Abstract
Background
Post-transplant focal segmental glomerulosclerosis (FSGS) is associated with renal allograft loss. Currently, optimal treatment remains controversial.
Methods
The aim of our study was to examine the efficacy and safety of therapeutic plasma exchange (TPE), and rituximab (RTX), in the management of post-transplant FSGS. The treatment protocol consisted of RTX and monthly cycles of 5 plasma exchanges for 6 months. We treated 10 transplant recipients with biopsy-proven post-transplant FSGS. Lastly, we compared the studied group to a historic control group of nine patients with post-transplant FSGS.
Results
9 out of 10 patients achieved remission after the conclusion of treatment (4 complete and 5 partial), while 1 patient did not respond to treatment. During the follow-up period, there was one graft loss and one patient died while in remission from unrelated complications. There was a significant reduction in mean uPCR between diagnosis (517.4 ± 524.2 mg/mmol) and last follow-up (87 ± 121.6 mg/mmol) in the patients with sustained remission (p = 0.026). There was no significant decline in eGFR in the eight relapse-free responders at the end of follow-up. (54.4 ± 16.7 from 49.8 ± 20.4 ml/min) (p = 0.6) An increased response rate to the combined TPE and RTX treatment was demonstrated, when compared to a historic control group of nine patients with post-transplant FSGS, as only five out of nine patients achieved remission (two complete and three partial) in that group.
Conclusions
In this study, treatment with TPE and RTX appears to be safe, well tolerated and effective in the management of patients with post-transplant FSGS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Focal segmental glomerulosclerosis (FSGS) is defined by focal segmental sclerotic glomerular lesions on histology and proteinuria [1]. FSGS has an estimated incidence of 8 cases/million/year [2, 3]. In primary FSGS, 40–60% of patients develop ESRD within 10–20 years from diagnosis.
Following transplantation, approximately 30% (range 15–50%) of patients [4,5,6] will have recurrence of FSGS. Outcomes of recurrent FSGS typically range from chronic proteinuria to allograft dysfunction and loss [7]. Patients with post-transplant FSGS have 52% 5-year graft survival compared to 83% in the patients without recurrence [8] and when compared to patients with recurrence of other types of glomerulonephritis, they have double the risk of losing their graft over 10 years [9]. Currently, optimal post-transplant FSGS treatment remains controversial. Successful use of therapeutic plasma exchange (TPE) and rituximab (RTX) for the treatment of recurrent FSGS has been described only in case reports and small case series [10,11,12,13,14].
In the literature, 63% of adults and 70% of children will have some response to TPE [15]. TPE is thought to remove a putative plasma-permeability increasing factor, leading to reduction of proteinuria [16]. Other therapies, including high doses of calcineurin inhibitors [17], cyclophosphamide, as well as immune adsorption, have been tried with variable results [10]. Despite the absence of controlled trials, and the scarcity of prospective data [18, 19], TPE is widely employed to treat FSGS in kidney transplant recipients [20].
RTX, an anti CD-20 monoclonal antibody, is another therapeutic option for post-transplant FSGS, based on reports of successful treatment of FSGS with RTX. It has been proposed that B cells may be involved in the pathogenesis of FSGS through an abnormal cross-talk with T cells or by directly releasing a permeability factor [21,22,23]. A systematic review of 39 reported cases of recurrent FSGS treated with RTX showed that remission occurred in 64% of patients [24]. Recent evidence has shown that RTX can directly target podocytes in recurrent FSGS [25]. According to case series, combined treatment of post-transplant FSGS with TPE and RTX may potentiate the efficacy of both treatments [14].
In order to investigate the potential benefit of combination treatment with TPE and RTX for post-transplant FSGS, we reviewed retrospectively the outcomes of the management protocol that is currently in use in our institution.
Materials and methods
This was a study aiming to examine the efficacy and safety of TPE and RTX in the treatment of post-transplant FSGS. This was a retrospective review meeting the criteria for a service evaluation study and hence did not require approval from a Research Ethics Committee. This study was approved by the Departmental Transplant Research Group. All patients gave their consent for treatment and received standard care according to our accepted unit protocol. This therapeutic protocol for the management of post-transplant FSGS was introduced in our institution in 2011 and became the standard treatment for this clinical condition as approved by the Transplant Clinical and Research Group in our Centre. Our retrospective study is in compliance with the Helsinki Declaration.
Patients
We reviewed the outcomes of 10 adult ESRD patients who received a live or deceased donor transplant between 2010 and 2015 in our center. All the patients received a steroid-sparing immunosuppressive regimen (7-day course of steroids) with alemtuzumab induction and tacrolimus monotherapy.
Post-transplant FSGS diagnosis
Transplant recipients presenting with an increase in urine protein/creatinine ratio (uPCR) of over 100 mg/mmol, were subjected to an indication allograft biopsy. Post-transplant FSGS was diagnosed by renal histopathology, in the presence of new onset of proteinuria.
For the purpose of this study, we utilised the term post-transplant FSGS to include both patients with biopsy-proven FSGS as their primary disease as well as transplant recipients with unknown or non-biopsy proven primary diagnosis that presented with histologically proven FSGS post-transplantation which was classified as non-secondary. Non-secondary refers to the fact that cases with an identifiable potential cause for secondary FSGS were excluded, specifically, those with evidence of past or current glomerulonephritis or with past or current alloimmune transplant glomerulopathy, as well as cases with moderate or severe tubulointerstitial scarring. Foot process effacement was qualitatively described as minor (< 10% capillary loops involved), segmental (10–70%), fairly extensive (70–90%) or extensive (> 90%).
Treatment protocol
The post-transplant FSGS treatment protocol consisted of RTX (total of 2 g over 2 infusions, 2 weeks apart) and monthly cycles of 5 TPE (against 3 l of 5% human albumin) over 7 days for 6 months. During and post-treatment the patients were followed up using uPCR, renal function and lymphocyte subsets. Partial remission was defined as 50% reduction of proteinuria, and complete remission as proteinuria < 0.3 g/day or uPCR < 30. Remission was defined as sustained when continued for more than 1 year. Cameron’s classification was applied to define time of recurrence: immediate (< 48 h), early (< 3 months) and late recurrence (> 3 months) [26]. A post-treatment allograft biopsy including EM was performed. Following the end of treatment, patients in complete remission with stable allograft function were actively monitored without further TPE or RTX. The management protocol is illustrated in Table 1.
Statistics
Continuous variables are presented as mean ± standard deviation. For nominal or non-parametric variables, Chi square test was performed. Confidence interval was set to 95% and p was considered significant at < 0.05. Analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp).
Results
Patients
Ten transplant recipients (8 male) with a mean age of 51 years (range 23–67) with biopsy-proven post-transplant FSGS were treated. Demographics are shown in Table 2. Four of ten patients had biopsy-proven FSGS as their primary disease. Two of ten had unknown primary diagnosis, while the remaining four had either presumed diabetic nephropathy or hypertension as the primary diagnosis. It should be noted that P3 lost his first transplant due to post-transplant FSGS.
4/10 had early (< 3 months) and 6/10 patients had late (> 3 months) diagnosis of post-transplant FSGS. The mean time to diagnosis was 6.8 (0.1–34.6) months. 5/10 patients presented with nephrotic range proteinuria. Mean uPCR on diagnosis was 509 ± 482 mg/mmol, mean albumin 33.9 ± 4.9 mg/dl and mean eGFR 49.1 ± 19.4 ml/min.
Histopathology
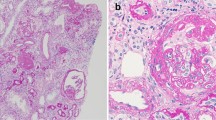
All patients underwent indication kidney biopsies (mean glomeruli per biopsy sampled = 20.4 ± 11) that included tissue processed for EM (Table 3). On histology, there was no glomerulitis, peritubularcapillaritis, transplant glomerulopathy, thrombotic microangiopathy, tubulitis, vasculitis or C4d deposition. Tubular atrophy was mild (mean 8.5% of cortex, range 0–15%). The histology diagnoses included three tip lesion, one collapsing and three NOS variants of FSGS. P1, P8 and P10 were diagnosed as podocytopathies, as extensive foot podocyte effacement (FPE) was found on EM, without segmental sclerosis on light microscopy. Histopathology is summarised in Table 3. A repeat biopsy after the completion of treatment was performed in 9/10 patients (mean glomeruli sampled = 12.1 ± 6.1). In the repeat biopsies, P1, P3, P7 and P8 showed no segmental sclerosis on light microscopy (Table 3). EM was performed in 8/9 patients with a post-treatment biopsy and revealed improvement in FPE in P1, P2, P5, P7 and P9 (Fig. 1).
Treatment and outcome
All patients received treatment with at least 2 g of RTX in total and remained B-cell deplete for 6.4 ± 3.5 months. Mean time from diagnosis to initiation of treatment was 39.4 (range 2–131) days. Eight patients completed six cycles of 5TPE as intended. P4 had only one cycle of TPE, as he did not tolerate further treatment and received 4 g of RTX in total. P10 had five cycles of TPE, after which complete remission was achieved and further treatment was stopped after she developed acute obstruction due to ureteric stricture and urosepsis. P9 had an episode of line sepsis, which was treated with 2 weeks of IV antibiotics and the infected line was replaced.
Mean follow-up after FSGS diagnosis was 20 ± 9.3 months. Nine out of ten patients achieved remission after the conclusion of treatment; four patients achieved complete remission (P1, P7, P8, P10) and five partial (P2, P3, P4, P6, P9), while one patient did not respond to treatment (P5). During the follow-up period, P3 relapsed, and required dialysis, despite further TPE, at 11 months post-diagnosis. It should be noted that this was the second transplant for P3 and the second allograft lost to FSGS. P6 died from unrelated complications (cardiac cause) while still in remission, at 16 months post-diagnosis. Overall, 9/10 patients responded to treatment with 8 of them achieving sustained remission of over a year (5 partial and 3 complete) (Table 4).
There was no significant decline in eGFR in the eight relapse-free responders at the end of follow-up (54.4 ± 16.7 from 49.8 ± 20.4 ml/min) (p = 0.6). For the full responders there was an improvement in mean eGFR from 39 (± 24.6) to 59 (± 12.5) ml/min, although it did not reach statistical significance (p = 0.35) (Fig. 2). There was a significant reduction in mean uPCR between diagnosis (517.4 ± 524.2 mg/mmol) and last follow-up (87 ± 121.6 mg/mmol) in patients with sustained remission (p = 0.026) (Fig. 3). On review of potential predictive factors for response, the eight relapse-free responders had post-transplant FSGS diagnosed and treated earlier, at a mean of 25.7 ± 19.6 vs 79.5 ± 72.8 days (p < 0.001) and 3.8 ± 3.05 vs 18.9 ± 22.1 months (p = 0.001), respectively.
Lastly, we compared the study group to a historic control group of nine KTRs with post-transplant FSGS (Table 2). The mean time to diagnosis was longer at 13.5 (1.5–40.3) months for the historic control group. There was no difference in uPCR at diagnosis between the two groups (509.5 ± 482.4 mg/mmol vs 518.9 ± 599.9 mg/mmol, p = 0.48), while the control group had lower mean eGFR at diagnosis (49.1 ± 19.34 ml/min vs 31.2 ± 8.534 ml/min, p = 0.02). The historic group of patients received a variety of treatments; IVIG + TPE (n = 4), TPE (n = 4) or no treatment (n = 1). 9 out of 10 patients treated with TPE and RTX achieved remission after the conclusion of treatment (4 complete and 5 partial), while in the historic group only 5 out of 9 patients achieved remission (2 complete and 3 partial). 1 patient from each group relapsed, and ended up requiring dialysis at 11 and 24 months post-diagnosis, respectively. At 1 year post-diagnosis 8/10 patients (80%) treated with TPE and RTX were in remission (5 partial and 3 complete), while 5/9 patients (56%) from the historic group were in partial remission. In relapse-free responders there was a significant reduction in mean uPCR between diagnosis (645 ± 667 mg/mmol) and 1 year (126 ± 130 mg/mmol) in the group treated with TPE and RTX (p = 0.026), but not in the historic control group (777 ± 867 mg/mmol vs 152 ± 158 mg/mmol, p = 0.17) (Fig. 4).
Discussion
Our results suggest that first-line combined TPE and RTX treatment is safe and achieves an increased rate of remission in post-transplant FSGS. Our treatment protocol resulted in the majority of treated patients achieving sustained remission without ongoing TPE treatment or augmentation of maintenance immunosuppression, strategies which has been described as successful in the literature, but can increase adverse effects. To our knowledge, none of the reported treatment protocols in the literature have achieved remission rates in excess of 60–70% [17, 27]. In our cohort remission was achieved 90% of patients and sustained remission at 1 year in 80%. Overall, our results suggest an increased response rate compared to that previously reported in the literature, with remission rates of adults receiving either RTX at 58% [24] or TPE at 63% [15].
Comparison to a historic control group of patients from our institution treated with TPE and/or IVIG showed an improved rate of remission with the combined first-line treatment of TPE and RTX. TPE and RTX as first-line treatment also resulted in a significant and sustained reduction in proteinuria, as well as preserved renal function. In a recent meta-analysis of 77 case reports and case series on treatment of post-transplant FSGS with TPE, the overall remission rate in 423 patients with outcome data was 71%. In that analysis, concurrent treatment with RTX was not statistically associated with remission, but RTX was administered in just 4.3% of cases reviewed [28]. The added effect of RTX has been recently reported in a multicenter retrospective study of 19 patients with post-transplant FSGS that received RTX in addition to TPE. Garrouste et al. showed that RTX may be beneficial for cases that have failed initial treatment or are TPE dependent [27].
In our study, combined TPE and RTX treatment proved to be safe with just 2 adverse events; P10 suffered from an episode of urosepsis on a background of complicated ureteric anatomy and P9 had blood stream infection due to line sepsis. One of the patients (P5) did not respond to treatment. P5 had 4/18 segmentally sclerosed glomeruli with extensive foot process effacement on EM. Post-transplant FSGS was diagnosed late (34.6 months) in this patient and initiation of treatment was delayed significantly due to patient’s non-adherence. P3 achieved partial remission, but relapsed and eventually lost his allograft. Recurrence on a previous graft is known to be one of the most powerful predictors of relapse, and this patient lost his previous graft due to collapsing FSGS.
In our study, the diagnosis of FSGS was based on clinical presentation together with histopathology findings. Evidence of segmental or focal glomerulosclerosis on light microscopy and/or diffuse effacement of podocyte foot processes on EM were considered diagnostic for FSGS in patients with proteinuria. Patients who showed evidence of an underlying process (CNI toxicity, rejection, glomerulonephritis, extensive tubular atrophy) suggesting secondary FSGS were excluded after careful consideration at a multi-disciplinary meeting. The absence of a fully constituted FSGS lesion in P1, P8 and P10 may be due to the short natural course between the onset of proteinuria and the diagnosis. It should be also noted that foot process effacement alone was the main finding in 3/4 patients that achieved full remission. Previously published data have suggested that TPE appears to be more effective in cases where the treatment is started early, when the only finding on biopsy is foot process effacement. Our findings are consistent with this notion that TPE is more efficacious prior to the development of glomerular sclerosis on LM [29].
The rationale of our choice of treatment protocol was based on targeting two distinct pathophysiological mechanisms: the elusive permeability factor thought to cause recurrent FSGS and the stabilisation of the podocyte cytoskeleton. The exact mechanism causing post-transplant FSGS is unknown. Post-transplant FSGS is thought more likely to be caused by a circulating glomerular permeability factor (or factors) that induce podocyte injury. Supportive of this theory is that application of FSGS patient plasma to human podocytes in vitro results in rapid derangement of the cellular cytoskeleton [30]. It has been argued that the podocyte dysfunction in post-transplant FSGS could be also due to the lack of a normal circulating factor, since replacement of FSGS plasma with normal plasma allows podocyte cytoskeleton recovery in vitro [31]. However, post-transplant FSGS is also responsive to TPE with albumin as the replacement fluid, which is supportive of the existence of a toxic circulating factor, as shown also in our study [17]. A number of potential candidates have been suggested as the toxic circulating factor, including permeability factors [32,33,34] and autoantibodies [35,36,37]. Initially, it was assumed that this factor was a T-cell derived cytokine. This proposed mechanism was based on case studies of relapsing nephrotic syndrome and T-cell malignancy that resolved following successful chemotherapy of the malignancy [38]. In support of this idea, animal studies have also indicated a possible link between this elusive circulating factor and T cells [39, 40]. More recently, serum soluble urokinase receptor (suPAR) has been implicated in the pathogenesis of FSGS and has even been proposed as a clinical marker to treatment response [32, 41, 42]. suPAR is elevated in two-thirds of subjects with primary FSGS, and patients with recurrent FSGS had higher levels of suPAR pre-transplantation and during the course of FSGS recurrence post-transplant [41, 42]. However, suPAR is elevated in all patients with chronic kidney disease and not just patients with FSGS. Recent evidence has shown that RTX can directly target podocytes in recurrent FSGS. Fornoni et al. have demonstrated that RTX can bind to molecules expressed in human podocytes, such as SMPDL-3b, a protein that is down-regulated upon in vitro exposure of podocytes to sera of FSGS patients. This effect on SMPDL-3b (a protein implicated in actin remodeling) can be reversed by RTX [25].
Our study has limitations. Firstly, this is a retrospective study. In addition, because of the small number of patients, we were not able to comment on predictors of response to treatment.
In conclusion, this study shows that combined first-line treatment with RTX and TPE can have a beneficial effect on post-transplant FSGS in adult kidney transplant recipients. These promising preliminary results will have to be confirmed in a larger population and over a longer follow-up but may provide a basis for effective treatment for this challenging condition.
Abbreviations
- FSGS:
-
Focal segmental glomerulosclerosis
- uPCR:
-
Urine protein creatinine ratio
- eGFR:
-
Estimated glomerular filtration rate
- EM:
-
Electron microscopy
- FPE:
-
Foot process effacement
- MMF:
-
Mycophenolate mofetil
- TPE:
-
Therapeutic plasma exchange
- ESRD:
-
End-stage renal disease
- RTX:
-
Rituximab
- HAS:
-
Human albumin solution
References
D’Agati VD, et al. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43(2):368–82.
McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–30.
Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–5.
Vincenti F, Ghiggeri GM. New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant. 2005;5(6):1179–85.
Choy BY, Chan TM, Lai KN. Recurrent glomerulonephritis after kidney transplantation. Am J Transplant. 2006;6(11):2535–42.
Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5(12):2363–72.
Chadban S. Glomerulonephritis recurrence in the renal graft. J Am Soc Nephrol. 2001;12(2):394–402.
Francis A, Trnka P, McTaggart SJ. Long-term outcome of kidney transplantation in recipients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2016;11(11):2041–6.
Briganti EM, et al. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347(2):103–9.
Dantal J, et al. Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N Engl J Med. 1994;330(1):7–14.
Gungor O, et al. Plasmapheresis therapy in renal transplant patients: five-year experience. Transplant Proc. 2011;43(3):853–7.
Hristea D, et al. Successful treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation by plasmapheresis and rituximab. Transpl Int. 2007;20(1):102–5.
Rodriguez-Ferrero M, Ampuero J, Anaya F. Rituximab and chronic plasmapheresis therapy of nephrotic syndrome in renal transplantation patients with recurrent focal segmental glomerulosclerosis. Transplant Proc. 2009;41(6):2406–8.
Tsagalis G, et al. Combination treatment with plasmapheresis and rituximab for recurrent focal segmental glomerulosclerosis after renal transplantation. Artif Organs. 2011;35(4):420–5.
Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant. 2010;25(1):25–31.
Laufer J, et al. Plasma exchange for recurrent nephrotic syndrome following renal transplantation. Transplantation. 1988;46(4):540–2.
Canaud G, et al. Intensive and prolonged treatment of focal and segmental glomerulosclerosis recurrence in adult kidney transplant recipients: a pilot study. Am J Transplant. 2009;9(5):1081–6.
Gohh RY, et al. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am J Transplant. 2005;5(12):2907–12.
Valdivia P, et al. Plasmapheresis for the prophylaxis and treatment of recurrent focal segmental glomerulosclerosis following renal transplant. Transplant Proc. 2005;37(3):1473–4.
Cravedi P, Kopp JB, Remuzzi G. Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant. 2013;13(2):266–74.
Iharada A, et al. Increased nitric oxide production by T- and B-cells in idiopathic nephrotic syndrome. Pediatr Nephrol. 2009;24(5):1033–8.
Benz K, et al. Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol. 2004;19(7):794–7.
Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med. 2006;354(18):1961–3.
Araya CE, Dharnidharka VR. The factors that may predict response to rituximab therapy in recurrent focal segmental glomerulosclerosis: a systematic review. J Transplant. 2011;2011:374213.
Fornoni A, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3(85):85ra46.
Cameron JS, et al. Focal segmental glomerulosclerosis in fifty-nine renal allografts from a single centre; analysis of risk factors for recurrence. Transplant Proc. 1989;21(1 Pt 2):2117–8.
Garrouste C, et al. Rituximab for recurrence of primary focal segmental glomerulosclerosis after kidney transplantation: clinical outcomes. Transplantation. 2017;101(3):649–56.
Kashgary A, et al. The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: a systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol. 2016;17(1):104.
D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365(25):2398–411.
Coward RJ, et al. Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, Podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol. 2005;16(3):629–37.
Sharma R, et al. Components of normal serum block the focal segmental glomerulosclerosis factor activity in vitro. Kidney Int. 2000;58(5):1973–9.
Wei C, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17(8):952–60.
Alachkar N, et al. Podocyte effacement closely links to suPAR levels at time of posttransplantation focal segmental glomerulosclerosis occurrence and improves with therapy. Transplantation. 2013;96(7):649–56.
Yu H, et al. Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol Cell Biol. 2013;33(23):4755–64.
Alachkar N, Gupta G, Montgomery RA. Angiotensin antibodies and focal segmental glomerulosclerosis. N Engl J Med. 2013;368(10):971–3.
Musante L, et al. Circulating anti-actin and anti-ATP synthase antibodies identify a sub-set of patients with idiopathic nephrotic syndrome. Clin Exp Immunol. 2005;141(3):491–9.
Delville M, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6(256):256ra136.
Orman SV, et al. Nephrotic syndrome associated with a clonal T-cell leukemia of large granular lymphocytes with cytotoxic function. Arch Intern Med. 1986;146(9):1827–9.
Le Berre L, et al. Induction of T regulatory cells attenuates idiopathic nephrotic syndrome. J Am Soc Nephrol. 2009;20(1):57–67.
Bao L, et al. Decay-accelerating factor expression in the rat kidney is restricted to the apical surface of podocytes. Kidney Int. 2002;62(6):2010–21.
Wei C, et al. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23(12):2051–9.
Fujimoto K, et al. Clinical significance of serum and urinary soluble urokinase receptor (suPAR) in primary nephrotic syndrome and MPO-ANCA-associated glomerulonephritis in Japanese. Clin Exp Nephrol. 2015;19(5):804–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no competing interest.
Research involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors. This was a retrospective review meeting the criteria for a service evaluation study and hence did not require approval from a Research Ethics Committee. This study was approved by the Departmental Transplant Research Group. This therapeutic protocol for the management of post-transplant FSGS was introduced in our institution in 2011 and became the standard treatment for this clinical condition as approved by the Transplant Clinical and Research Group in our Centre. Our retrospective study is in compliance with the Helsinki Declaration.
Informed consent
All patients gave their consent for treatment and received standard care according to our accepted unit protocol.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Koutroutsos, K., Charif, R., Moran, L. et al. Successful management of post-transplant focal segmental glomerulosclerosis with therapeutic plasma exchange and rituximab. Clin Exp Nephrol 23, 700–709 (2019). https://doi.org/10.1007/s10157-019-01690-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01690-0