Abstract
Purpose
The purpose of our study is to carry out a Bayesian network meta-analysis comparing the efficacy of different antimicrobial lock solutions (ALS) for prevention of catheter-related infections (CRI) in patients with hemodialysis (HD) and ranking these ALS for practical consideration.
Methods
We searched six electronic databases, earlier relevant meta-analysis and reference lists of included studies for randomized controlled trials (RCTs) that compared ALS for preventing episodes of CRI in patients with HD either head-to-head or against control interventions using non-ALS. Two authors independently assessed the methodological quality of included studies using the Cochrane risk of bias tool and extracted relevant information according to a predesigned extraction form. Data were analysed using the WinBUGS (V.1.4.3) and the Stata (V.13.0).
Results
Finally, 18 studies involving 2395 patients and evaluating 9 ALS strategies were included. Network meta-analysis showed that gentamicin plus citrate (OR 0.07, 95% CrI 0.00–0.48) and gentamicin plus heparin (OR 0.04, 95% CrI 0.00–0.23) were statistically superior to heparin alone in terms of reducing CRBSI. For exit site infection and all-cause mortality, no significant difference in the intervention effect (p > 0.05) was detected for all included ALS when compared to heparin. Moreover, all ALS were similar in efficacy (p > 0.05) from each other for CRBSI, exit site infection and all-cause mortality.
Conclusions
Our findings indicated that gentamicin plus heparin may be selected for the prophylaxis of CRI in patients undergoing HD with CVCs. Whether this strategy will lead to antimicrobial resistance remains unclear in view of the relatively short duration of included studies. More attentions should be made regarding head-to-head comparisons of the most commonly used ALS in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For chronic hemodialysis (HD) treatment, a functioning arteriovenous fistula or synthetic graft is preferred for vascular access [1, 2]. However, in current practice, central venous catheters (CVCs) remain a common form of access for many patients [3, 4]. They are temporarily used in acute kidney injury and in a substantial proportion of incident and prevalent HD patients until their permanent vascular access becomes available. However, CVCs are prone to catheter malfunction and catheter-related infection (CRI), which includes catheter-related bloodstream infection (CRBSI) and exit site infection. It has been estimated that the relative risk (RR) for infection in HD catheters when compared with native arteriovenous fistulae is 15.5 and 25.5 [5].
CRI is associated with a substantial morbidity and mortality. According to the US Renal Data System, infection is the second leading cause of death in patients with end-stage renal disease, and the leading cause of catheter removal and morbidity in dialysis patients [6–8]. Besides, the costs to the health care system are also substantial [9]. Therefore, the prevention and management of CRI remains a significant clinical challenge in the management of this form of vascular access.
Over recent years, interest has focused on the use of antimicrobial lock solutions (ALS) of the catheters between each HD session. It is known from in vitro studies that solutions containing antimicrobials can prevent biofilm formation [10]. The biofilm constitutes a permanent source of bacteraemia, as well as a key factor favouring bacterial resistance [11, 12]. Also there were some clinical researches reports that ALS is significantly more effective in reducing the occurrence of CRI when used in HD patients [13–15]. These available antimicrobials include gentamicin, taurolidine, minocycline and so on. Similarly, several recent meta-analyses confirmed that prophylactic antimicrobial locks are effective in preventing such infections when compared to heparin locks [16–20].
However, the most appropriate antimicrobial agent has not been determined to date, as few head-to-head comparisons among different ALS have been published and the traditional meta-analysis only focuses on comparing two alternatives. In this situation, a network meta-analysis is urgently needed in this area. Network meta-analysis methods enabled us to simultaneously compare more than two interventions in the same analysis; provided relative effect estimates for all intervention comparisons, even those in which there was no direct evidence available; enabled the estimation of the probability that each intervention is best; and reduced the uncertainty in the intervention effect estimates [21–23]. Thus, we carried out this network meta-analysis to compare the efficacy of different ALS for prevention of CRI for HD patients based on existing RCT and ranking these ALS for practical consideration.
Subjects and methods
Protocol and registration
This protocol has been registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO/) under registration number CRD42015027010.
Eligibility criteria
-
1.
Type of study
Any relevant RCTs were included.
-
2.
Participants
The participants must be adults, aged at least 18 years, who had or were about to commence either short-term or maintenance hemodialysis using tunnelled or non-tunnelled CVC as vascular access.
-
3.
Type of interventions
RCTs of ALS used to prevent CRI in HD patients were included, regardless of whether the antimicrobials were tested between themselves (head-to-head) or against placebo/control intervention such as heparin.
-
4.
Outcomes of interest
The primary outcome was CRBSI. The Centers for Disease Control (CDC) definitions for CRBSI were used [24]. Only RCTs that used this definition, or RCTs whose results were detailed enough to be re-adjudicated according to the aforementioned definition, were included. In cases when a study separately reported definite, probable and possible CRBSI, we chose not to include ‘possible’ blood stream infection (defined as the absence of laboratory confirmation of blood stream infection).
The secondary outcomes were exit site infection (defined as the development of a purulent exudates or redness around the site not resulting from residual stitches), all-cause mortality and adverse events as reported by study authors.
-
5.
Other criteria
Other inclusion criteria: the RCTs must report sufficient data for calculating the risks of CRBSI in the intervention and control group. Other exclusion criteria were (1) duplicated or redundant studies, (2) combined interventions with multiple antimicrobial solutions and (3) studies dealing with the treatment of CRI rather than with prophylaxis.
Information sources and search
We systematically performed an electronic search of PubMed, Cochrane Library, Embase (via Embase.com platform), Sciences Citation Index (via Web of knowledge platform), CINAHL (via EBSCO platform) and Chinese Biomedical Literature Database from their inception to September 2015 with no language restrictions. In addition, we searched unpublished theses and dissertations via Conference Proceedings Citation Index, China Proceeding of Conference Full-text Database, China Doctoral Dissertation Full-text Database, China Master’s Theses Full-text Database and the System for Information on Gray Literature database in Europe (SIGLE). We also searched the World Health Organization International Clinical Trials Registry Platform Search Portal (www.who.int/trialsearch/) for ongoing trial registers. Relevant systematic reviews and meta-analyses from these databases were identified and bibliographies were scrutinised for further relevant trials, as well as those of RCTs included in the review. The search method included relevant text words and medical subject headings related to HD, infection, CVC and RCT. The exact search strategy used in the PubMed database is provided as an example in Online Resource 1.
Study selection
Literature search results were imported into ENDNOTE X7 literature management software. Two authors independently reviewed the literature searches from the title, abstract or descriptors and excluded the study that clearly does not meet the inclusion criteria. After excluding the duplicated and apparently irrelevant studies, the remaining studies were reviewed in full text to assess eligibility for inclusion. Any disagreements were resolved by discussion or by seeking an independent third opinion. Excluded trials and the reason for their exclusion were listed and examined by a third reviewer.
Data collection process and data items
Two authors independently extracted the data from each study using a standardized data extraction checklist, which include study characteristics (e.g. first author’s name, publication year, journal, country where the study was conducted), characteristics of study subjects (e.g. number of participants, age, gender distribution), characteristics of catheter (e.g. type of catheters, number of catheters), interventions details (e.g. type and concentration of lock solutions, patient involvement, duration of hemodialysis, number of catheter days), outcome variables (e.g. number of episodes) and any additional prophylactic measures used that may have affected outcomes (e.g. catheter care). Outcomes were extracted preferentially by intention to treat (ITT) at the end of follow-up. Quantitative data were extracted to calculate effect sizes. Data on effect size that could not be obtained directly were recalculated, when possible. Any discrepancy was resolved by consensus.
Risk of bias within individual studies
Two authors independently evaluated the methodological quality of the included studies for major potential sources of bias by using the Cochrane Collaboration’s risk of bias tool [25], which include method of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data selective reporting, and other sources of bias. We evaluated methodological quality of each study on each criterion as low, high or unclear risk of bias. Any disagreements was resolved through discussion, if need be, with another reviewer.
Statistical analysis
Bayesian network meta-analysis was performed by using the Markov chain Monte Carlo method in WinBUGS 1.4.3. The other analyses were performed and presented by the Stata 13.0 using the mvmeta command. Treatment effects of dichotomous outcomes were reported as posterior means of odds ratio (OR) with corresponding 95% credible intervals (CrI), which can be interpreted similarly to conventional 95% confidence intervals (CI). Both random effects and fixed effects models with vague priors for multiarm trials were used. The main difference between these two types of model is that the former takes into account the between-study variance, thereby producing wider credible intervals. Model fit was determined based on the deviance information criteria (DIC) for each outcome measure [26]. Three Markov chains were run simultaneously with different arbitrarily chosen initial values. To ensure convergence, Brooks–Gelman–Rubin method was assessed [27]. By this process, a potential scale reduction factor (PSRF) was calculated by comparing within-chain and between-chain variance. A PSRF very close to 1 was considered to indicate an approximate convergence. Convergence were found to be adequate after running 20,000 samples for three chains. These samples were then discarded as ‘burn-in’, and posterior summaries were based on 100,000 subsequent simulations. When a loop connected three treatments, it was possible to evaluate the inconsistency between direct and indirect evidence. The node-splitting method was used to calculate the inconsistency of the model, which separated evidence on a particular comparison into direct and indirect evidence. Significant inconsistency was indicated if node-splitting analysis derived p < 0.05.
The ranking probabilities of all interventions were used to calculate a summary numerical value: the surface under the cumulative ranking curve (SUCRA). SUCRA values are expressed as percentages; if an intervention is certainly the best, its SUCRA value would be 100%, and if an intervention is certainly the worst, its SUCRA value would be 0%.
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. The I 2 statistic was used to assess heterogeneity among the studies and in the entire network and considered values over 50% to represent high heterogeneity. We conducted a sensitivity analysis by excluding trials with a total sample size of less than 50 randomized patients. A comparison-adjusted funnel plot was used to assess whether smaller studies produced larger treatment effects.
Results
Study selection
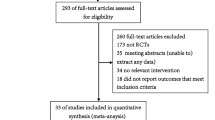
Figure 1 presents a flow diagram illustrating the studies selection process. The electronic searches identified 1467 studies, of which 572 duplicates were excluded by Endnote software and 793 articles were clearly not relevant after the first screening. 102 were retrieved in full text for in-depth consideration, and then 84 were excluded. Finally, 18 studies [13–15, 28–42] were included in our analysis. References cited in published original and review papers were examined until no further studies were found.
Characteristics of included studies
The characteristics of studies included in the network meta-analysis are presented in Table 1.
Of these included studies, four [33, 34, 38, 42] were performed in China, three [15, 31, 40] in the USA, two [37, 41] in Greece, two [28, 35] in the United Kingdom, two [13, 39] in Australia, two [14, 30] in Netherlands, one [32] in Canada, one [29] in Saudi Arabia and one [36] in Brazil. The combined sample size across 18 included studies [13–15, 28–42] was 2395 participants with a total of 2548 catheters. The interventions used in studies were highly variable. The antimicrobials assessed were gentamicin in 8 trials [13, 28, 31, 33, 34, 37, 38, 40], taurolidine in 3 trials [14, 35, 37], minocycline-EDTA in 3 trials [15, 31, 36], citrate in 2 trials [30, 32], cefotaxime in 1 trial [29], vancomycin in 1 trial [41], cefazolin in 1 trial [42] and ethanol in 1 trial [39]. Nori et al. [31] and Sofroniadou et al. [41] used two active comparator groups in their studies in addition to the control group. Of note, we were unable to include in our analysis the linezolid arm of the study conducted by Sofroniadou et al. [41] because of missing data for patients in this group. Comparison treatments were somewhat less varied with most trials using heparin as controls. There was the only one study in which both study groups included an antimicrobial agent. The concentration of antimicrobial lock solutions and heparin used in individual studies were presented in Online Resource 2. Not all studies used the same type of catheter. Nine [13, 28, 31–35, 39, 40] assessed tunnelled catheters only, 2 [29, 41] assessed non-tunnelled catheters only, 4 [14, 15, 30, 36] assessed both types of catheters and the remainders [37, 38] were unclear in the reported paper. Catheter care is of crucial importance to patients using intravascular catheters. Fourteen studies [13, 14, 28–30, 32, 34–41] described catheter care procedures. Cleaning the catheter site with iodine or chlorhexidine solutions and changing of dressing after each dialysis session appeared to be the most common catheter care. All trials evaluated the incidence of CRBSI and some of them assessed the incidence of exit site infection. All-cause mortality at the end of follow-up was reported in 7 trials [30–34, 40, 41].
Methodological quality of included studies
Table 2 shows the quality assessment of the studies in this network meta-analysis. All included studies were stated to be randomized, but based on the Cochrane Collaboration tool, only 10 [13, 14, 29, 30, 34, 35, 37, 39–41] specified the method of randomization and 7 [13, 28–30, 35, 36, 39] had a clearly defined method to conceal allocation of patients. Three trials [13, 29, 30] had blinding of patient, personnel and outcome assessors. The patients and personnel providers alone were blinded in 4 trials [15, 34, 35, 41], and one trial [40] had blinding of patients alone. Seven trials [14, 28, 31, 32, 36, 37, 39] were open-label trials. Description of drop-outs was adequate in 14 trials [13–15, 28, 30–33, 35–39, 41], and the remainders were unclear in the reported paper [29, 34, 40, 42].
The results of the network meta-analysis
We performed a Bayesian network meta-analysis to assess the relative outcomes of different ALS and control conditions with each other from all direct and indirect comparisons. The random effects model was used for CRBSI because the DIC for this outcome measure favoured this model over the fixed effects model. So, the analysis of CRBSI was based on the results generated by the former. For exit site infection and all-cause mortality, the fixed effects model showed a better fit than the random-effect one.
Figure 2 showed the network structure of the comparisons among the different interventions for the outcomes. The lines between interventions nodes indicate the direct comparisons made within randomized trials. The width of the lines is proportional to the number of trials comparing each pair of interventions, and the size of each node is proportional to the number of randomly assigned participants. All these network plots have a star geometry, with heparin acting as the common comparator. The missing links between active interventions reflect the scarcity of direct comparisons.
Effects on CRBSI
The results of network meta-analysis about 10 interventions (including the control group) comparing with each other were reported in Fig. 3. A total of 18 trials [13–15, 28–42] (2395 participants, 2496 catheters) contributed to the analysis of CRBSI. It indicated that only gentamicin plus citrate (OR 0.07, 95% CrI 0.00–0.48) and gentamicin plus heparin (OR 0.04, 95% CrI 0.00–0.23) were statistically significantly more effective in terms of reducing CRBSI when compared to heparin alone. Besides that, there was no difference in effects between active interventions.
The results of the evaluation of heterogeneity showed a moderate to high level of statistical heterogeneity for many of the pair-wise comparisons (Online Resource 3), as well as across all studies as a whole (I 2 = 50%). However, given the low number of eligible studies for the majority of comparisons, we didn’t perform meaningful subgroup analysis, which is important given the heterogeneity of the study.
Table 3 shows which ALS had the greatest possibility of being the most efficacious intervention based on an analysis of the area under the SUCRA curve, which was drawn according to the cumulative probabilities, with the percentage of the area under each curve shown (larger area signifying a better result). Based on SUCRA, gentamicin plus heparin ranked the first, the second was vancomycin plus heparin and the last was heparin alone. As shown in Figure S1, the inspection of the ‘comparison-adjusted’ funnel plot did not show small study bias (Online Resource 4).
Effects on exit site infection
Figure 4 summarizes the overall efficacy to reduce exit site infection of the included antimicrobial categories. A total of 11 trials [13, 14, 29, 30, 32, 34–36, 38, 40, 41] (1891 participants, 1961 catheters) contributed to this analysis. The results showed that none of the intervention arms were found to be statistically significantly different when compared with each other. According to SUCRA values (Table 3), vancomycin plus heparin got the highest probability (69.0%) and taurolidine plus citrate got the second highest (66.8%) among all the eight treatments. No significant evidence of heterogeneity was observed in the evaluation of heterogeneity on this outcome (I 2 = 0%) (Online Resource 3); therefore, we did not perform subgroup analyses in relation to heterogeneity. As shown in Figure S2, the inspection of the ‘comparison-adjusted’ funnel plot did not show small study bias (Online Resource 4).
Effects on all-cause mortality
Seven trials [30–34, 40, 41] (1131 participants, 1096 catheters) provided data for the analysis of all-cause mortality. The analysis results suggested that there was no difference in reducing all-cause mortality between included interventions (Fig. 5). Similarly, substantial heterogeneity was not present, therefore we did not perform subgroup analyses (I 2 = 0%) (Online Resource 3). In the rank probability test (Table 3), gentamicin plus citrate had the highest rank (74.7%), followed by heparin (69.2%). As shown in Figure S3, the inspection of the ‘comparison-adjusted’ funnel plot did not show small study bias (Online Resource 4).
Adverse events
A summary of adverse events reported in the included trials is presented in Table 4. A total of eleven trials [13, 14, 28, 30, 33, 34, 37–39, 41, 42] reported adverse effects in their results. Five [14, 28, 37, 38, 41] of them claimed no adverse effects related to taurolidine plus citrate, gentamicin plus heparin and vancomycin plus heparin, respectively. However, two other studies [33, 34] reported adverse effect of tinnitus related to gentamicin plus heparin. One trial [30] reported a higher frequency of adverse events, mainly bleeding, in the heparin group, and 1 trial [30] reported a higher rate of paraesthesia with citrate compared with heparin. In Broom’s study [39], in the ethanol group there was 1 patient who complained of stinging at the catheter exit site and another patient complained of dry lips and being thirsty.
Sensitivity analysis
In order to assess the robustness of results, we conducted a sensitivity analysis by excluding trials with a total sample size of less than 50 randomized patients. A total of 16 trials [13–15, 28–38, 40, 41] (2321 participants, 2422 catheters) contributed to the analysis. The results of the sensitivity analysis did not show a major influence on the primary outcome (Fig. 6; Table 3). We were not able to recalculate effect sizes for the other two outcomes due to the small number of studies that were examined. Similarly, given the low number of eligible studies, we did not perform another meaningful sensitivity analysis by excluding trials that the criterion of CRBSI diagnosis does not meet the Infectious Diseases Society of America (IDSA) guidelines.
Inconsistency and convergence
In network (a) and network (d), 2 triangle loops (gentamicin + heparin vs heparin vs taurolidine + citrate and gentamicin + citrate vs heparin vs minocycline-EDTA) were found. In network (c), 1 triangle loop (gentamicin + citrate vs heparin vs minocycline-EDTA) was found. There was no loop in networks (b). In these 3 loops, the direct comparisons and indirect comparisons did not show any major inconsistence. That is, all the p values of node-splitting analysis were not statistically significant at the 5% significance level (Online Resource 5).
The PSRFs of parameters were all unlimitedly close to 1 without exception, which represented good convergence.
Discussion
The objective of this study was to compare the efficacy of different kinds of ALS in terms of CRBSI, exit site infection, all-cause mortality and adverse events among HD patients. This is the first systematic review that has included almost all ALS categories for hemodialysis in the same analysis using a network meta-analysis method that includes indirect comparisons.
Summary of the results
In terms of reducing the occurrences of CRBSI, gentamicin plus citrate and gentamicin plus heparin had a positive effect in comparison with heparin alone. However, after conducting the rank probability test, the effectiveness of vancomycin plus heparin and ethanol appeared to be better than gentamicin plus citrate in this outcome measure. Sensitive analysis was conducted to assess the robustness of results by excluding trials with a total sample size of less than 50 randomized patients. The results of the sensitivity analyses showed broad agreement with the main analyses, and the rank probability for sensitive analysis was also consistent with the previous probability. Overall, gentamicin plus heparin is most likely (highest probability) to be the best intervention option among the nine ALS in preventing CRBSI for HD patients. These findings barely changed in sensitivity analysis. In this network meta-analysis, no significant difference in the intervention effect was detected with respect to either the exit site infection or the all-cause mortality for all included ALS. Based on the rank probability, vancomycin plus heparin was associated with lowest exit site infection and gentamicin plus citrate was associated with lowest all-cause mortality.
In the past few years, some pair-wise meta-analyses regarding these techniques have been published [16, 18, 43]. From these studies, it is concluded that ALS reduces the risk of CRBSI for HD patients with CVCs. However, it is notable that these studies did not distinguish the types of antimicrobials analysed by each domain. Thus, it is unclear from these meta-analyses whether particular antimicrobial agent or certain combinations of antimicrobial agents may have been responsible for the observed treatment effects. Besides, no evidence focused on the efficacy of these ALS when compared to each other due to few RCT compared them directly. In this case, our study adds to these previous efforts by providing a more complete understanding of the current body of evidence on comparative effectiveness of ALS for the prevention of CRBSI.
Exit site infections are an additional cause of morbidity in patients with CVCs, and these infections may contribute to the pathogenesis of CRBSI [44]. Pooled analysis from eleven trials in our network meta-analysis indicated that all included ALS did not significantly reduce the exit site infections when compared to heparin alone and also no significant difference was observed among them. But results from Zacharioudakis et al.’s [43] and Rabindranath et al.’s [17] studies suggested that ALS have a significant effect in reducing the risk of these infections. In these two meta-analyses [17, 43], pooling data on exit site infections were provided by 12 included RCTs. Based on the results of these individual RCTs presented, we found that none of the ALS was significantly superior to heparin except for citrate in Weijmer’s study [30]. However, Zhao et al.’s meta-analysis [20] showed no statistical significance between citrate and heparin in the prevention of exit site infection.
Seven RCTs [30–34, 40, 41] involving 5 different ALS provided a measure of all-cause mortality. In these, no statistically significant difference was observed. Results from the previous meta-analyses also confirmed that prophylaxis against CRI with ALS may not reduce all-cause mortality for patients [43, 45].
It must be noted that despite the accumulation of RCTs supporting the efficacy of gentamicin prophylaxis for CRBSI, the implementation of this agent in clinical practice should be prudent due to the potential for development of bacterial antibiotic resistance. Admittedly, gentamicin-resistant CRIs have been observed among HD patients who are receiving gentamicin lock therapy [46]. However, none of the included studies in our analysis reported the emergence of drug-resistant bacterial isolates. The major reason may be the relatively short duration of follow-up of the included studies. It is preferable that this type of research question be addressed using long-term prospective studies.
Besides, a decision regarding use of the gentamicin-containing lock solutions should be based on potential adverse events. It is known that gentamicin has been linked to ototoxicity. In the studies using gentamicin in our analysis, the formal audiology testing of ototoxicity was not performed. Dogra et al. [13] reported deafness in almost 10% of patients in the study arm using high-concentration gentamicin plus citrate in the context of a significant increase in serum gentamicin levels. Moreover, there was 1 patient who complained of mild tinnitus that might be attributable to gentamicin, respectively, in Tan’s [33] and Zhang et al.’s studies [34]. However, this adverse effect was not seen in 5 other studies [28, 31, 37, 38, 40], which also used a low concentration of gentamicin. Therefore, low-dose gentamicin appears safe. If possible, we proposed further studies should monitor serum gentamicin levels with the detailed documentation of safety parameters.
Limitation
In this study, a comprehensive literature search with several databases and sources was performed to cover as many eligible trials as possible. In order to minimize possible concerns about heterogeneity and transitivity, we selected all relevant studies strictly according to the pre-specified inclusion criteria. The review was conducted according to modern methodological standards. But, as is the case with all systematic reviews, several important limitations of this study should be noted.
First, reporting of the included studies themselves was incomplete. The majority of trials were failed to specify the method of randomization, use appropriate allocation concealment procedures and ensure blinding of relevant personnel, representing significant methodological limitations that may have biased the results.
Second, both the number of included studies and the sample size were small. Some comparisons were performed based on only 1 RCT, so the potential for bias should not be neglected. Moreover, the small number of studies that evaluated each particular pair of treatments limits performing a meaningful subgroup meta-analysis. An additional question caused by the low number of eligible studies and small sample sizes within trials was the wide CrI. Moreover, when event rates are very low, CrI around relative effects may also be wide. Wide CrI represent the high uncertainty of results. The conclusion drawn from such meta-analysis remains preliminary. More RCTs in this field are definitely needed to allow more solid conclusions about most moderating factors. However, we have confidence in the estimates of efficacy of gentamicin prophylaxis for CRBSI in our analysis. The 95% CrI excluding no effect indicates a robust effect when compared to heparin alone.
Third, we excluded trials investigating ALS with more than one antimicrobial agent, although combination intervention is common. The large number of intervention combinations and scarcity of trials comparing these interventions prompted us to restrict our analysis to mono-therapies. The decision to exclude combination interventions was also intended to minimize heterogeneity among interventions because it was believed that combination therapies would probably vary considerably, which would preclude meaningful pooling of data.
Finally, antimicrobial resistance remains an unresolved issue in view of the relatively short duration of included studies.
Fortunately, no obvious evidence of inconsistency was observed in this network meta-analysis. Secondly, the low level of methodological quality and the limitation in sample size of some studies could be a potential threat to the validity of results. However, the stability of the results of the sensitivity analyses confirms that the main findings of this research are robust and justified.
Conclusions
We compared the most commonly used ALS for HD patients via a Bayesian network meta-analysis. The use of this method has enabled us to provide new information on the relative effectiveness of ALS for the management of CRI in HD patients. Based on the results of network meta-analysis and probability rank, gentamicin plus heparin may be the best way to prevent CRBSI, vancomycin plus heparin might lead to the lowest incidence of exit site infection and gentamicin plus citrate might had the lowest risk of all-cause mortality for HD people. However, evidence is scant, mostly indirect and often derived from small trials with an unclear risk of bias. A certain degree of caution should be taken with interpretation of these results. Our network meta-analysis therefore has implications for future research efforts and highlights the need for properly designed RCTs and more head-to-head comparisons of the most commonly used ALS in this field.
References
Rehman R, Schmidt RJ, Moss AH (2009) Ethical and legal obligation to avoid long-term tunneled catheter access. Clin J Am Soc Nephrol 4:456–460
Rayner HC, Besarab A, Brown WW et al (2004) Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines. Am J Kidney Dis 44:22–26
Pisoni RL, Young EW, Dykstra DM et al (2002) Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int 61:305–316
Lacson E Jr, Lazarus JM, Himmelfarb J et al (2007) Balancing fistula first with catheters last. Am J Kidney Dis 50:379–395
Taylor G, Gravel D, Johnston L et al (2002) Prospective surveillance for primary bloodstream infections occurring in Canadian hemodialysis units. Infect Control Hosp Epidemiol 23:716–720
Silva TN, de Marchi D, Mendes ML et al (2014) Approach to prophylactic measures for central venous catheter-related infections in hemodialysis: a critical review. Hemodial Int 18:15–23
Katneni R, Hedayati SS (2007) Central venous catheter-related bacteremia in chronic hemodialysis patients: epidemiology and evidence-based management. Nat Clin Pract Nephrol 3:256–266
Ishani A, Collins AJ, Herzog CA et al (2005) Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int 68:311–318
Engemann JJ, Friedman JY, Reed SD et al (2005) Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol 26:534–539
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Donlan RM (2001) Biofilm formation: a clinically relevant microbiologic process. Clin Infect Dis 33:1387–1392
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007
Dogra GK, Herson H, Hutchison B et al (2002) Prevention of tunneled hemodialysis catheter-related infections using catheter-restricted filling with gentamicin and citrate: a randomized controlled study. J Am Soc Nephrol 13:2133–2139
Betjes MG, van Agteren M (2004) Prevention of dialysis catheter-related sepsis with a citrate-taurolidine-containing lock solution. Nephrol Dial Transplant 19:1546–1551
Bleyer AJ, Mason L, Russell G et al (2005) A randomized, controlled trial of a new vascular catheter flush solution (minocycline-EDTA) in temporary hemodialysis access. Infect Control Hosp Epidemiol 26:520–524
Snaterse M, Rüger W, Scholte Op Reimer WJ et al (2010) Antibiotic-based catheter lock solutions for prevention of catheter-related bloodstream infection: a systematic review of randomised controlled trials. J Hosp Infect 75:1–11
Rabindranath KS, Bansal T, Adams J et al (2009) Systematic review of antimicrobials for the prevention of hemodialysis catheter-related infections. Nephrol Dial Transplant 24:3763–3774
Yahav D, Rozen-Zvi B, Gafter-Gvili A et al (2008) Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Clin Infect Dis 47:83–93
Liu H, Liu H, Deng J et al (2014) Preventing catheter-related bacteremia with taurolidine-citrate catheter locks: a systematic review and meta-analysis. Blood Purif 37:179–187
Zhao Y, Li Z, Zhang L et al (2014) Citrate versus heparin lock for hemodialysis catheters: a systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis 63:479–490
Lu G, Ades AE (2004) Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 23:3105–3124
Jansen JP, Crawford B, Bergman G et al (2008) Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health 11:956–964
Catalá-López F, Tobías A, Cameron C et al (2014) Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int 34:1489–1496
O’Grady NP, Alexander M, Dellinger EP et al (2002) Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep 51:1–29
Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Shriner D, Yi N (2009) Deviance information criterion (DIC) in Bayesian multiple QTL mapping. Comput Stat Data Anal 53:1850–1860
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7:434–445
McIntyre CW, Hulme LJ, Taal M et al (2004) Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney Int 66:801–805
Saxena AK, Panhotra BR (2005) The impact of catheter-restricted filling with cefotaxime and heparin on the lifespan of temporary hemodialysis catheters: a case controlled study. J Nephrol 18:755–763
Weijmer MC, van den Dorpel MA, Van de Ven PJ et al (2005) Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J Am Soc Nephrol 16:2769–2777
Nori US, Manoharan A, Yee J et al (2006) Comparison of low-dose gentamicin with minocycline as catheter lock solutions in the prevention of catheter-related bacteremia. Am J Kidney Dis 48:596–605
Macrae JM, Dojcinovic I, Djurdjev O et al (2009) Citrate 4% versus heparin and the reduction of thrombosis study (CHARTS). Clin J Am Soc Nephrol 3:369–374
Tan HZ (2008) A randomized controlled study on prevention of catheter-related bacteremia with gentamicin-heparin lock solution. Dissertation, Zhejiang University
Zhang P, Yuan J, Tan H et al (2009) Successful prevention of cuffed hemodialysis catheter-related infection using an antibiotic lock technique by strictly catheter-restricted antibiotic lock solution method. Blood Purif 27:206–211
Solomon LR, Cheesbrough JS, Ebah L et al (2010) A randomized double-blind controlled trial of taurolidine-citrate catheter locks for the prevention of bacteremia in patients treated with hemodialysis. Am J Kidney Dis 55:1060–1068
Campos RP, do Nascimento MM, Chula DC et al (2011) Minocycline-EDTA lock solution prevents catheter-related bacteremia in hemodialysis. J Am Soc Nephrol 22:1939–1945
Filiopoulos V, Hadjiyannakos D, Koutis I et al (2011) Approaches to prolong the use of uncuffed hemodialysis catheters: results of a randomized trial. Am J Nephrol 33:260–268
Lu DS (2011) Gentamicin lock solution prevents central venous catheter-related infections in hemodialysis. Med Inform 24:123–124
Broom JK, Krishnasamy R, Hawley CM et al (2012) A randomised controlled trial of heparin versus ethanol lock therapy for the prevention of catheter associated infection in hemodialysis patients–the HEALTHY-CATH trial. BMC Nephrol 13:146
Moran J, Sun S, Khababa I et al (2012) A randomized trial comparing gentamicin/citrate and heparin locks for central venous catheters in maintenance hemodialysis patients. Am J Kidney Dis 59:102–107
Sofroniadou S, Revela I, Smirloglou D et al (2012) Linezolid versus vancomycin antibiotic lock solution for the prevention of nontunneled catheter-related blood stream infections in hemodialysis patients: a prospective randomized study. Semin Dial 25:344–350
Hu ZX, Xiao HQ, Fei P (2013) Application of the lock solution containing l% cefazolin/30% sodium citrate for long-term indwelling catheters in maintenance hemodialysis patient. Chin J Blood Purif 12:602–604
Zacharioudakis IM, Zervou FN, Arvanitis M et al (2014) Antimicrobial lock solutions as a method to prevent central line-associated bloodstream infections: a meta-analysis of randomized controlled trials. Clin Infect Dis 59:1741–1749
Oliver MJ, Callery SM, Thorpe KE et al (2000) Risk of bacteremia from temporary hemodialysis catheters by site of insertion and duration of use: a prospective study. Kidney Int 58:2543–2545
Wang AY, Ivany JN, Perkovic V et al (2013) Anticoagulant therapies for the prevention of intravascular catheters malfunction in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Nephrol Dial Transplant 28:2875–2888
Landry DL, Braden GL, Gobeille SL et al (2010) Emergence of gentamicin-resistant bacteremia in hemodialysis patients receiving gentamicin lock catheter prophylaxis. Clin J Am Soc Nephrol 5:1799–1804
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, B., Li, R. et al. Does antimicrobial lock solution reduce catheter-related infections in hemodialysis patients with central venous catheters? A Bayesian network meta-analysis. Int Urol Nephrol 49, 701–716 (2017). https://doi.org/10.1007/s11255-016-1490-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1490-x