Abstract
Background
Sodium–glucose cotransporter 2 inhibitors (SGLT2i) improve outcomes of patients with type 2 diabetes at high cardiovascular risk and chronic kidney disease. Recent studies showed an increase in hemoglobin and hematocrit after SGLT2i treatment.
Materials and methods
We did a systematic review and meta-analysis of randomized, double-blind, placebo-controlled studies of SGLT2i in patients with type 2 diabetes. We searched through PubMed/Medline, Web of Science, Embase (Elsevier), and the Cochrane Central Register of Controlled Trials (Wiley) from January 2010 to January 2021.
Results
We included seventeen randomized, double-blind, placebo-controlled studies. The total number of evaluated patients was 14,748. The treatment arm consisted of canagliflozin, dapagliflozin, empagliflozin and ipragliflozin. SGLT2i therapy significantly increased hemoglobin levels when compared to placebo (MD 5.60 g/L, 95% CI 3.73–7.47 g/L, P < 0.00001, considerable heterogeneity—I2 = 94%). Each SGLT2i also led to a significant increase in the hematocrit level when compared to placebo (MD 1.32%, 95% CI 1.21–1.44, P < 0.00001, considerable heterogeneity—I2 = 99%).
Conclusions
SGLT2i led to significant increases in hemoglobin and hematocrit levels when compared to placebo. In addition to their cardiovascular effect, SGLT2i also increases hemoglobin and hematocrit levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sodium–glucose cotransporter 2 inhibitors (SGLT2i) are a class of glucose-lowering drugs that increase urinary glucose excretion by inhibiting glucose reabsorption in the proximal tubule. SGLT2i decrease blood pressure and blood glucose levels and contribute to weight loss [1, 2]. There is also strong evidence demonstrating the cardioprotective and renoprotective effects of this class of glucose-lowering drugs. Different mechanisms are thought to contribute to organ protection, including the activation of tubuloglomerular feedback leading to reduced intraglomerular pressures, diuresis, lower blood pressure, and weight loss [1,2,3]. They also impact anti-inflammatory pathways which may contribute to cardiorenal protection [1, 2].
Anemia worsens the prognosis of many diseases such as type 2 diabetes mellitus and chronic kidney disease leading to increased morbidity and mortality [4,5,6]. Kidneys have regulatory effects on red blood cell production through erythropoietin release in response to hypoxia [7]. Type 2 diabetes mellitus is a major risk factor for chronic kidney disease, which can potentially lead to anemia development. Previous studies have reported an increase in hemoglobin and hematocrit levels with the initiation of SGLT2i in type 2 diabetes mellitus patients [8,9,10,11]. Although this could be explained by a decrease in plasma volume leading to hemoconcentration, additional mechanisms may contribute to the increase in hemoglobin and hematocrit values following the administration of SGLT2i [8, 12]. There are also reports explaining the relationship between SGLT2i and increased red blood cell parameters [9, 11,12,13]. In this meta-analysis, we investigate the effects of different SGLT2i administered in varying doses on hemoglobin and hematocrit levels in type 2 diabetes mellitus patients.
Methods
Our study investigated the impact of SGLT2i on hemoglobin and hematocrit levels. We selected the included studies from various databases according to predefined inclusion and exclusion criteria. We followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines to report this meta-analysis (Supplementary Table S1).
Literature search and inclusion/exclusion criteria
In this systematic review and meta-analysis, we performed a literature search through four databases, including PubMed/Medline, Web of Science, Embase (Elsevier), and the Cochrane Central Register of Controlled Trials (Wiley) from January 2010 to January 2021 using the following keywords: ‘‘SGLT2i’’, ‘‘SGLT2 inhibitor’’, ‘‘type 2 diabetes mellitus’’, ‘‘hematocrit’’, ‘‘hemoglobin’’, ‘‘anemia”, “cardiovascular disease”, “chronic kidney disease”, and “hypoxia-inducible factor”.
We independently assessed the titles and the abstracts of each study. We discussed and reexamined each article in detail until reaching a consensus if any conflicts were present. We also analyzed the references of all selected studies. After the preliminary selection, we independently evaluated the full-text versions of the selected studies.
The inclusion criteria for our systematic review and meta-analysis were as follows: we included studies that provided data on SGLT2i and red blood cell parameters, hemoglobin and hematocrit levels. Studies with retrospective or prospective design irrespective of randomization were included and cross-sectional studies were excluded. All included studies were in English and published in a peer-reviewed journal until January 2021.
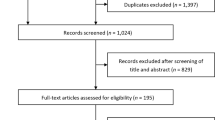
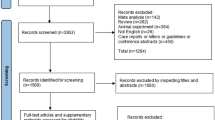
We excluded studies with missing data or inadequate description of outcomes. Studies not classified as original articles (e.g. reviews, meta-analyses, editorials, commentaries), study designs that were not listed in our inclusion criteria (e.g. case reports, case series), and unpublished data were also excluded from our study. Our search algorithm is presented in Fig. 1.
Quality assessment
We assessed the quality of each included study in accordance with the Newcastle–Ottawa Scale [14] which uses the selection of study groups as the main criteria, assessment of outcomes, and comparability of the groups (Tables 1, 2). The Newcastle–Ottawa Scale scores a study out of nine stars, the maximum score representing the highest quality research [14]. We reached a consensus decision on the quality assessment of each study.
We used the Cochrane risk-of-bias tool for the risk of bias assessment in the included studies (supplementary table 2). The Cochrane risk-of-bias tool was used to evaluate randomization, masking of treatment allocation, blinding, adherence and withdrawals for each of the randomized controlled trials [15].
Statistical analysis
We used a random-effects model in an inverse variance analysis and expressed treatment effects as mean difference (MD) for continuous outcomes with 95% CI (hemoglobin, hematocrit). Treatment effect was significant if P < 0.05. When the results were expressed as standard error, we converted standard error to standard deviation using a standard formula [16].
We used the I2 statistic to assess inconsistency across individual studies [17]. An I2 > 50% indicated a large heterogeneity which was not explained by chance.
If a sufficient number of studies were identified, subgroup analysis was used to explore possible sources of heterogeneity. All statistical analyses were performed using Review Manager (RevMan) Version 5.3 (The Cochrane Collaboration 2012).
Results
We included, in our final analysis, seventeen randomized, double-blind, placebo-controlled studies [9,10,11, 18,19,20,21,22,23,24,25,26,27,28,29,30,31] (Tables 1, 2). The total number of evaluated patients was 14,748 (with a minimum of 180 [21] and a maximum of 7020 patients [31]). All studies enrolled type 2 diabetes patients. Estimated glomerular filtration rate was above 30 ml/min/1.73 m2 in all studies. The treatment arm consisted of canagliflozin [13, 22, 25, 27, 30], dapagliflozin [10, 11, 19,20,21, 23, 24, 29], empagliflozin [9, 18, 31] and ipragliflozin [28]. The doses of the different SGLT2i were as follows: canagliflozin 50 mg [13], 100 mg [22, 25, 27, 30], 200 mg [13], 300 mg [13, 22, 25, 27, 30] and 300 mg [13]; dapagliflozin 1 mg [11, 24], 2.5 mg [10, 11, 19, 20, 23, 24, 30], 5 mg [10, 11, 19, 20, 23, 24, 29], 10 mg [10, 19,20,21, 23, 24, 29], and 20 mg and 50 mg [10]; empagliflozin 10 mg and 25 mg [9, 18, 31]; ipragliflozin 12.5 mg, 50 mg, 150 mg and 300 mg [28].
All the included studies reported the outcomes as MD between baseline and post-intervention values measured at different timings across the study (at 12 weeks [10, 13, 24, 28], at 24 weeks [9, 11, 18,19,20,21, 23], at 26 weeks [22, 27, 30], at 48 weeks [29, 31] and at 52 weeks [25].
We evaluated all the included studies in terms of the risk of bias using the Cochrane risk of bias tool (supplementary table 2). All of the studies were double-blind trials. Seven of the included studies did not report the details of allocation concealment. There were no incomplete outcomes and selective reporting in the seventeen studies.
Outcome measures reporting
Effect of SGLT2i on hemoglobin
There were seven studies, five with canagliflozin [13, 22, 25, 27, 30], one with empagliflozin [31] and one with ipragliflozin [28] that evaluated the effect of SGLT2i therapy on hemoglobin levels. As shown in Fig. 2, SGLT2i therapy was shown to significantly increase hemoglobin when compared to placebo (MD 5.60 g/L, 95% CI 3.73–7.47 g/L, P < 0.00001, considerable heterogeneity—I2 = 94%) at 12–48 weeks of follow-up. Given the large heterogeneity, we, therefore, analyzed the effect on hemoglobin levels by SGLT2i class. As shown in Fig. 3, empagliflozin, canagliflozin and ipragliflozin all significantly increased hemoglobin levels, with a trend for a further increase in hemoglobin for empagliflozin (MD 6.24 g/L, 95% CI 3.08–9.40, P < 0.00001, significant heterogeneity—I2 = 98%).
When analyzing separately the studies in which canagliflozin was administered, no significant differences were observed between the 100 mg and the 300 mg (MD = − 0.25 g/L, 95% CI − 0.91–0.41, P = 0.46, reduced heterogeneity—I2 = 7%) [13, 22, 25, 27].
Therefore, the large heterogeneity may be explained by the use of different molecules at different doses. Moreover, the included patients had different baseline eGFR, varying from normal renal function to stage 3 CKD. Another possible explanation is represented by the fact that none of the included studies was originally designed to assess the impact of SGLT2i on hemoglobin levels. The follow-up periods were also different and the effect may be time-dependent.
Effect of SGLT2i on hematocrit
In total, thirteen studies reported the mean change in hematocrit level after treatment with SGLT2i: eight with dapagliflozin [10, 11, 19,20,21, 23, 24, 29], two with canagliflozin [13, 30], three with empagliflozin [9, 18, 31] and one with ipragliflozin [28]. SGLT2i treatment was significantly associated with increased hematocrit levels when compared to placebo (MD 1.32%, 95% CI 1.21–2.44, P < 0.00001, considerable heterogeneity—I2 = 99%) (Fig. 4) at 12–48 weeks of follow-up.
When analyzed individually, each SGLT2i led to a significant increase in the hematocrit level when compared to placebo (MD 2.19%, 95% CI 0.28–4.10, P < 0.00001, considerable heterogeneity—I2 = 100%) (Fig. 5) at 12–48 weeks of follow-up.
To determine whether there was a dose-dependent effect, we then analyzed each SGLT2i by dose.
For dapagliflozin, the 2.5 mg was non inferior to the 5 mg dose (MD 0.00%, 95% CI 0.00–0.01, P = 0.07, insignificant heterogeneity—I2 = 0%), but inferior to the 10 mg dose (MD 0.34%, 95% CI 0.00–0.67, P = 0.05, moderate heterogeneity—I2 = 55%) (Supplementary Fig. 1) [10, 11, 19, 20, 23, 24, 29]. Additionally, there were six studies [10, 19, 20, 23, 24, 29] which compared the 5 mg with the 10 mg. This analysis showed no significant difference in hematocrit levels with the 10 mg dose (MD 0.00%, 95% CI 0.00–0.41, P = 0.37, insignificant heterogeneity—I2 = 0%).
For canagliflozin, there was no significant difference between the doses of 100 mg and the 300 mg, in the two studies that reported data on hematocrit (MD − 0.12%, 95% CI − 0.97–0.73, P = 0.78, insignificant heterogeneity—I2 = 0%) [13, 30].
Similarly, no significant dose-dependent effect was noted with the 25 mg of empagliflozin, when compared to the 10 mg (MD 0.20%, 95% CI − 0.08–0.48, P = 0.99, insignificant heterogeneity—I2 = 0%) [9, 31].
Discussion
In this systematic review and meta-analysis, we investigated the effects of different types and doses of SGLT2i on hemoglobin and hematocrit levels. The treatment arm consisted of canagliflozin [13, 22, 25, 27, 30], dapagliflozin [10, 11, 19,20,21, 23, 24, 29], empagliflozin [9, 18, 31] and ipragliflozin [28]. We showed that hemoglobin and hematocrit levels were significantly increased with SGLT2i therapy when compared to placebo.
In addition to their glucose-lowering effects, SGLT2i reduce blood pressure levels and contribute to weight loss [1, 2]. SGLT2i exert these effects through several mechanisms such as the activation of tubuloglomerular feedback leading to decreased intraglomerular pressures and diuresis leading to calorie and sodium losses [32]. They also have anti-inflammatory effects and reduce the fibrotic, and hyperplastic responses of proximal tubular cells through the prevention of hyperfiltration and glucose reabsorption in the renal proximal tubule [32].
Previous studies have shown that anemia worsens the prognosis of many diseases such as type 2 diabetes mellitus, chronic kidney disease, chronic heart failure, chronic obstructive pulmonary disease, thus leading to increased morbidity and mortality [4, 33]. In this analysis, SGLT2i therapies raise hemoglobin and hematocrit levels [8], an effect that has been linked with cardiorenal protection, possibly by improving tissue oxygen delivery. The rise in hemoglobin and hematocrit could be partially explained by a decrease in plasma volume due to the diuretic effects of SGLT2i. Among the included studies Bailey et al. [11], Rosenstock et al. [13] and Kovacs et al. [9] have all argued that the increase in hematocrit and hemoglobin levels could be explained by circulating volume contraction effects of SGLT-2i.
In contrast, several studies implied that other mechanisms engaged by SGLT2i could explain the increase in hemoglobin and hematocrit levels, besides volume depletion [30]. One postulated mechanism would be the correction of pathologically decreased erythropoietin levels. Erythropoietin is a hormone synthesized mainly by renal interstitial fibroblasts, in an oxygen-dependent manner, via hypoxia-inducible factor (HIF) [8, 34]. Glucose is cotransported with sodium (Na+) ions via SGLT2 channels located on proximal renal tubules [12]. This process is highly dependent on the Na+ ion gradient between tubule lumen and renal proximal tubular cells [12]. The Na+ ion gradient is maintained by Na+/K+/ATPase channels consuming a significant amount of ATP [12]. In patients with type 2 diabetes, higher amounts of glucose are reabsorbed causing increased stress in renal interstitial cells due to relative depletion of oxygen levels, as most are consumed by the proximal renal tubular epithelium [12]. This causes ischemia and further fibrosis of renal interstitial cells, leading to decreased erythropoietin levels, which could explain anemia in patients with kidney diseases [12]. SGLT2i could prevent damage to the renal interstitial cells by reducing the activity of SGLT2 channels on proximal tubular cells, which would result in the preservation of adequate erythropoietin levels and subsequent increase in hemoglobin and hematocrit levels [12]. In any case, this hypothesis is marred by the fact that hypoxia would acutely induce erythropoietin synthesis and the effects of SGLT2i on hemoglobin occurred relatively early [35].
SGLT2 may also have direct effects on HIF metabolism. The two types of HIFs: HIF-1α or HIF-2α, although similar, possess different cellular actions and distribution patterns. Indeed, these two isoforms often have opposing actions. While HIF-1α decreases oxygen use and increases angiogenesis, HIF-2α is the primary stimulus for erythropoietin synthesis. It was suggested that SGLT2i inhibit HIF1α, but may increase SIRT1-mediated activation of HIF-2α [36]. Thus, SGLT2i may increase erythropoietin secretion directly and indirectly by decreasing renal fibrosis and enhancing the viability of erythropoietin secreting cells. Indeed, a recent study has shown that HIF-1α is the therapeutic target of SGLT2i for diabetic kidney disease and tubulointerstitial fibrosis [37].
Last but not least, there are also studies that showed that the anti-inflammatory actions of SGLT2i may contribute to increase hemoglobin and hematocrit. Dapagliflozin reduced circulating hepcidin and ferritin concentrations while increasing levels of the hepcidin inhibitor erythroferrone, and transiently increasing erythropoietin. Additionally, dapagliflozin increased plasma transferrin levels and expression of transferrin receptors 1 and 2 but there was no change in the expression of the iron cellular transporter, ferroportin [38].
The important question is that to what extent these different mechanisms contribute to hemoglobin and hematocrit elevation. In fact, the mechanisms by which SGLT2 inhibitors improve hemoglobin levels in patients with diabetes and chronic kidney disease are not fully understood and there are also other suggested mechanisms. For example, it is postulated that SGLT2i have diuretic-like effects and reduce plasma volume (a probable cause of hemoconcentration and hemoglobin elevation) and increase EPO secretion by renal fibroblasts [39]. In addition, SGLT2i increase 5′ AMP-activated protein kinase and Sirtuin 1 which activates HIF-2 alpha the isoform responsible for the synthesis of EPO. Further studies are needed to highlight the contributions of these different mechanisms on anemia correction and whether mechanisms differed in diabetic and non-diabetic patients [40].
We also analyzed each SGLT2i by dose to determine whether there was a dose-dependent effect on hemoglobin and hematocrit levels. The change in hematocrit and hemoglobin levels differed according to the type of SGLT2i used. Our analysis showed that only dapagliflozin led to a significantly greater increase in hematocrit levels when administered in higher doses [10, 11, 23, 24]. However, there were no significant differences in blood parameters between the varying doses of canagliflozin, empagliflozin and ipragliflozin [9, 13, 28, 30].
It needs to be mentioned that the beneficial pleiotropic effects are valid for SGLT2i as a class effect. These effects (anti-inflammatory, anti-fibrotic, hemoglobin and hematocrit elevation) are independent of blood glucose lowering. It is probable that patients without diabetes and with kidney disease probably get a similar benefit from SGLT2 inhibition. Indeed it was already demonstrated that SGLT2i also improved outcomes in non-diabetic CKD patients [2].
Limitations
We acknowledge several limitations of our meta-analysis. The changes in hemoglobin and hematocrit levels after SGLT2i administration were not investigated as primary outcomes in the included studies. Furthermore, studies had not reported baseline and/or follow-up levels of erythropoietin, hepcidin and inflammatory markers following SGLT2i treatments. There was a wide heterogeneity between study populations and treatment protocols. Patients were not evaluated for different stages of kidney disease.
Conclusion
In conclusion, SGLT2i led to significant increases in hemoglobin and hematocrit levels when compared to placebo. These drugs can be used to prevent the adverse consequences of anemia and contribute to a better prognosis in patients with diabetes mellitus. Studies are needed to assess the impact of SGLT2i on anemia in patients with different stages of CKD and to further characterized the interaction of SGLT2i with iron availability. Furthermore, the potential relationship of changes in hemoglobin with outcomes should be explored in already available large clinical trials having clinical events as primary endpoints.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Cherney DZ, Kanbay M, Lovshin JA (2020) Renal physiology of glucose handling and therapeutic implications. Nephrol Dial Transplant 35:i3–i12
Fernandez-Fernandez B, Sarafidis P, Kanbay M, Navarro-Gonzalez JF, Soler MJ, Gorriz JL, Ortiz A (2020) SGLT2 inhibitors for non-diabetic kidney disease: drugs to treat CKD that also improve glycaemia. Clin Kidney J 13:728–733
Kanbay M, Ertuglu LA, Afsar B, Ozdogan E, Kucuksumer ZS, Ortiz A, Covic A, Kuwabara M, Cherney DZI, van Raalte DH, de Zeeuw D (2019) Renal hyperfiltration defined by high estimated glomerular filtration rate: a risk factor for cardiovascular disease and mortality. Diabetes Obes Metab 21:2368–2383
Yilmaz MI, Solak Y, Covic A, Goldsmith D, Kanbay M (2011) Renal anemia of inflammation: the name is self-explanatory. Blood Purif 32:220–225
Kanbay M, Perazella MA, Kasapoglu B, Koroglu M, Covic A (2010) Erythropoiesis stimulatory agent- resistant anemia in dialysis patients: review of causes and management. Blood Purif 29:1–12
Solak Y, Cetiner M, Siriopol D, Tarim K, Afsar B, Covic A, Kanbay M (2016) Novel masters of erythropoiesis: hypoxia inducible factors and recent advances in anemia of renal disease. Blood Purif 42:160–167
Copur S, Onal EM, Afsar B, Ortiz A, van Raalte DH, Cherney DZ, Rossing P, Kanbay M (2020) Diabetes mellitus in chronic kidney disease: Biomarkers beyond HbA1c to estimate glycemic control and diabetes-dependent morbidity and mortality. J Diabetes Complicat 34:107707
Maruyama T, Takashima H, Oguma H, Nakamura Y, Ohno M, Utsunomiya K, Furukawa T, Tei R, Abe M (2019) Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther 21:713–720
Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, Broedl UC, investigators E-RPt (2014) Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 16:147–158
List JF, Woo V, Morales E, Tang W, Fiedorek FT (2009) Sodium–glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32:650–657
Bailey CJ, Iqbal N, T’Joen C, List JF (2012) Dapagliflozin monotherapy in drug-naive patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obes Metab 14:951–959
O’Neill J, Fasching A, Pihl L, Patinha D, Franzen S, Palm F (2015) Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 309:F227-234
Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W, Canagliflozin DIASG (2012) Dose-ranging effects of canagliflozin, a sodium–glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 35:1232–1238
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2019) Cochrane handbook for systematic reviews of interventions, 2nd edn. Wiley, Chichester
DerSimonian R, Kacker R (2007) Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 28:105–114
Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC, Investigators E-RMT (2013) Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 36:3396–3404
Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S (2011) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 13:928–938
Bailey CJ, Gross JL, Pieters A, Bastien A, List JF (2010) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375:2223–2233
Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S (2012) Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 97:1020–1031
Bode B, Stenlof K, Sullivan D, Fung A, Usiskin K (1995) Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract 2013(41):72–84
Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF (2010) Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33:2217–2224
Kaku K, Inoue S, Matsuoka O, Kiyosue A, Azuma H, Hayashi N, Tokudome T, Langkilde AM, Parikh S (2013) Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 15:432–440
Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G (2013) Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 56:2582–2592
Rosenstock J, Vico M, Wei L, Salsali A, List JF (2012) Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 35:1473–1478
Stenlof K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G (2013) Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 15:372–382
Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A (2013) Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab 15:403–409
Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S, Dapagliflozin 006 Study G (2012) Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 156:405–415
Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G (2013) Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 15:463–473
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128
Rangaswami J, Bhalla V, de Boer IH, Staruschenko A, Sharp JA, Singh RR, Lo KB, Tuttle K, Vaduganathan M, Ventura H, McCullough PA, American Heart Association Council on the Kidney in Cardiovascular D, Council on Arteriosclerosis T, Vascular B, Council on C, Stroke N, Council on Clinical C, Council on L, Cardiometabolic H (2020) Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the American Heart Association. Circulation 142:e265–e286
Yerlikaya A, Bulbul MC, Afsar B, Dagel T, Aslan G, Voroneanu L, Siriopol D, Covic A, Kanbay M (2018) Iron in kidney and heart failure: from theory to practice. Int Urol Nephrol 50:481–493
Afsar B, Kanbay M, Afsar RE (2020) Hypoxia inducible factor-1 protects against COVID-19: a hypothesis. Med Hypotheses 143:109857
Lawler PR, Liu H, Frankfurter C, Lovblom LE, Lytvyn Y, Burger D, Burns KD, Brinc D, Cherney DZI (2021) Changes in cardiovascular biomarkers associated with the sodium–glucose cotransporter 2 (SGLT2) inhibitor ertugliflozin in patients with chronic kidney disease and type 2 diabetes. Diabetes Care 2021 Mar;44(3):e45-e47. https://doi.org/10.2337/dc20-2265
Packer M (2020) Mutual antagonism of hypoxia-inducible factor isoforms in cardiac, vascular, and renal disorders.JACC Basic Transl Sci 5:961–968
Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, Sakagami H, Ota T (2019) Hypoxia-inducible factor-1alpha is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep 9:14754
Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, Chaudhuri A, Dandona P (2020) Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 2020 Apr 1; 105(4):dgaa057
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J (2013) Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15:853–862
Afsar B, Hornum M, Afsar RE, Ertuglu LA, Ortiz A, Covic A, van Raalte DH, Cherney DZI, Kanbay M (2021) Mitochondrion-driven nephroprotective mechanisms of novel glucose lowering medications. Mitochondrion 58:72–82
Acknowledgements
MK gratefully acknowledges the use of the services and facilities of the Koc University Research Center for Translational Medicine (KUTTAM), funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget.
Funding
AC, LT and CU gratefully acknowledge the grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI-UEFISCDI, project number PN-III-P4-ID-PCE-2020-2393, within PNCDIII.
Author information
Authors and Affiliations
Contributions
Contributed substantially to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: AC, EC, CT, AD, CU, LT and MK. Drafted the work or revised it critically for important intellectual content: BA, AC, DC, AO and MK. Approved the final version to be published: AC, EC, CT, AD, CU, LT, DC and MK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanbay, M., Tapoi, L., Ureche, C. et al. Effect of sodium–glucose cotransporter 2 inhibitors on hemoglobin and hematocrit levels in type 2 diabetes: a systematic review and meta-analysis. Int Urol Nephrol 54, 827–841 (2022). https://doi.org/10.1007/s11255-021-02943-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-02943-2