number of uneaten seeds and seedlings at the middle-end of autumn under 520 female Paraná pine trees across the species’ distribution and identified signs of the species consuming seeds from each tree through direct observations combined with camera trapping. Most of the sampled trees (98%) were visited by at least one seed consumer species, and over 60% were visited by at least one non-native mammal. Seed surplus strongly declined in the presence of cattle, horses and wild boars, their impacts being additive, whereas the number of seedlings declined in the presence of European hares. Our results emphasize the importance of Paraná pine seeds for native fauna and the additive impact of invaders in a species-rich ecosystem. Seed predation by non-native species reduces the potential regeneration of Paraná pine forests, and may severely reduce food supply for its native consumers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assessing the effects of biological invasions is challenging due to the potentially multifaceted impact of introduced non-native species on native biota and ecosystems, including changes in the abundance and distribution of common species, ecological properties and interactions that tend to pass unnoticed (Simberloff et al. 2013). Moreover, recent reviews have stressed the importance of considering the impacts of multiple co-occurring invasive species, rather than focusing on single-species invasions (Kuebbing et al. 2013; Jackson 2015; Ballari et al. 2016). This shift is important because over two-thirds of habitats of conservation concern have been invaded by multiple species, and interactions among co-occurring invaders may strongly influence their invasiveness and overall impact (Kuebbing et al. 2013). This was illustrated by a recent meta-analysis of the combined impact of 45 pairs of co-occurring invasive animals (Jackson 2015), finding that impacts were generally antagonistic (i.e. less than predicted based on their individual effects) but that they become additive when the impact of multiple invaders on autotrophs was examined. Much more information is however required to fully understand the combined impacts of multiple invaders, as the above conclusions mostly come from aquatic ecosystems (43 out of the 45 species pairs studied, Jackson 2015). Even less is known on the combined impact of more than two co-occurring invaders, and on their impacts on different life stages of native biota.
Latorre et al. (2013) found combined and complex impacts of three introduced mammals on three life stages (seeds, seedlings and saplings) of an endangered perennial shrub (Medicago citrina), and Tella et al. (2016a) found combined impacts of nine non-native mammals on the seeds and seedlings of a long-lived endangered tree (Araucaria araucana). However, these studies were conducted in ecosystems with a naturally-poor community of native seed predators (a small Mediterranean island and the Andean Patagonia, respectively), and thus the combined impact of multiple invaders could differ when examined in species-rich ecosystems. Elton (1958) postulated that species-rich communities might be more resistant to invasion than those that are species-poor given the scarcity of vacant niches in the former (biotic resistance hypothesis). In such a case, a rich community of native species would leave few food resources available to invaders, translating into negative impacts of one invader on another (Jackson 2015) and on an overall reduced impact of multiple invasions. Seven decades later, however, there is still little information for assessing this hypothesis regarding the susceptibility of forests to multiple mammal invasions (Latham et al. 2017).
In this study, we replicated the assessment of the combined impacts of multiple non-native mammals on two life stages (seeds and seedlings) of Araucaria araucana (Tella et al. 2016a) using as study model a sister species, the Paraná pine (Araucaria angustifolia). Contrarily to the Andean A. araucana forests, where the community of its seed predators is reduced to a parakeet and a few mice species (Tella et al. 2016a), the seeds of A. angustifolia in the Brazilian Atlantic Forest constitute a key food resource for a rich community of mammals and birds (Vieira and Iob 2009). First, we complemented published information with direct observations and the use of camera traps for a better knowledge of the native community of seed consummers. Thereafter, we recorded signals of native and four non-native species visiting 520 fruiting female Paraná pines for testing the hypotheses that (H1) non-native species would negatively influence seed availability and the number of seedlings under female parent trees through seed predation and seedling browsing (Sanguinetti and Kitzberger 2010; Tella et al. 2016a, b; Zamorano-Elgueta et al. 2012), predicting that impact on both life stages of the tree would increase as the number of co-occurring non-native species (i.e., species richness) increases; and that (H2) the identity of non-native consumers would influence their impact on seed availability and regeneration (Latorre et al. 2013), predicting that the occurrence of cattle, horse, wild boar (Sus scrofa) and European hare (Lepus europaeus) would have different negative effects on the two life stages of the tree. In concert with the results found by Jackson (2015) on the joint impacts of multiple non-native consumers on a native autotroph, we also posited that (H3) interactions between introduced mammals would be mostly neutral (i.e. no synergistic or antagonistic interactions), resulting in an overall additive impact. Finally, applying the biotic resistance hypothesis (Elton 1958) at the smaller geographic scale, we expected a negative interaction between native and non-native species on their impact on individual trees (H4). This scenario can easily be envisioned if, for example, a high number of native species that pick up Paraná pine seeds before fruits fall to the ground (e.g. parrots and monkeys, Vieira and Iob 2009) would leave few resources available for non-native mammals, which can only forage under the tree canopy. We discuss the implications of this study for the management of multiple invasive mammals and conservation of the Paraná pine and the native species that depend on it across its range.
Methods
Study area and species
Distributed throughout southern and southeastern Brazil, the Paraná pine has great economic and ecological importance (Pereira and Ganade 2008; Thomas 2013). The species defines the Araucaria Forest ecoregion of the Atlantic Forest biome, but it is also found in montane moist forests and grasslands (Thomas 2013). Its range declined by more than 97% in the last century due to massive exploitation for timber, generating a mosaic of relatively small forest patches (Thomas 2013). Females produce on average 13–20 cones, each yielding 80–90 large (c. 7 g), highly nutritious seeds after 20–24 months of maturation (Mantovani et al. 2004). Seed maturation and seedfall occur from March to June, a period of great fruit scarcity, thus offering a food resource for a variety of mammals and birds, including threatened species such as Vinaceous-breasted Amazon (Amazona vinacea; endangered), Red-spectacled Amazon (A. pretrei; vulnerable), and Azure Jay (Cyanocorax caeruleus; near-threatened) (Vieira and Iob 2009; IUCN 2016). In turn, several of these consumers offer important seed-dispersal services over short (rodents) and long distances (jays, parrots; up to approx. 500 m) (Vieira and Iob 2009; Tella et al. 2016b). With a mix of pioneer and late-successional-type features, such as the ability to germinate and establish in open areas and in shaded conditions (Duarte and Dillenburg 2000), the Paraná pine has the capacity to regenerate and establish populations in a variety of natural and anthropogenic vegetation types (Pereira and Ganade 2008). Although small juveniles tend to be more frequent in microsites with increased light levels, the main factors limiting regeneration and seedling establishment patterns are biotic barriers such as seed predation and dispersal (Pereira and Ganade 2008; Souza et al. 2008).
We selected four distant large areas across the distribution of the Paraná pine to cover spatial variability in habitats and the diversity of seed consumers (Fig. 1, Martinez and Prestes 2008; Prestes et al. 2008, 2014): Serra da Mantiqueira (SM), in the municipalities of Gonçalves, Sapucaí-Mirim, Camanducaia (Minas Gerais state), Campos do Jordão and São Bento do Sapucaí (São Paulo); Serra Catarinense (SC), in Painel and Urupema, (Santa Catarina); Muitos Capões (MC, Rio Grande do Sul [RS]); and São Francisco de Paula (SF, in RS).
Location of the study region in southern Brazil (A), the original distribution (dark grey) of Paraná pine forests (B), and the location of the four selected study areas (C: Serra da Mantiqueira [SM], D: Serra Catarinense [SC], E: Muitos Capões [MC], F: São Francisco de Paula [SF]) showing the location of surveyed Paraná pine females (white dots, note many of them overlap), the patchy distribution of forests (light grey), and protected areas (dark grey). Protected areas visited were: (C) Parque Estadual de Campos do Jordão and Monumento Natural Estadual da Pedra do Baú; Estação Ecológica de Aracuri-Esmeralda (E); and Floresta Nacional de São Francisco de Paula and Centro de Pesquisas e Conservação da Natureza Pró-Mata PUC-RS (F)
Field sampling
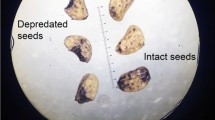
Fieldwork was conducted between 11 May and 6 June 2015, during the middle–end of the seed production period (Mantovani et al. 2004; Vieira and Iob 2009). Within study areas, we sampled 520 mature female Paraná pine trees to assess seed availability and identification of seed consumers (212 in SM, 121 in SC, 46 in MC and 141 in SF). We selected trees (Fig. 1) to cover a wide environmental gradient, from 802 to 1820 m a.s.l., distributed through early to late-successional and old growth forest patches differing in their size and connectivity, and also including isolated trees in grasslands, pastures and cultivated landscapes. Additionally, all selected trees were far enough apart (minimum distance = 40 m) to avoid confusion when assigning fallen seeds to parental trees (Solórzano-Filho 2001). To estimate seed surplus (a measure of seed availability) and count the number of yearling seedlings (young unbranched plants usually < 20 cm tall) under each tree, 2–3 persons inspected the ground within a radius of 10–20 m (depending on tree canopy size) for 10–15 min. We recorded the number of seedlings (λ) and an index of seed surplus (σ), obtained by scoring the quantity of seeds found under each tree among 10 categories—0: 0 seeds, 1: 1–10 seeds, 2: 11–20 seeds, …, 10: 91–100 seeds. See Tella et al. (2016b) for a detailed description of this sampling approach.
We employed a variety of methods to identify seed consumers visiting each tree (Tella et al. 2016b). Any species directly observed feeding on seeds on the ground or the tree crown during sampling was recorded. In addition, we searched under each tree for evidence of seed predation such as partically-eaten seeds (on which consumers regularly leave tooth and beak marks with characteristic shapes, Pereira and Ganade 2008; Tella et al. 2016a, b), feces, footprints and diggings. We also employed passive infrared-triggered camera traps on a subsample (n = 53) of trees distributed across sampled sites (16 in SM, 7 in SC, 14 in MC and 16 in SF), which operated for periods of 2–7 days (depending on logistic limitations), to record both diurnal and nocturnal consumers and inform the identification of markings and other tracks. Cameras were set up so that their detection zones were directly below the tree crown and overlooking an area with numerous and clearly visible seeds, and allowed recording of both medium and small-sized consumers.
As noted by Tella et al. (2016a, b), identification of consumers by feces, tracks and photographs (when the photographs show animals but not that they were actually feeding on seeds) is a presence-based approach and, as such, cannot assure that animals always consumed seeds when they were recorded visiting trees, although that seems likely for most species besides those with highly-specialized or carnivorous diets (e.g. felids). We may have failed to record consumers on occasions when no feces or other signs of presence were left, or if animals removed or ate seeds without leaving parts of consumed seeds as evidence. Consequently, our results regarding diversity of seed-consumer species at each tree and the proportion of trees visited by consumers should not be viewed as exact measurements of these parameters, but as conservative estimates.
To complement our field observations and provide an extensive summary of the diversity of Paraná pine seed consumers throughout its range, we performed a non-systematic review of the literature for records of species reported feeding on this resource (Table S1 in Supporting Information). We then classified them according to the type of interaction (seed predator or secondary consumer), whether or not they act as seed dispersers, and where they obtain seeds (on the ground or up on tree crowns). We also attempted to group consumers according to the importance of the Paraná pine seed for each species (when the resource is available), based on information on their diets in the literature and our own observations and long-term knowledge from studies in Paraná pine forests (e.g. Martinez and Prestes 2008; Prestes et al. 2008, 2014).
Modeling approach
We employed generalized linear models (GLM) to assess the potential effects of non-native seed consumers on seed availability (i.e. seed surplus [σ]) and seedling abundance (i.e. number of seedlings [λ]) under each female tree, the response variables. We developed a candidate set of biologically-plausible models to test our a priori hypotheses and predictions, verifying the slope (β), 95% confidence interval (CI), and the explained deviance for each variable, calculated using deviance partitioning analysis (Hastie and Pregibon 1992). We assessed the effect of non-native species on the response variables (Hypotheses 1 and 2, see Introduction) by either including the number of non-native species recorded under each tree (E) or the occurrence (a binary variable indicating presence or absence) of cattle, horse, wild boar and European hare (respectively, Ca, Ho, Wb and Ha). To examine whether multiple non-native consumers have an additive impact on response variables (Hypothesis 3), we included pairwise interaction terms among the four binary occurrence predictors species. An additive impact of two non-native species would be supported if their interaction term is not retained in the best-supported models. Finally, the interaction between the number of native (N) and number of non-native species (E) was assessed for testing our Hypothesis 4.
Both the number of seeds and seedlings per tree can be affected by several factors that need to be controlled for when testing the above mentioned hypotheses. Native species can have an impact on seed availability and number of seedlings, which could confound estimates of the impact of non-native seed/seedling predators. We controlled for this process quantitatively, by including richness of native seed consumers recorded at each tree (N). Recognizing the variability in the size of Paraná pines surveyed, the large geographical extent of our survey, and the heterogeneity in habitats (Solórzano-Filho 2001; Dillenburg et al. 2009; Vieira and Iob 2009), we controlled for their effects on response variables by including predictors identifying tree sizes (i.e. tree girth at breast height, G, centered and scaled to unit SD), study area (site factor with 4 levels; Fig. 1) and habitat (factor with 3 levels: forest [F], forest edge [Fe] and agro-pastoral [Ap]). These variables were included as fixed effects. We also considered interaction terms between study areas and occurrence of non-native consumers to assess potential spatial variations in consumer impacts. To avoid the problems derived from obtaining an excessive number of candidate models (Grueber et al. 2011), we initially fitted and compared models without interactions terms. After finding the best models from this initial candidate set, we obtained and compared additional models by fitting each possible interaction among the predictor variables indicating occurrence of non-native species present in the model.
Preliminary analysis revealed that Poisson GLMs showed residual overdispersion for both response variables, so we fitted models with negative-binomial distribution with a log link (see Tella et al. 2016a, b for the same approach). Models were ranked with Akaike’s information criterion (AIC), selecting the model with the lowest AIC value as the most parsimonious. Models within 2.0 ΔAIC of the best-ranked model were considered as equally supported. Then, we examined these alternative models for the identification of an eventual “pretending variable” (i.e. a noninformative variable that enters as one additional parameter and therefore incurs only a small “penalty” of about 2 AIC, but does not decrease the deviance; Anderson 2008). In such a case, we followed recommendations by Grueber et al. (2011) and disregarded models including pretending variables that are complex versions of the simplest one within the top model set (ΔAIC < 2); see also Arnold (2010) for the recommendation of reporting all models but dismissing those with uninformative variables when dealing with small sets of a priori-defined hypotheses. The difference in AIC values (ΔAIC) between the best-supported model and other models was used to calculate model weights (AIC wgt), which indicates the relative weight of evidence of the model given the data and the model set (Anderson 2008). Statistical analyses were done in R (R Development Core Team 2016) with packages MASS (Venables and Ripley 2002), AER (Kleiber and Zeileis 2008), and bbmle (Bolker and R Development Core Team 2017). Figures were made using R packages ggplot2 (Wickham 2009), hexbin (Carr 2018), gridExtra (Auguie 2017), and ArcGIS (ESRI 2013).
Results
Paraná pine seed consumers
Combining field observations with an extensive literature review, we identified 70 native species as confirmed (66) or potential (4) consumers of Paraná pine seeds, including 35 birds, 33 mammals and two insects (Table S1, Fig. 2). Of these, 32 species (45%) were detected with camera traps (9 exclusively) and 49 (59%) by direct observations (21 exclusively) at our sampling locations (Table S1). We identified 45 species (63%) as seed predators and 21 (30%) as secondary consumers—i.e. feeding on the remains of seeds opened, partly consumed, wasted, or dropped by seed predators (Fig. 2). We also identified four mammals (3 felids and the Brazilian cottontail Sylvilagus brasiliensis) that we recorded but could not confirm as seed predators or secondary consumers (Table S1). Two mammals, 13 birds, and the tortricid moth Cydia araucariae were only recorded feeding on Paraná pine seeds that were still on the tree canopies (i.e., cones in tree branches), while 51 other species (71%) were only recorded feeding on seeds fallen to the ground and only three bird species were recorded on both (Table S1, Fig. 2). Paraná pine seed dispersers, based on our observations and the literature, amounted to 23 species (33%, Table S1, Fig. 2). Paraná pine seeds were considered essential (40–100% of the diet during the period of seed production) for seven species (including three classified as threatened; Table S1, IUCN 2016), complementary (39–10% of the diet) for 20 species, and occasional (< 10% of the diet) for 22 species. We excluded 20 species from these groups due to lack of information. Moreover, we confirmed that the four non-native mammal species considered in this study—cattle, horse, wild boar and European hare—are consumers of Paraná pine seeds, and that the first three completely destroy seeds when consuming them, thus precluding the possibility of dispersal.
Diversity of Paraná pine seed consumers according to class (B birds, M mammals, I insects); type of interaction with seeds; whether the species has been recorded dispersing seeds; the relative importance of Paraná pine seeds in the diet (essential: 100–40% of the diet; complementary: 39–10%; occasional < 10%); and whether species forage seeds on the ground, tree crown, or both
Most of the 520 female Paraná pine trees sampled (98.4%) were visited by at least one seed-consumer species. Over 60% of trees were visited by at least one non-native mammal (i.e. European hare, horse, wild boar and cattle). The most frequent seed consumers were cattle (~ 50% of trees), Cricetid rodents (considered as a single group due to the difficulties of distinguishing each species, ~ 40%), wild boar (~ 20%), azure jay (~ 20%), and maroon-bellied parakeet (Pyrrhura frontalis; ~ 20%; Fig. 3).
We found a negative correlation (β = −0.45 [CI −0.55, −0.35], ΔAIC in relation to null model = −88.1) between the numbers of native and non-native seed consumers (Figure S1). The former occurred in higher numbers in forests (βFe = −0.45 [CI −0.60, −0.31], βAp = −0.69 [CI −0.88, −0.50], ΔAIC in relation to null model = −69), and the latter in forest edge and agropastoral habitat (βFe = 0.66 [CI 0.43, 0.89], βAp = 0.76 [CI 0.51, 1.01], ΔAIC in relation to null model = −42.1; Figure S2).
Impacts of non-native consumers on seed surplus and number of seedlings
Our hypotheses-based modeling approach for assessing variability in seed surplus and seedling abundance (Table S2) gave support for differences among non-native species in their impact (Hypothesis 2) and for additive impacts when some of them co-occur (Hypothesis 3). These effects varied depending on the life stage of the tree (seeds, seedlings) assessed. However, we found little or no support for an effect of non-native species richness (Hypothesis 1) and for the interaction between the richness of native and non-native species co-occurring at particular trees (Hypothesis 4).
The best-ranked seed surplus model (σ1, pseudo-R2 = 0.49) included occurrence of cattle, wild boars and horses, study area, tree girth, richness of native consumers, interaction terms between wild boar occurrence and study area, and between horse occurrence and study area (Table S2). Occurrence of cattle, wild boars, and horses had negative effects on seed surplus, with approximately 25, 13 and 3% of the deviance explained, respectively (summing to 41% of the explained deviance, Table 1). The fact that no interaction term between non-native consumers was selected indicates that their combined impacts should be considered as additive (Table 1). Predictions based on the best model indicated strong seed surplus declines in the presence of cattle, wild boars, horses, and especially in combinations thereof, in all four study areas (Fig. 4a), with the exception of horses in the Muitos Capões area, where we estimated a wide CI due to low number (n = 1) of horse records.
Predictions of seed surplus (a) and number of seedlings (b) with 95% confidence intervals for each study area, in different scenarios of non-native consumer occurrence according to variables present in the best-ranked models (Ca cattle, Ho horse, Wb wild boar, Ha European hare). All predictions are for forest habitat. Other numeric predictor variables in the models were set to their median observed values: tree girth (147 cm) and native consumer richness (2)
Six of the models for number of seedlings were within 2.0 ΔAIC (Table S2). However, they only differed from each other due to the inclusion of one or two mostly uninformative predictors or interaction terms, all of which explained less than 5% of the deviance (Tables S3–S7). As recommended by Arnold (2010), we report all of the models within 2.0 ΔAIC but dismiss those with uninformative variables. That leaves a single model (λ3, pseudo-R2 = 0.17) which includes study area, habitat, tree girth, and occurrence of European hare as predictors (Tables 1 and S2). Occurrence of European hare had a negative effect with 10% of the explained deviance (Table 1). Predictions from the best model indicated a decline in the number of seedling in the presence of European hares in all the study areas (Fig. 4b).
Regarding the potential impact of the richness of non-native consumers on seed surplus and seedling abundance (Hypothesis 1), models including this predictor (E) did not rank within 2.0 ΔAIC for both response variables (Table S2). Nonetheless, the best-ranked model among those including this predictor (σ13: ΔAIC = 17.7) did show a negative effect on seed surplus (βE = −0.52 ± 0.06), with 56.84% of the explained deviance (Table S8). Among seedling abundance models that included this predictor (Table S2), the best-ranked one (λ10: ΔAIC = 3.2) also indicated a negative effect (βE = −0.38 ± 0.20), but it only explained 1.13% of the deviance.
A potential interaction between the richness of native (N) and non-native (E) species (Hypothesis 4) received even less support. Models including this interaction for variability in seed surplus and seedling abundance were poorly ranked (σ13: ΔAIC = 17.7, and λ20: ΔAIC = 6.7 respectively, Table S2). Moreover, this interaction only explained 0.65% of the deviance for seed surplus (Table S8) and 0.22% for seedling abundance (Table S9).
Discussion
Paraná pine seeds as an underestimated food resource
The diversity of Paraná pine native seed consumers revealed by our field observations and literature review, resulting in the identification of 70 species as confirmed or potential consumers, differs drastically from that of previous assessments. In a review of Paraná pine seed predators, Vieira and Iob (2009) only listed 21 species, and 40 species that we recorded had not been listed as Paraná pine seed consumers. We believe this difference is due to several factors. First, our list includes seed predators and secondary consumers that feed on seeds on the ground or still in cones on tree branches, whereas previous assessments have focused on seed predators and on predation by mammals on the ground (Iob and Vieira 2008; Vieira et al. 2011) or by a few selected bird species (Anjos 1991; Prestes et al. 2008); second, our multi-region study included areas on the edge of the original range of the Paraná pine (areas C and F, Fig. 1), increasing the probability of observing seed consumers whose ranges now only marginally overlap with the Paraná pine’s; third, by surveying trees in forest-edge and agro-pastoral habitats, we also increased opportunities for recording species of open-habitats that usually avoid forests; and fourth, the combination of sampling methodologies, particularly the use of camera traps, allowed us to record elusive species that are rarely directly observed while foraging.
These results emphasize the key role Paraná pines play in providing a highly-nutritious resource to a previously underestimated number of species. This role may be even more important when one considers that the peak of Paraná pine seed production occurs during the months when the proportion of Angiosperms producing zoochoric fruits is lowest and, in at least one studied area, no fruits are produced by other trees (Paise and Vieira 2005). They also highlight the myriad interactions among this tree, its seed predators, dispersers, and secondary consumers. In contrast to the mutualisms between Paraná pine and seed dispersers, which are recognized for several species (Vieira and Iob 2009; Tella et al. 2016b), the interactions between Paraná pine seed predators and secondary consumers remain unexplored. Our observations indicate the latter are mostly small birds that cannot eat entire seeds and lack the beak strength to open them (e.g. passerines, doves, rails). That secondary consumers are unable to directly access this resource should not be taken as evidence that it is unimportant to them: many small obligate scavengers, for example, depend on larger species to open carcasses, especially in regions with low winter productivity (Selva et al. 2003; Moleón et al. 2014).
Combined impacts of non-native mammals on Paraná pine regeneration
Most research on the ecological impacts of non-native species focus on single-species invasions, yet a better understanding of the combined effects of multiple co-occurring invaders is necessary (Kuebbing et al. 2013; Jackson 2015; Ballari et al. 2016). The Paraná pine is now widely in contact with four non-native mammals across its range, as indicated by the high proportion (ca. 65%) of seeding female Paraná pine trees visited by non-native mammals. Cattle and wild boars rank first and third among its most frequent seed consumers, respectively. Contrarily to previous works that examined the combined impact of multiple non-native species in species-poor communities of native consumers (Latorre et al. 2013; Tella et al. 2016a), Paraná pine forests hold a community of native consumers even richer than previously thought. We found that richness of native seed consumers was positively related to seed surplus. This result could be related to the fact that at least some seed predators forage more often on trees bearing larger crop sizes (Ragusa-Netto 2014; Tella et al. 2016a) and are swamped by the availability of more seeds than they could consume, resulting in a negative relationship between crop sizes and predation rates (Ragusa-Netto 2014). We did not find, however, support for the hypothesis that this rich community of native consumers could reduce the impact of non-native species. Overall, evidence for the biotic resistance hypothesis is equivocal (Levine and D’Antonio 1999; Jeschke et al. 2012), and there is the possibility that even species-rich forest communities offer vacant niches for non-native mammals (Latham et al. 2017). In this sense, we failed to record large-bodied native mammals known to be consumers of Paraná pine seeds (e.g. collared peccary Pecari tajacu; lowland tapir Tapirus terrestris; Table S1) because they were already very rare or locally extinct (Dirzo et al. 2014; Galetti et al. 2015). As such, our study cannot tease apart whether seed supply and number of seedlings in the presence of non-native species is lower than what would have been observed prior to such local extinctions and invasion by non-native species. However, the densities of large-bodied native mammals likely never reached that currently observed for non-native species, possibly because the former was controlled by large predators that are now also rare or locally extinct (i.e. puma Puma concolor, jaguar Panthera onca; Paviolo et al. 2008), or because the density of non-native domestic species such as cattle is artificially maintained through ranching practices. Thus, it is unlikely that the impacts of large-bodied native seed consumers were ever as high as that of non-native species such as wild boar, cattle and horses. This is in agreement with the results of Brocardo et al. (2017), who quantified Paraná pine seed predation rates by several native consumers (Table S1) and wild boars in forest fragments in the northern part of the species’ range, and found that the latter showed the highest predation rate, while a similar native species (white-lipped peccary Tayassu pecari) consumed less than half of the seeds consumed by wild boars. On the other hand, habitat segregation appears to drive the negative correlation between richness of native and non-native seed consumers observed in this study. This is somewhat expected, since two of the four non-natives (cattle and horses) are free-ranging domestic species mostly restricted to agropastoral habitat, or forest edges adjacent to pastures that are not fenced, whereas the majority of native consumers are mostly restricted to forest and edge habitats.
As predicted, attending to results obtained from a sister species (Tella et al. 2016a), seed surplus and number of seedlings were negatively affected by the occurrence of non-native mammals, which exhibited varying effects depending on species and on the life stage of the plant. While cattle, horses and wild boars impacted seed surplus, European hares reduced seedling abundance. These results highlight the need of assessing the complex impacts of multiple invaders on different plant life stages (Latorre et al. 2013). The fact that seed surplus was not influenced by interactions between non-native seed predators indicated that their combined effects were additive, supporting predictions for impacts of co-occurring pairs of non-native species on autotrophs (Jackson 2015). On the other hand, interactions between the occurrence of wild boar and horse and study area likely indicate geographic variation in the strength of their impacts on seed supply. Importantly, our models show a steep decline in seed surplus as non-native consumers co-occur, almost to the point of full depletion, as reported by Tella et al. (2016a) for monkey puzzle seeds subject to similar pressures in the Andean Patagonia. It is worth noting, however, that while variability in seed surplus was reasonably explained by our model (pseudo-R2 = 0.49) little variation was explained for seedling abundance (pseudo-R2 = 0.17) (see however Møller and Jennions 2002, showing that the mean amount of variance explained in ecological studies ranges between 2.5 and 5.4%). A large amount of unexplained variance could be due to our study design, where we could record the occurrence of species but not their actual predation rates, but also to variance in our response variables. While there was large variation in our seed index (mean = 3, quartiles = 1–6, range 0–10), the extremely low variability in seedling abundance (mean = 0, quartiles = 0–0, range 0–19) (Figure S3) made difficult the statistical assessment of sources of variation.
We found that non-native mammals mostly prey upon and usually completely destroy the Paraná pine seeds (see also Tella et al. 2016a for Araucaria araucana). Paraná pine seeds have the ability to germinate and establish in a variety of natural and anthropogenic vegetation types, including formations where it is the dominant species (Pereira and Ganade 2008), even after being partially consumed (Tella et al. 2016b). Once on the ground, seeds may have three different outcomes: (1) they can serve as food for a variety of animals that cannot access the tree to consume seeds before the cone falls to the ground; (2) they can eventually be dispersed by these seed consumers—secondary dispersion—over short and long distances (Pereira and Ganade 2008; Vieira and Iob 2009); and (3) the remaining seeds can germinate and reach adult age under or close to the parent tree (Souza et al. 2008). As such, any seed found on the ground under parent trees is a potential representative of the future adult population, local or otherwise. It follows that the depletion of seeds on the ground by non-native mammals, as indicated by our results, drastically reduces the potential for germination, secondary dispersal and, ultimately, on-site regeneration and colonization of new sites mediated by ground dispersers.
Conservation implications and management
While an estimated reduction of 97% in the global distribution of Paraná pine over three generations justified its inclusion as Critically Endangered in the IUCN Red List, the potential impact of non-native mammals was not considered as a current nor potential threat for the species (Thomas 2013). Our results reverse this notion and are somewhat alarming, since ca. 65% of the trees sampled across a large part of its distribution were visited by non-native mammals, and such visits were related to a drastic decrease of seeds available for in situ germination or dispersal at the end of the seeding period. Therefore, seed predation—and to a lesser extent seedling predation—by non-native mammals seem to be compromising the regeneration of a highly threatened species with a fragmented and restricted range, and this threat is expected to increase as the non-native mammal populations—both domestic and wild—are still spreading and increasing in Brazil (Pedrosa et al. 2015).
Our study is but a first approach to this conservation problem, and thus several knowledge gaps and research needs remain regarding the impacts of non-native mammals and the conservation of the Paraná pine and its ecological function. Previous studies emphasize the importance of Paraná pine seeds in the diet and population dynamics of their main predators/dispersers (e.g. Anjos 1991; Prestes et al. 2008, 2014; Vieira et al. 2011; Ribeiro and Vieira 2014). Our results on the diversity of primary and secondary consumers of these seeds reinforce its importance for a much larger portion of the animal community during a period of food scarcity in the Brazilian Atlantic Forest.
Ameliorating the impact on forests of non-native mammals is challenging, even more when multiple invaders co-occur (Latham et al. 2017). Recent reviews on the management and removal of invasive species show that removing only one of the co-existing invaders may have unexpected consequences (Ballari et al. 2016), and that the likelihood of ecological recovery decreases in areas with several invaders and anthropogenic disturbance, being more difficult to restore plant communities and ecological processes (Prior et al. 2018). Unfortunately, it seems to be the case of the yet highly-fragmented and disturbed Paraná pine forests, where the combination of wild and domestic non-native mammals complicates their joint removal. Supporting the economy of many rural communities, cattle ranching is among the most important agricultural activities in Brazil (Barretto et al. 2013). In addition to the habitat loss associated with the conversion of forests into pastures, free ranging livestock not only prevent regeneration and ground-based secondary dispersion of the Paraná pine but ultimately may also deny a key resource for a large portion of the native fauna (i.e. species that forage seeds on the ground). This is disquieting, given the challenges in managing such an important economic activity for conservation purposes (e.g. cattle eradication is not a realistic solution in most situations), but opportunities to reduce the impacts of cattle and horses exist across a range of scales. At local scales (e.g. properties that include pastures and forest fragment habitats), regulation of livestock densities and management (i.e. fencing) to ensure that cattle do not enter forest fragments could prove an effective and relatively low cost measure (Zamorano-Elgueta et al. 2012). At regional scales, strategic land-use planning should incentivize the replacement of pastures surrounding large Araucaria forest fragments in protected areas (or otherwise) with buffer zones consisting of Paraná pine tree plantations for timber and seed harvesting. Regarding wild non-native mammals, the Paraná pine forests are among the areas most affected by wild boar and European hare invasions in Brazil (Hegel and Marini 2013; Pedrosa et al. 2015; de Faria et al. 2016). Among the ten Brazilian protected areas with the highest occurrence of invasive species, five harbor Paraná pine forests and have been invaded by both species (Sampaio and Schmidt 2014). Although the creation of regulations to control the spread of wild boars is an important advance to mitigate its damage to natural habitats, environmental agencies should take on a more active role by promoting (and not only regulating) wild boar management, especially in protected areas. While there is still a lack of information regarding the impacts of the European hare invasion in Brazilian ecosystems (de Faria et al. 2016), our results indicate that it may hinder regeneration of Paraná pines through seedling predation. Therefore, the regulation of livestock densities and ranching practices, combined with increased efforts to control the invasion of wild boars and European hares should be priority actions for the conservation of this critically-endangered Atlantic Forest species and its important ecological function.
References
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer, New York
Anjos L (1991) O ciclo anual de Cyanocorax caeruleus em Floresta de Araucária (Passeriformes: Corvidae). Ararajuba 2:19–23
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildl Manag 74:1175–1178
Auguie B (2017) gridExtra: Miscellaneous functions for “grid” graphics. R package version 2.3. https://CRAN.R-project.org/package=gridExtra
Ballari SA, Kuebbing SE, Nuñez MA (2016) Potential problems of removing one invasive species at a time: a meta-analysis of the interactions between invasive vertebrates and unexpected effects of removal programs. PeerJ 4:e2029
Barretto AGOP, Berndes G, Sparovek G, Wirsenius S (2013) Agricultural intensification in Brazil and its effects on land-use patterns: an analysis of the 1975–2006 period. Glob Change Biol 19:1804–1815. https://doi.org/10.1111/gcb.12174
Bolker B, R Development Core Team (2017) bbmle: Tools for general maximum likelihood estimation. R package version 1.0.20. https://CRAN.R-project.org/package=bbmle
Brocardo CR, Pedrosa F, Galetti M (2017) Forest fragmentation and selective logging affect the seed survival and recruitment of a relictual conifer. For Ecol Manag 408:87–93. https://doi.org/10.1016/j.foreco.2017.09.046
Carr D (2018) hexbin: Hexagonal binning routines. R package version 1.27.2. https://CRAN.R-project.org/package=hexbin
de Faria GMM, Rosa CA, Castro Corrêa GL et al (2016) Geographic distribution of the European hare (Lepus europaeus) in Brazil and new records of occurrence for the Cerrado and Atlantic Forest biomes. Mammalia 80:497–505. https://doi.org/10.1515/mammalia-2015-0036
Dillenburg L, Franco AMS, Coutinho AL et al (2009) Aspectos ecofisiológicos da regeneração de Araucaria angustifolia. In: Fonseca CR, Souza AF, Leal-Zanchet AM et al (eds) Floresta com Araucária: Ecologia, Conservação e Desenvolvimento Sustentável. Holos, Ribeirão Preto, pp 57–65
Dirzo R, Young HS, Galetti M et al (2014) Defaunation in the Anthropocene. Science 345:401–406. https://doi.org/10.1126/science.1251817
Duarte LS, Dillenburg A (2000) Ecophysiological responses of Araucaria angustifolia seedlings to different irradiance levels. Aust J Bot 48:531. https://doi.org/10.1071/BT98046
Elton CS (1958) The ecology of invasions by animals and plants. Chapman and Hall, London
Galetti M, Bovendorp RS, Guevara R (2015) Defaunation of large mammals leads to an increase in seed predation in the Atlantic forests. Glob Ecol Conserv 3:824–830. https://doi.org/10.1016/j.gecco.2015.04.008
Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24:699–711
Hastie TJ, Pregibon D (1992) Generalized linear models. In: Chambers JM, Hastie TJ (eds) Statistical models in S. Wadsworth & Brooks/Cole, Pacific Grove
Hegel CGZ, Marini MÂ (2013) Impact of the wild boar, Sus scrofa, on a fragment of Brazilian Atlantic Forest. Neotrop Biol Conserv 8:17–24. https://doi.org/10.4013/nbc.2013.81.03
Iob G, Vieira EM (2008) Seed predation of Araucaria angustifolia (Araucariaceae) in the Brazilian Araucaria Forest: influence of deposition site and comparative role of small and “large” mammals. Plant Ecol 198:185–196. https://doi.org/10.1007/s11258-007-9394-6
IUCN (2016) The IUCN red list of threatened species. Version 2016-2. http://www.iucnredlist.org. Accessed 7 Sep 2016
Jackson MC (2015) Interactions among multiple invasive animals. Ecology 96:2035–2041. https://doi.org/10.1890/15-0171.1
Jeschke J, Aparicio LG, Haider S, Heger T, Lortie C, Pyšek P, Strayer D (2012) Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 14:1
Kleiber C, Zeileis A (2008) Applied econometrics with R. Springer, New York. https://CRAN.R-project.org/package=AER
Kuebbing SE, Nuñez MA, Simberloff D (2013) Current mismatch between research and conservation efforts: the need to study co-occurring invasive plant species. Biol Conserv 160:121–129. https://doi.org/10.1016/j.biocon.2013.01.009
Latham ADM, Warburton B, Byrom AE, Pech RP (2017) The ecology and management of mammal invasions in forests. Biol Invasions 19:3121–3139
Latorre L, Larrinaga AR, Santamaría L (2013) Combined impact of multiple exotic herbivores on different life stages of an endangered plant endemism, Medicago citrina. J Ecol 101:107–117
Levine JM, D’Antonio CM (1999) Elton revisited: a review of evidence linking diversity an dinvasibility. Oikos 87:15–26
Mantovani A, Morellato LPC, Dos Reis MS (2004) Fenologia reprodutiva e produção de sementes em Araucaria angustifolia (Bert.) O. Kuntze. Rev Bras Bot 27:787–796. https://doi.org/10.1590/S0100-84042004000400017
Martinez J, Prestes NP (2008) Tamanho populacional, tamanho médio de bando e outros aspectos demográficos do papagaio-charão (Amazona pretrei). In: J Martinez, Prestes NP (eds) Biologia da Conservação - estudo de caso com o papagaio-charão e outros papagaios brasileiros. UPF Editora, Passo Fundo, pp 178–206
Moleón M, Sánchez-Zapata JA, Selva N et al (2014) Inter-specific interactions linking predation and scavenging in terrestrial vertebrate assemblages. Biol Rev 89:1042–1054. https://doi.org/10.1111/brv.12097
Møller A, Jennions MD (2002) How much variance can be explained by ecologists and evolutionary biologists? Oecologia 132:492–500
Paise G, Vieira EM (2005) Produção de frutos e distribuição espacial de angiospermas com frutos zoocóricos em uma Floresta Ombrófila Mista no Rio Grande do Sul, Brasil. Rev Bras Bot 28:615–625. https://doi.org/10.1590/S0100-84042005000300017
Paviolo A, De Angelo CD, Di Blanco YE, Di Bitetti MS (2008) Jaguar Panthera onca population decline in the Upper Paraná Atlantic Forest of Argentina and Brazil. Oryx 42:554. https://doi.org/10.1017/S0030605308000641
Pedrosa F, Salerno R, Padilha FVB, Galetti M (2015) Current distribution of invasive feral pigs in Brazil: economic impacts and ecological uncertainty. Nat Conserv 13:84–87. https://doi.org/10.1016/j.ncon.2015.04.005
Pereira F, Ganade G (2008) Spread of a Brazilian keystone-species in a landscape mosaic. For Ecol Manag 255:1674–1683. https://doi.org/10.1016/j.foreco.2007.11.026
Prestes NP, Martinez J, Rosa AV (2008) Dieta alimentar do papagaio-charão (Amazona pretrei). In: J Martinez, Prestes NP (eds) Biologia da Conservação: um estudo de caso do papagaio-charão e de outros papagaios brasileiros. UPF Editora, Passo Fundo, pp 88–104
Prestes NP, Martinez J, Kilpp JC et al (2014) Ecologia e conservação de Amazona vinacea em áreas simpátricas com Amazona pretrei. Ornithologia 6:109–120
Prior KM, Adams DC, Klepzig KD, Hulcr J (2018) When does invasive species removal lead to ecological recovery? Implications for management success. Biol Invasions 20:267–283
R Development Core Team (2016) R: a language and environment for statistical computing. R Development Core Team, Vienna
Ragusa-Netto J (2014) Crop damage of Eriotheca gracilipes (Bombacaceae) by the blue-fronted Amazon (Amazona aestiva, Psittacidae), in the Brazilian Cerrado. Braz J Biol 74:837–843
Ribeiro JF, Vieira EM (2014) Interactions between a seed-eating neotropical rodent, the Azara’s agouti (Dasyprocta azarae), and the Brazilian pine’ Araucaria angustifolia. Austral Ecol 39:279–287. https://doi.org/10.1111/aec.12077
Sampaio AB, Schmidt IB (2014) Espécies exóticas invasoras em unidades de conservação federais do Brasil. Biodivers Bras 3:32–49
Sanguinetti J, Kitzberger T (2010) Factors controlling seed predation by rodents and non-native Sus scrofa in Araucaria araucana forests: potential effects on seedling establishment. Biol Invasions 12:689–706. https://doi.org/10.1007/s10530-009-9474-8
Selva N, Jedrzejewska B, Jedrzejewski W, Wajrak A (2003) Scavenging on European bison carcasses in Bialowieza Primeval Forest (eastern Poland). Ecoscience 10:303–311
Simberloff D, Martin JL, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66. https://doi.org/10.1016/j.tree.2012.07.013
Solórzano-Filho JA (2001) Demografia, fenologia e ecologia da dispersão de sementes de Araucaria angustifolia em uma população relictural em Campos do Jordão. University of São Paulo, São Paulo
Souza AF, Forgiarini C, Longhi SJ, Brena DA (2008) Regeneration patterns of a long-lived dominant conifer and the effects of logging in southern South America. Acta Oecol 34:221–232. https://doi.org/10.1016/j.actao.2008.05.013
Tella JL, Lambertucci SA, Speziale KL, Hiraldo F (2016a) Large-scale impacts of multiple co-occurring invaders on monkey puzzle forest regeneration, native seed predators and their ecological interactions. Glob Ecol Conserv 6:1–15. https://doi.org/10.1016/j.gecco.2016.01.001
Tella JL, Dénes FV, Zulian V et al (2016b) Endangered plant-parrot mutualisms: seed tolerance to predation makes parrots pervasive dispersers of the Parana pine. Sci Rep 6:31709. https://doi.org/10.1038/srep31709
Thomas P (2013) Araucaria angustifolia. IUCN red list threat species 2013 e.T32975A2829141
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Vieira EM, Iob G (2009) Dispersão e predação de sementes de Araucaria angustifolia. In: Fonseca CR, Souza AF, Leal-Zanchet AM et al (eds) Floresta com Araucária: Ecologia, Conservação e Desenvolvimento Sustentável. Holos, Ribeirão Preto, pp 85–95
Vieira EM, Ribeiro JF, Iob G (2011) Seed predation of Araucaria angustifolia (Araucariaceae) by small rodents in two areas with contrasting seed densities in the Brazilian Araucaria forest. J Nat Hist 45:843–854. https://doi.org/10.1080/00222933.2010.536265
Wickham H (2009) ggplot2: Elegant graphics for data analysis. Springer, New York
Zamorano-Elgueta C, Cayuela L, González-Espinosa M et al (2012) Impacts of cattle on the South American temperate forests: challenges for the conservation of the endangered monkey puzzle tree (Araucaria araucana) in Chile. Biol Conserv 152:110–118. https://doi.org/10.1016/j.biocon.2012.03.037
Acknowledgements
We thank G. Ferraz and managers and staff of Estação Ecológica de Aracuri-Esmeralda, Floresta Nacional de São Francisco de Paula and Centro de Pesquisas e Conservação da Natureza Pró-Mata (PUC-RS). We are grateful to James Russell, and an anonymous reviewer for their helpful comments and suggestions. We also thank Andrew Crosby for the English review. Funds were provided by Fundación Repsol. F.V.D. was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (206107/2014-5). N.P. and J.M. thank the Fundação Grupo Boticário de Proteção à Natureza for financial support of long-term work on parrots in southern Brazil.
Data accessibility
The dataset with seed and seedling counts and covariates for all observations, and the analysis R code file are uploaded as online supporting information.
Author information
Authors and Affiliations
Contributions
FH, FVD and JLT designed the work; FH, FVD, JLT, VZ, NM and JM conducted field work; FVD and JLT analysed the data; FVD led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix S2
CSV dataset with seed and seedling counts and covariates used in modeling analysis (CSV 23 kb)
Rights and permissions
About this article
Cite this article
Dénes, F.V., Tella, J.L., Zulian, V. et al. Combined impacts of multiple non-native mammals on two life stages of a critically endangered Neotropical tree. Biol Invasions 20, 3055–3068 (2018). https://doi.org/10.1007/s10530-018-1758-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1758-4