Abstract
Purpose
To demonstrate the efficacy of combined rituximab and plasmapheresis (PP)/plasma exchange (PE) therapy for focal segmental glomerulosclerosis in transplanted kidneys (ptFSGS).
Methods
We searched MEDLINE, SCOPUS, and Cochrane Library for eligible publications. Only observational studies or clinical trials containing patients’ age > 18 years were included for full-text extraction.
Results
A total of eight observational studies (n = 85) were included in meta-analyses. With a median follow-up of 18 months (IQR 4.4), combination therapy of RTX-PP/PE in patients with ptFSGS resulted in overall remission rate of 72.7% (95% CI 52.3–86.6%) with a significant reduction of proteinuria and serum creatinine levels. Complete remission was 41.0%, while partial remission was 31.7%. The mean difference of serum creatinine levels between pre- and post-treatment was − 0.65 mg/dL (95% CI − 1.15 to − 0.14). The mean difference of the degree of proteinuria between pre- and post-treatment was − 4.79 g/day (95% CI − 7.02 to − 2.56). Subgroup analyses were performed after adjusted for study year, type of intervention, and primary pre-transplant lesion. Patients with recurrent FSGS tended have lesser reduction in the degree of proteinuria compared to patients with de novo FSGS. Incidence of serious adverse events with combined RTX-PP/PE therapy was 0.12 event/year.
Conclusion
We conclude that combined RTX-PP/PE therapy may be considered as an alternative treatment of ptFSGS in achieving remission by lowering proteinuria and serum creatinine levels. However, the efficacy of combined RTX-PP/PE therapy must be confirmed in randomized-controlled trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Focal segmental glomerulosclerosis (FSGS) is a common cause of nephrotic syndrome in adults [1]. The incidence of end-stage kidney disease (ESKD) due to FSGS in the United States has dramatically increased in the past decades, from 0.2% in 1980 to 2.3% in 2000 [2]. This increase in incidence is likely multifactorial as FSGS can develop following numerous secondary causes [3]. Interestingly, recurrence of FSGS in renal allografts occurs in 30–50% and is associated with reduced graft survival [4]. The treatment for recurrent FSGS in kidney transplant recipients is difficult, since these patients are already on immunosuppressants, such as calcineurin inhibitors (CNI).
Steroids have been the mainstream therapy for FSGS in adults, but approximately one-half of patients achieved remission [5]. Moreover, partial remission is more common than complete remission for FSGS. Moreover, a large proportion of patients relapse and later become steroid resistant [6]. The use of other immunosuppressive agents, such as CNI, has been shown to induce remission in resistant or steroid-dependent FSGS [6]. Nonetheless, the use of steroids and CNI can be limited in some patients due to their both short-term and long-term side effects. Rituximab (RTX), an anti-CD20 antibodies, has been introduced for treatment of FSGS in children [7]. However, clinical evidence is limited to establish its role in adults. Likewise, plasmapheresis (PP) has been shown to be effective in treatment of recurrent FSGS post-transplant with reported 50–60% remission rate in most studies [8, 9]. However, relapses are common after discontinuing PP. These data suggested that recurrent FSGS in renal allografts is difficult to treat with the current treatment regimens.
In 2011, Damodar et al. were among the first researchers to introduce the use of combined rituximab and plasmapheresis (RTX-PP) in the treatment of post-transplant FSGS (ptFSGS) [10]. In this study, combined RTX-PP resulted in reduction of serum creatinine levels and degree of proteinuria in all patients. Later, several groups of researchers have attempted to demonstrate the efficacy of combined rituximab and plasmapheresis as well as plasma exchange for ptFSGS. The sample size, however, was small and the results were inconclusive across studies. Thus, we conducted this meta-analysis to elaborate the treatment outcomes of combined RTX-PP/PE therapy for ptFSGS in adults. The knowledge obtained from this study would help guide the design of randomized-controlled trial and support the treatment decision for patients who develop FSGS in renal allografts.
Materials and methods
Information sources and search strategy
The protocol of this systematic review is registered with ResearchRegistry.com (registration number: reviewregistry843). We conducted a systematic literature search on Ovid MEDLINE, SCOPUS, and the Cochrane Library from database inception through September 2019. The literature search was conducted by two independent authors (P.H. and N.G.) using the following search approach: “focal segmental glomerulosclerosis” OR “minimal change disease” AND “rituximab”. The search strategy for each database is elaborated in Supplemental Document 1. Additional articles were obtained through manual reference search of the included studies. This systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement [11].
Study selection
Only articles available in English were included for further screening. Other inclusion criteria include clinical trials, or observational studies that enrolled patients age ≥ 18 years with post-kidney transplant FSGS who were treated with combined rituximab-plasmapheresis/plasma exchange therapy. Studies containing secondary FSGS were excluded. Case reports or studies demonstrating either rituximab or plasmapheresis/plasma exchange alone were excluded. Eligible studies needed to provide the following outcomes: degree of proteinuria, serum creatinine levels, remissions, and relapses. Moreover, this systematic review and meta-analysis is limited to patients who developed FSGS after kidney transplantation regardless of pre-transplant primary lesions. Retrieved articles were independently examined for eligibility by two authors (P.H. and N.G.). Conflicts were resolved by consensus between the authors or by consulting the third physician or a biostatistician. All references were managed through Endnote X9.2 software (Clarivate Analytics, Philadelphia, PA, USA).
Data collection process
A structured data collecting form was developed to gather the following data from each included study: title, name of authors, publication year, and country where the study was conducted, type of study, patients’ diagnosis, sample size, intervention, total dosage of rituximab, treatment outcomes, follow-up duration, CD19/20 depletion rate, and serious adverse events. Complete remission is defined by a reduction in proteinuria to less than 1 g/g upon completion of treatment course. Partial remission is defined by a reduction in proteinuria > 50% from peak proteinuria level, but still above 1 g/g upon completion of treatment. Risk of bias was assessed using ROBINS-I tool for non-randomized studies of interventions [12] with the following category; participants, intervention(s), comparator, co-intervention(s), and outcome(s). Quality of studies fulfilled inclusion criteria which was rated as low, moderate, or high risk of bias.
Sensitivity analysis and publication bias
Sensitivity analyses and subgroup analyses were performed to minimize the heterogeneity between studies. Sensitivity analyses were performed by removing one study at a time. Subgroup analyses were preformed based on the study date (prior to 2015 vs. after 2015), and type of intervention (rituximab–plasmapheresis vs. rituximab–plasma exchange). Publication bias was analysed by Egger’s regression intercept and Funnel plot if the number of included studies is greater than 10 [13].
Statistical analysis
We used the Comprehensive Meta-Analysis software version 3.3.070 (Biostat Inc, Englewood, NJ, USA) to conduct meta-analyses and SPSS version 23.0 (IBM Corp., Armonk, NY, USA) for descriptive analyses. Study with immunoadsorption will be presented in the systematic review table, but will not be included in the meta-analysis. Statistical heterogeneity of studies was assessed using Cochran’s Q test and I2 (≤ 25%, insignificant heterogeneity; 26–50%, low heterogeneity; 51–75%, moderate heterogeneity; and ≥ 75%, high heterogeneity) [14]. Note that I2 reported in this study was derived from fixed-effects model of analysis. We analysed the results using random-effects model or mixed-effects to minimize the heterogeneity or between-study variance. For descriptive analyses, continuous data were reported in mean ± standard deviation (SD) or median ± interquartile range (IQR), depending on data distribution. P value less than 0.05 is considered statistically significant.
Results
Study characteristics
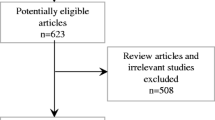
A total of 699 potential articles were identified from our literature search. Exclusion criteria were applied to limit only studies elaborating the effect of RTX-PP/PE combination therapy in patients with post-transplant FSGS. The flowchart of systematic literature search and review is demonstrated in Fig. 1. A total of eight studies were included in the systematic review. Alachkar, 2018 comprised of both retrospective and prospective cohort. All included studies were observational studies. Four of eight cohorts were in prospective design. The treatment outcomes (degree of proteinuria and serum creatinine levels) were available in all cohorts to be included for meta-analyses. Overall remission was reported in six studies. The median follow-up duration was 18 months (IQR 4.4). Note that 81/89 (91%) patients had the primary lesion of FSGS while 8/89 (9%) had primary disease other than FSGS, including hypertension, diabetes, or unknown. Mean pre-treatment and post-treatment serum creatinine level were 2.37 ± 0.48 mg/dL and 1.86 ± 0.45 mg/dL, respectively. Mean pre-treatment and post-treatment urine protein were 7.96 ± 3.40 g/g and 3.53 ± 2.39 g/g, respectively. Study characteristics are illustrated in Table 1.
Combined RTX-PP/PE therapy on overall remission
Overall remission was analysed from six studies (n = 51). We demonstrated that the overall remission rate of ptFSGS was 72.7% (95% CI 52.3–86.6%; I2 = 28.6%) following combined RTX-PP/PE therapy. Event rates for complete remission and partial remission are similar. Complete remission was achieved in 41.0%, while partial remission was achieved in 31.7%. Figure 2 illustrates the Forest plot of the meta-analysis for overall remission rate.
Combined RTX-PP/PE therapy on proteinuria
Of 85 patients, combined RTX-PP/PE therapy resulted in a significant reduction of proteinuria in post-transplant FSGS. The mean difference of the degree of proteinuria between pre- and post-treatment is − 4.43 g/day (95% CI − 6.81 to − 2.04; I2 = 98.7%). The Forest plot of combined RTX-PP/PE therapy on the degree of proteinuria is illustrated in Fig. 3.
Combined RTX-PP/PE therapy on serum creatinine levels
RTX-PP/PE combination therapy resulted in a significant reduction of serum creatinine levels in post-transplant FSGS. The mean difference of serum creatinine levels between pre- and post-therapy was − 0.49 mg/dL (95% CI − 0.84 to − 0.14; I2 = 0%). The Forest plot of combined RTX-PP/PE therapy on serum creatinine levels is illustrated in Fig. 4.
Subgroup analyses
Subgroup analyses were performed based on study date (prior to 2015 vs. year 2015 and later), type of intervention (rituximab plus plasmapheresis vs. rituximab plus plasma exchange), and pre-transplant disease (FSGS vs. non-FSGS). One study with immunoadsorption was excluded from the meta-analysis. There were no significant differences in the degree of proteinuria (Q = 1.44, mixed-effects; p = 0.23) as well as serum creatinine levels (Q = 0.55, mixed-effects; p = 0.46) between studies prior to 2015 vs. after 2015. After adjusted for type of intervention, the reduction of proteinuria and serum creatinine level was similar between RTX-PP and RTX-PE groups (Q = 1.19, mixed effect; p = 0.28 for proteinuria and Q = 0.21, mixed effect; p = 0.65 for serum creatinine). Our subgroup analysis showed that the reduction of proteinuria was significantly greater among patients who had non-FSGS in pre-transplant lesion (− 8.13 g/day; 95% CI − 9.60 to − 6.65) compared to patients who had FSGS pre-transplant (− 2.52 g/day; 95% CI − 3.09 to − 1.95) (Q = 48.35, mixed-effects; p < 0.001). Nonetheless, there was no significant difference in serum creatinine reduction after adjusted for pre-transplant lesion (Q = 1.55, mixed-effects; p = 0.21).
Adverse events
Only two studies reported serious adverse effect from RTX-PP/PE combination therapy. One study stated that sepsis occurred in 20% of patients, while the other study reported severe infection in up to 73% of patients. From our analysis, the incidence of serious adverse events following combined RTX-PP/PE therapy was 0.12 event per year.
Sensitivity analysis and publication bias
Sensitivity analyses were performed by removing one study at a time for both meta-analyses of overall remission, proteinuria, and serum creatinine levels. We found that overall remission, reductions in both proteinuria and serum creatinine levels remained statistically significant in all sensitivity analyses. Publication bias was evaluated by Egger’s regression intercept. The Funnel plots for publications cannot be performed as the number of included studies is less than 10. Egger’s regression intercept for overall remission, difference in mean proteinuria, and mean serum creatinine did not indicate the possibility of publication bias (p = 0.354, p = 0.438 and p = 0.205, respectively).
Discussion
Up to 70% of patients with ptFSGS achieved remission with a significant reduction of proteinuria (− 4.4 g/day) and serum creatinine (− 0.49 mg/dL) following combined RTX-PP/PE therapy. A systematic review of case reports and case series of patients with ptFSGS (n = 77) treated with PP alone showed that the remission was achieved in 71% [15]. This review, however, contained mixed adult and pediatric population and lack of control group which limit the conclusions on causality. Additional study showed that relapses were common in patients treated with PP and those who responded to treatment were likely PP-dependent [16]. Our study suggested that adding rituximab to plasmapheresis/plasma exchange treatment resulted in a significant lower serum creatinine and proteinuria leading to disease remission.
Recent progress in the pathophysiology of recurrent FSGS suggested that glomerular permeability to albumin was associated with some plasma-borne factors, known as circulating permeability factors [17]. Examples of these factors include cardiotrophin-like cytokine 1 (CLC-1) and soluble urokinase receptor (suPAR). Dysregulation of these substances leads to podocyte injury and increased permeability [18, 19]. Thus, it has been proposed that plasmapheresis or plasma exchange therapy helps to eliminate circulatory permeability factors as the treatment of primary FSGS. Rituximab is a monoclonal antibody directed against CD20-positive lymphocytes. Histological study in transplanted kidneys affected by FSGS recurrence revealed some degrees of lymphocytic infiltration [20]. This finding might suggest that FSGS is an antibody-mediated disease. Thus, the rationale of adding rituximab to plasmapheresis/plasma exchange in the treatment of FSGS is to inhibit antibody production as well as to eliminate circulatory permeability factors and disease-mediated antibodies.
From subgroup analyses, the reduction of proteinuria was significantly greater in patients who had non-FSGS in pre-transplant lesion compared to patients who had recurrent FSGS. Although, this could be secondary to selection bias as patients with recurrent FSGS tended to have greater proteinuria at baseline; however, there are emerging evidence that recurrent FSGS is associated with poorer treatment outcomes and graft outcomes in comparison to de novo FSGS [21]. The treatment outcomes of either RTX-PP or RTX-PE were similar. It is widely reported that recurrent FSGS is associated with an increased risk of allograft loss up to 18.7% [22]. Autoantibodies directed against actin, angiotensin II type 1 receptor, adenosine triphosphate synthase, nephrin, and Thy1 have been implicated in the pathogenesis of FSGS recurrence [22]. However, it is not fully understood if primary FSGS and recurrent FSGS share a common pathophysiology. Furthermore, it is also possible that recurrent FSGS is usually resistant to treatment as most patients already underwent and completed the standard treatment. Bench research and clinical studies are needed to conclude the underlying mechanism of recurrent FSGS as opposed to primary FSGS.
We found that the incidence of serious adverse effects from combined RTX-PP/PE therapy was relatively low compared to what previously described in the literature. World Apheresis Registry reported the extent of side effects during apheresis to be approximately 5% [23]. One explanation is patients with post-transplant FSGS received cumulative dose of plasma with shorter duration of treatment compared to patients with the other diagnoses. Malignancies, neurological disorders, and haematological disorders are the most common indications for plasmapheresis/plasma exchange [24]. These patients generally required a higher dose of replaced plasma and longer duration of treatment. However, it is worth noting that our finding might be underpowered given its relatively small pooled sample size and only two studies reported adverse events. We advised interpreting our finding with caution.
There are some limitations to our study. First, all included studies were observation studies without comparative control group making it difficult to draw a conclusion. Second, the pooled sample size remains small with moderate heterogeneity. We encouraged the audience to apply the findings from our study with caution. Third, relapses were not reported in all studies. Having missing data could underpower the analyses. Fourth, the subtype of FSGS was not identified in all studies. As suggested by D’Agati [25], the response to treatment of FSGS is dependent on its subtype from the biopsy. Fifth, the use of pre-transplant plasmapheresis to prevent recurrence of ptFSGS was demonstrated in several studies to date; however, it is beyond the scope of this research [26, 27]. Sixth, our results could be subjected to possible confounders, such as immunosuppressive therapy, pre-transplant prophylactic PP/PE, and ABO incompatibility of kidney transplantation. These factors, however, will be eliminated by randomization in future clinical trials. Finally, data from unpublished studies or studies in non-English language were not reviewed. However, we identified no potential publication bias in our analyses. In spite of these limitations, this is the first meta-analysis supporting a randomized-controlled trial comparing the treatment outcomes of combination therapy of RTX and PP/PE versus rituximab or PP/PE alone for ptFSGS.
Post-transplant FSGS is a disease entity that is difficult to treat. In this meta-analysis, we showed that combined RTX-PP/PE therapy resulted in a significant reduction in proteinuria and serum creatinine levels leading to remissions. However, the data are still immature in preventing relapses after treatment.
References
D'Agati V (1994) The many masks of focal segmental glomerulosclerosis. Kidney Int 46(4):1223–1241. https://doi.org/10.1038/ki.1994.388
Kitiyakara C, Eggers P, Kopp JB (2004) Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44(5):815–825
Beaudreuil S, Lorenzo HK, Elias M, Nnang Obada E, Charpentier B, Durrbach A (2017) Optimal management of primary focal segmental glomerulosclerosis in adults. Int J Nephrol Renovasc Dis 10:97–107. https://doi.org/10.2147/ijnrd.S126844
Choy BY, Chan TM, Lai KN (2006) Recurrent glomerulonephritis after kidney transplantation. Am J Transplant 6(11):2535–2542. https://doi.org/10.1111/j.1600-6143.2006.01502.x
Cattran DC, Rao P (1998) Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis 32(1):72–79. https://doi.org/10.1053/ajkd.1998.v32.pm9669427
Korbet SM (2002) Treatment of primary focal segmental glomerulosclerosis. Kidney Int 62(6):2301–2310. https://doi.org/10.1046/j.1523-1755.2002.00674.x
Iijima K, Sako M, Nozu K (2017) Rituximab for nephrotic syndrome in children. Clin Exp Nephrol 21(2):193–202. https://doi.org/10.1007/s10157-016-1313-5
Andresdottir MB, Ajubi N, Croockewit S, Assmann KJ, Hibrands LB, Wetzels JF (1999) Recurrent focal glomerulosclerosis: natural course and treatment with plasma exchange. Nephrol Dial Transplant 14(11):2650–2656. https://doi.org/10.1093/ndt/14.11.2650
Davenport RD (2001) Apheresis treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation: re-analysis of published case-reports and case-series. J Clin Apher 16(4):175–178. https://doi.org/10.1002/jca.10007
Damodar A, Mustafa R, Bhatnagar J, Panesar M, Gundroo A, Zachariah M, Blessios G, Tornatore K, Weber-Shrikant E, Venuto R (2011) Use of anti-CD20 antibody in the treatment of post-transplant glomerulonephritis. Clin Transplant 25(3):375–379. https://doi.org/10.1111/j.1399-0012.2010.01245.x
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. (1756–1833 (Electronic))
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR (1991) Publication bias in clinical research. Lancet 337(8746):867–872
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Kashgary A, Sontrop JM, Li L, Al-Jaishi AA, Habibullah ZN, Alsolaimani R, Clark WF (2016) The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: a systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol 17(1):104. https://doi.org/10.1186/s12882-016-0322-7
Matalon A, Markowitz GS, Joseph RE, Cohen DJ, Saal SD, Kaplan B, D'Agati VD, Appel GB (2001) Plasmapheresis treatment of recurrent FSGS in adult renal transplant recipients. Clin Nephrol 56(4):271–278
Cravedi P, Kopp J, Fau-Remuzzi G, Remuzzi G. Recent progress in the pathophysiology and treatment of FSGS recurrence. (1600–6143 (Electronic))
Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal M, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi M, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. (1546–170X (Electronic))
McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. (1555–905X (Electronic))
de Oliveira JG, Xavier P, Carvalho E, Ramos JP, Magalhaes MC, Mendes AA, Faria V, Guerra LE (1999) T lymphocyte subsets and cytokine production by graft-infiltrating cells in FSGS recurrence post-transplantation. Nephrol Dial Transplant 14(3):713–716. https://doi.org/10.1093/ndt/14.3.713
Kienzl-Wagner K, Waldegger S, Schneeberger S. Disease recurrence-the Sword of damocles in kidney transplantation for primary focal segmental glomerulosclerosis. (1664–3224 (Electronic))
Abbott KC, Sawyers E, Oliver J, Ko C, Kirk A, Welch P, Peters T, Agodoa LY. Graft loss due to recurrent focal segmental glomerulosclerosis in renal transplant recipients in the United States. (1523–6838 (Electronic))
Stegmayr B, Ptak J, Wikström B, Berlin G, Axelsson CG, Griskevicius A, Centoni P, Liumbruno G, Molfettini P, Audzijoniene J, Mokvist K, Sojka BN, Norda R, Knutson F, Ramlow W, Blaha M, Witt V, Evergren M, Tomaz J (2008) World apheresis registry 2003–2007 data. Transfus Apheres Sci 39(3):247–254. https://doi.org/10.1016/j.transci.2008.09.003
Rock G (2016) Note from the editor in chief. Transfus Apheres Sci 54(1):1. https://doi.org/10.1016/j.transci.2016.01.002
D'Agati V (2003) Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol 23(2):117–134. https://doi.org/10.1053/snep.2003.50012
Valdivia P, Gonzalez Roncero F, Gentil MA, Jimenez F, Algarra G, Pereira P, Rivera M, Suner M, Cabello V, Toro J, Mateos J (2005) Plasmapheresis for the prophylaxis and treatment of recurrent focal segmental glomerulosclerosis following renal transplant. Transpl Proc 37(3):1473–1474. https://doi.org/10.1016/j.transproceed.2005.02.061
Alasfar S, Matar D, Montgomery RA, Desai N, Lonze B, Vujjini V, Estrella MM, Manllo Dieck J, Khneizer G, Sever S, Reiser J, Alachkar N (2018) Rituximab and therapeutic plasma exchange in recurrent focal segmental glomerulosclerosis postkidney transplantation. Transplantation 102(3):e115–e120. https://doi.org/10.1097/tp.0000000000002008
Rodríguez-Ferrero M, Ampuero J, Anaya F (2009) Rituximab and chronic plasmapheresis therapy of nephrotic syndrome in renal transplantation patients with recurrent focal segmental glomerulosclerosis. Transpl Proc 41(6):2406–2408. https://doi.org/10.1016/j.transproceed.2009.06.044
Tsagalis G, Psimenou E, Nakopoulou L, Laggouranis A (2011) Combination treatment with plasmapheresis and rituximab for recurrent focal segmental glomerulosclerosis after renal transplantation. Artif Organs 35(4):420–425. https://doi.org/10.1111/j.1525-1594.2010.01068.x
Alachkar N, Wei C, Arend LJ, Jackson AM, Racusen LC, Fornoni A, Burke G, Rabb H, Kakkad K, Reiser J, Estrella MM (2013) Podocyte effacement closely links to suPAR levels at time of posttransplantation focal segmental glomerulosclerosis occurrence and improves with therapy. Transplantation 96(7):649–656. https://doi.org/10.1097/TP.0b013e31829eda4f
Lionaki S, Vlachopanos G, Georgalis A, Liapis G, Skalioti C, Zavos G, Boletis JN (2015) Individualized scheme of immunoadsorption for the recurrence of idiopathic focal segmental glomerulosclerosis in the graft: a single center experience. Ren Fail 37(5):777–783. https://doi.org/10.3109/0886022X.2015.1015366
Garrouste C, Canaud G, Büchler M, Rivalan J, Colosio C, Martinez F, Aniort J, Dudreuilh C, Pereira B, Caillard S, Philipponnet C, Anglicheau D, Heng AE (2017) Rituximab for recurrence of primary focal segmental glomerulosclerosis after kidney transplantation: clinical outcomes. Transplantation 101(3):649–656. https://doi.org/10.1097/TP.0000000000001160
Alachkar N, Li J, Matar D, Vujjini V, Alasfar S, Tracy M, Reiser J, Wei C (2018) Monitoring suPAR levels in post-kidney transplant focal segmental glomerulosclerosis treated with therapeutic plasma exchange and rituximab. BMC Nephrol. https://doi.org/10.1186/s12882-018-1177-x
Koutroutsos K, Charif R, Moran L, Moss J, Cook T, Roufosse C, Pusey C, Taube D, Loucaidou M (2019) Successful management of post-transplant focal segmental glomerulosclerosis with therapeutic plasma exchange and rituximab. Clin Exp Nephrol 23(5):700–709. https://doi.org/10.1007/s10157-019-01690-0
Acknowledgements
There is no institutional or national funding for this research.
Funding
None.
Author information
Authors and Affiliations
Contributions
PH and NG performed literature search, citation screening, and data collection. PH analysed the data and drafted the manuscript. PH and NG revised and edited the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest.
Ethics approval
This study does not involve human participants and/or animals. Thus, ethical approval is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hansrivijit, P., Ghahramani, N. Combined rituximab and plasmapheresis or plasma exchange for focal segmental glomerulosclerosis in adult kidney transplant recipients: a meta-analysis. Int Urol Nephrol 52, 1377–1387 (2020). https://doi.org/10.1007/s11255-020-02462-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02462-6