Abstract
Purpose
High levels of serum total homocysteine (tHcy), often observed in chronic kidney disease (CKD) patients, are a risk factor for cardiovascular disease. However, little is known about the relationship between tHcy and renal function in healthy individuals. We examined whether tHcy levels are related to renal function in Asian individuals without CKD.
Methods
This cross-sectional study examined 2032 subjects, aged 40–64 years. Individuals with kidney diseases or other conditions that could affect tHcy were excluded. Renal function was determined by estimated glomerular filtration rate (eGFR) from levels of serum creatinine (sCr) and cystatin C.
Results
Age, tHcy, sCr, and cystatin C of the subjects were 54.1 ± 6.0 years, 9.5 (8.0–11.4) μmol/L, 0.81 ± 0.1 mg/dL, and 0.82 ± 0.1 mg/L, respectively. In a multiple linear regression analysis, tHcy was a significant independent determinant of sCr and cystatin C in men (β = 0.206 and β = 0.282, respectively) and women (β = 0.247 and β = 0.229, respectively). Highest tHcy levels were independently associated with increased cystatin C (>s1.10 mg/L) with an odds ratio (OR) of 5.00 [95 % confidence interval (CI) 2.81–8.09] and decreased eGFR (<90 mL/min/1.73 m2) with an OR of 1.69 (95 % CI 1.36–2.11) compared to tHcy levels in the 1st–3rd quartiles.
Conclusions
Higher levels of tHcy are independently associated with sCr and cystatin C elevation. Our study suggests that tHcy levels may be influenced by renal function in Asian populations without CKD. Future studies are needed to define the role of tHcy in renal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an increasing prevalence of chronic kidney disease (CKD), a worldwide health problem with poor consequences and high cost [1]. Therefore, identification of factors responsible for the development of renal impairment at an early stage is important in preventing the progression towards CKD. It has been well documented that old age, diabetes mellitus (DM), hypertension (HTN), dyslipidaemia, and smoking are associated with subsequent reduction in glomerular filtration rate (GFR) [2, 3]. Despite prevention and treatment of these factors, the number of CKD patients is increasing [4]. This suggests that other factors are involved in renal function and they must be evaluated.
Homocysteine (Hcy) is a major metabolite produced by the transmethylation of methionine to cysteine [5]. The kidney is the major organ for the metabolism and removal of total homocysteine (tHcy) [6]. Therefore, the prevalence of hyperhomocysteinaemia is high in about 80 % of CKD patients [7]. However, the effects of hyperhomocysteinaemia in patients with normal renal function or in the earlier stages of CKD have not been studied in detail.
It is well known that high serum tHcy levels are associated with vascular damage, including cardiovascular, cerebral, and peripheral vessels, in the general population at a rate similar to that observed in CKD patients [8, 9]. This suggests that hyperhomocysteinaemia may lead to intrarenal arteriosclerotic change and a decline in renal function. In fact, several studies have showed that hyperhomocysteinaemia may be a predictor of CKD [10, 11]. However, the serum tHcy cut-off points, CKD prevalence, and selection of markers of renal function differed among the studies.
Meanwhile, even within the normal range, reduced GFR is known to be a major risk factor for mortality and cardiovascular disease (CVD) [12, 13]. This underlines the clinical importance of hyperhomocysteinaemia in the general population, and studies into the association between homocysteinaemia and renal function are required in individuals without CKD. Nevertheless, there are few reports exploring the influence of hyperhomocysteinaemia on renal function in the general population without CKD. Therefore, the objective of our study is to evaluate the association between serum Hcy levels and parameters for renal function in middle-aged individuals without CKD.
Methods
Study population
We conducted a cross-sectional study based on data obtained from medical records. We included participants aged 40–64 years who underwent a comprehensive health examination, including examination of serum tHcy levels (n = 2032) and kidney function, from July 2012 through June 2014. Exclusion criteria were as follows: individuals with CKD (diagnosed by a doctor or GFR < 60 mL/min/1.73 m2 for 3 months before the examination), gout, haematuria, pyuria, or other health conditions that may influence the tHcy level, such as cancer, liver diseases, gastrointestinal absorption disease, vegetarian lifestyle, or regular consumption of vitamin supplements, including vitamin B6, vitamin B12, or folate, in the 3 months prior to the examination. In addition, alcohol consumption may cause a decrease in vitamin B and an increase in plasma tHcy. Therefore, we excluded men with drinking intake >28 g/day and women with drinking intake >14 g/day [14]. The study protocol was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. E-2014153).
Data collection
Subjects were asked about medication history and health-related habits by a doctor. Smoking status was divided into “non-smoker” or “current smoker”. The type of alcohol, including the type of beverage, and frequency of drinking, as days per week, was recorded. Regular exercise was defined as moderate intensity exercise more than three times per week [15]. Patients with hypertension (HTN) were defined as those with a blood pressure (BP) > 140/90 mmHg or receiving antihypertensive medication. Patients with diabetes mellitus (DM) were those with haemoglobin A1c > 6.5 or fasting plasma glucose (FPG) > 126 mg/dL or those who were taking oral hypoglycaemic agents or insulin. Patients with dyslipidaemia were those with fasting total cholesterol (TC) > 240 mg/dL; those receiving statins or fenofibrates; or those with triglycerides greater than 200 mg/dL or high-density lipoprotein cholesterol less than 40 mg/dL in men or 50 mg/dL in women. While participants were seated after a 5-min rest, BP was checked twice using an automated measurement device (BP-203RV II, Colin Corp., Aichi, Japan) and the two results were averaged. Waist circumference (WC) was measured at the narrowest circumference between the lower margin of the rib cage and the crest of ilium. Body mass index (BMI) (kg/m2) was reported by dividing weight (kg) by height in squared metres (kg/m2). The percentage of body fat and total muscle mass (TMM) was assessed by bioelectric impedance analysis (Inbody 3.0, Biospace Co., Ltd, Seoul, Korea) in a standing position. All blood samples were obtained from the antecubital vein between 8 and 9 A.M. after overnight fasting for 12 h. We collected random midstream urine samples from all subjects. Lipid profiles and uric acid (UA) levels were analysed with the enzymatic colorimetric method using the Toshiba TBA200FR (Toshiba Co. Ltd., Tokyo, Japan). FPG was analysed using the glucose oxidase method using a Synchron LX 20 (Beckman Coulter, Fullerton, CA, USA). Blood urea nitrogen was analysed using a kinetic test by urease and glutamate dehydrogenase in a commercial enzymatic kit (Modular-DP, Roche, Basel, Switzerland).
Measurement of serum total homocysteine
To measure tHcy levels, venous blood samples were drawn from participants during the examination. Serum tHcy was measured by enzyme cycling using a Hitachi 7180 Automatic Analyzer (Hitachi, Japan). The Eone Reference Laboratory reported that the inter-assay and intra-assay coefficients of variation were less than 10 %. According to current clinical literature, hyperhomocysteinaemia was defined as a serum tHcy > 12 μmol/mL [16, 17].
Renal function measurement
Serum creatinine (sCr) was analysed by kinetic colorimetric assay based on a modified Jaffe method with a commercial enzymatic kit (Modular-DP, Roche, Basel, Switzerland). GFR estimated from sCr (eGFR), serum cystatin C, and urine protein were used for determination of renal function. We calculated the eGFR using the following equation from the Modification of Diet in Kidney Dysfunction Study (MDRD) [18].
Serum cystatin C was analysed by turbidimetric immunoassay (HBI Co., Ltd., Seongnam, Korea) using the Modular Analytics E170 (Roche Diagnostics, Basel, Switzerland).
Data analysis
Statistical analyses were conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Since tHcy and FPG levels were not normally distributed, the values were log-transformed. Each subject was divided into quartiles based on tHcy levels. For comparison of variables between four groups, we performed one-way analysis of variance (ANOVA) followed by Scheffe’s post hoc test. The Chi-squared test was used for analysis of the categorical variables. We also used Pearson’s correlation analysis to examine the association between sCr or cystatin C and tHcy. Simple linear regression analysis was conducted to determine the individual effects of sex, age, body composition on sCr or cystatin C. Subsequently, we performed a multiple linear regression analysis to identify whether tHcy levels are an independent determinant of sCr or cystatin C levels. Variables shown as P < 0.05 in the simple linear regression model were included as covariate factors in a multiple linear regression model. In addition, a cystatin C cut-off value of 1.10 mg/L was used to separate worse and better outcomes [19]. The odds ratio (OR) of cystatin C > 1.10 mg/L was computed using a multiple logistic regression analysis among the tHcy status groups after adjusting for confounders. If P values were <0.05, results were considered statistically significant.
Results
Clinical characteristics of the study subjects
A total of 2032 adults (1170 men and 862 women) aged 40–64 years were included in our study. The characteristics of the study subjects according to sex are presented in Table 1. On average, renal function of most study subjects was near normal. In comparison with women, men had higher prevalence of HTN, DM, and higher UA levels. Likewise, serum tHcy levels were higher in men than in women.
Differences among groups according to the quartile of serum total homocysteine
The clinical characteristics of the participants according to the quartile level of tHcy are presented in Tables 2, 3. Men with the highest tHcy levels had higher WC, body fat, SBP, DBP, and UA and were more likely to have HTN and be current smokers and alcohol drinkers (Table 2). Women with the highest tHcy levels tended to be older and more likely to have HTN, be alcohol drinkers, and have higher BMI, body fat, SBP, DBP, UA compared to women in the lowest group (Table 3).
Associations of renal function with serum total homocysteine
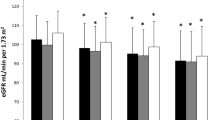
In evaluation of the association between tHcy status and renal function in both men and women, individuals with the highest tHcy levels had higher levels of sCr, cystatin C, and lower eGFR. However, these variables were within the normal range of values (Tables 2 and 3). tHcy showed a positive linear correlation with sCr (Fig. 1a) and cystatin C (Fig. 1b). In a multivariate linear regression analysis, sCr showed a significantly positive association with age, UA, and tHcy in both men and women (Table 4). Cystatin C also showed a significant positive correlation with age, UA, and tHcy in both men and women (Table 5).
Association of hyperhomocysteinaemia with reduced renal function
After having for sex, age, body composition, prevalence of chronic diseases, hyperuricaemia, TC, and FPG adjusted, ORs for risk of decreased eGFR (<90 mL/min/1.73 m2) and increased cystatin C (>1.10 mg/L) were 1.69 and 5.00 times greater in adults with the highest tHcy levels (tHcy > 12.6 μmol/L in men and tHcy > 9.2 μmol/L in women) compared to those with tHcy levels in the 1st–3rd quartiles (Table 6).
Discussion
The present study investigated the association between serum tHcy and renal function in middle-aged individuals without CKD. We found that tHcy had a positive linear association with sCr and cystatin C and a negative linear association with eGFR after adjustment for confounders. However, increases in cystatin C or sCr and decreases in eGFR with increasing tHcy levels were within the normal range of values.
In studies on the association between hyperhomocysteinaemia and renal function, most of the recent observational results were obtained from patients with advanced renal dysfunction [20–22]. These studies found an independent association of hyperhomocysteinaemia with decreasing GFR in CKD patients. Proposed mechanisms for hyperhomocysteinaemia in end-stage renal disease (ESRD) include reduced clearance of tHcy secondary to defective kidney or extra kidney metabolism of tHcy, and deficiencies of vitamin B12, folate, and pyridoxal 5-phosphate [6, 20–22]. Attention has focused on tHcy as a risk factor for CVD in CKD patients. Hyperhomocysteinaemia is of particular importance for CKD patients, in which CVD is the most common cause of death [9, 12]. The risk of CVD-related mortality in CKD patients is up to 30 times higher than for the general population [9]. Interestingly, traditional risk factors for CVD may not be able to explain this high mortality rate [23]. Instead, hyperhomocysteinaemia may contribute to increased CVD risk in CKD patients. Serum tHcy has atherogenic and prothrombotic properties [7, 8]. Moreover, tHcy triggers the biosynthesis of reactive oxygen species and damage endothelial function [16]. Hcy-induced vascular injuries cause smooth muscle hypertrophy, intimal thickening, elastic lamina disruption, marked platelet dysfunction, and formation of occlusive thrombi, all of which are associated with higher risk of CVD [24].
There have been few studies in the effect of increased tHcy values on renal function in populations with normal or mildly reduced renal function [25]. Because tHcy causes vascular damage, hyperhomocysteinaemia may have an adverse effect on kidney function. Supporting this hypothesis, our study found an inverse association between tHcy levels and renal function. Patients with mild degrees of renal dysfunction are also at increased CVD risk of a similar fate compared to those with ESRD [26]. Known CVD risk factors, such as HTN, DM, dyslipidaemia, cannot explain this higher risk [27]. Therefore, hyperhomocysteinaemia in individuals with mild renal dysfunction is of clinical importance.
Several studies attempted to find an inverse association between renal function and tHcy levels throughout the whole range of GFR values [28, 29]. However, most studies included patients with DM [28] or advanced age in their study subjects [29].
The mechanism underlying hyperhomocysteinaemia-related reduced renal function is unknown. It is possible that tHcy is easily oxidized into hydroxyl radicals and toxic superoxide anions [9]. Excessive formation of these oxidants would compromise the removal of free radicals by vascular endothelial cells and cause cell membrane lipid peroxidation. This membrane integrity damage would lead to microvascular endothelial dysfunction and degeneration [6]. Hyperhomocysteinaemia may also result in microvascular thrombosis by stimulating oxidative stress, the nitric oxide pathway, direct cytotoxicity, and coagulation factors [30]. The generation of microvascular thrombi causes damage to endothelial cells and reduces glomerular filtration. Meanwhile, although within normal range, decreased renal function may cause the retention of metabolites, influencing metabolism of sulphur amino acids, leading to high tHcy levels [25]. In the development of renal impairment, hyperhomocysteinaemia may therefore be an early marker for inflammatory processes.
Two longitudinal studies have addressed the association between hyperhomocysteinaemia and the development of CKD in populations with normal baseline renal function [10, 11]. Ninomiya et al. [10] examined 1477 Japanese individuals aged 40 years or older with 5 years of follow-up examinations and found that elevated tHcy levels were significantly associated with the incidence of CKD in women but not in men after adjustment for baseline sCr levels. However, serum tHcy levels were determined in this study using samples frozen for 12 years. Conversely, a second study on 3602 people (mean age, 44 ± 8.8 years) at a screening centre in Israel showed that both women and men with elevated tHcy level experienced accelerated renal function deterioration with a risk of about 4.85 over a median follow-up time of 7.75 years [11]. However, this result may not apply to the Asian population, since relevant factors of serum Hcy levels, such as dietary intake, vitamin consumption, and body composition, may differ between races [31]. Moreover, these two previous studies had a common limitation in the selection of eGFR as marker of renal function.
In our study particularly, tHcy was the strongest determinant of cystatin C. Our findings showed that the risk of impaired renal function (cystatin C > 1.10 mg/L) in a middle-aged population with tHcy level in the highest quartile was 5.0 times higher than in people with tHcy levels in the 1st–3rd quartiles. Results from the literature indicate that the effect of GFR based on cystatin C on tHcy differs with the population studied [29, 32]. To the best of our knowledge, we firstly found positive association between tHcy and cystatin C in large-scale population without CKD.
Cystatin C, also known as γ-microprotein, is an Hcy protease inhibitor produced by nucleated cells. This microprotein is a light molecular weight, non-glycosylated protein present in various nucleated cells and throughout the humoral fluids [19]. The synthesis of cystatin C is normally constant and not strongly affected by sex, age, diet, or inflammatory responses. Circulating cystatin C only is filtered by the kidneys and is almost completely reabsorbed and metabolized by the glomerular proximal tubule without returning to the blood circulation. Tubular epithelial cells cannot secrete cystatin C. Even minimal glomerular damage may cause a significant increase in serum cystatin C level [29]. Consistent studies suggest that cystatin C is better indicator of GFR than sCr, particularly for the early detection of GFR decreases [33]. Therefore, cystatin C is considered to be a more accurate marker for GFR. As a specific Hcy protease inhibitor, cystatin C can inhibit Hcy degradation and raise serum tHcy levels [34]. Cystatin C will synergize with Hcy and have an adverse impact on microvascular endothelia. Our finding suggests that elevated tHcy may contribute to early changes in renal dysfunction, leading to higher cystatin C in middle-aged populations without CKD.
Several factors such as vitamin deficiency and excessive alcohol drinking are known to cause the increase in the plasma level of tHcy [14, 31]. As hyperhomocysteinaemia is known as the marker for vascular disease, elevated level of tHcy may cause adverse effects in renal microvascular circulation and could possibly lead to the development of renal impairment. Early detection and interventions for reversible risk factors in mild stage of renal dysfunction are clinically important in order to prevent the progression to irreversible CKD. If hyperhomocysteinaemia may be served as the marker for decreased renal function as to our suggestion, tHcy lowering treatment can be recommended as the preventive strategy to reduce the risk of progression to CKD. In addition, our results propose that elevated tHcy may be a treatable risk factor in explanation of the heavy burden of CVD associated with kidney disease.
Our study has several limitations. First, we included only individuals who visited a local university hospital as study subjects, which may not represent the general population. Because it is a cross-sectional study, determination of a causal relationship among variables was difficult. Although we conducted careful chart review and 1:1 interview by doctor for exclusion individuals with CKD, it might be not sufficient enough to find the people with unknown CKD due to once laboratory examination. Moreover, we did not examine levels of vitamin B and folate directly. Vitamin deficiency contributes to a higher risk of hyperhomocysteinaemia [31]. However, there are contradictory findings from other investigators on the effects of renal function and vitamins on tHcy. This may be explained by a multitude of factors, including various dietary habit, different prevalence of vitamin deficiency in the investigated study groups, and the severity of renal dysfunction in the participants [14, 31, 35, 36].
Conclusions
In conclusion, higher levels of tHcy were associated with increased sCr and cystatin C in middle-aged individuals without CKD. These associations persisted after adjustment for age, body composition, chronic diseases, and health-related habits. Our results add credence to the evidence from previous studies that tHcy could be a possible biomarker for renal diseases. We suggest that, in populations without CKD, tHcy levels may be influenced by renal function and hyperhomocysteinaemia may be a significant early marker for the detection of renal dysfunction in the general population. To date, detection of CKD in the general population has been limited to measurement of microalbuminuria or sequential monitoring of GFR [2, 3]. If the mechanisms underlying the relationship between tHcy and renal function are clarified in prospective randomized clinical trials, timely intervention for hyperhomocysteinaemia at an early stage of renal dysfunction may be helpful in preventing and delaying the progression of CKD.
References
Obrador GT, Pereira BJ, Kausz AT (2002) Chronic kidney disease in the United States: an underrecognized problem. Semin Nephrol 22:441–448
Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J (2006) International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17(8):2275–2284
Ponte B, Pruijm M, Marques-Vidal P, Martin PY, Burnier M, Paccaud F, Waeber G, Vollenweider P, Bochud M (2013) Determinants and burden of chronic kidney disease in the population-based CoLaus study: a cross-sectional analysis. Nephrol Dial Transplant 28:2329–2339
Bommer Jurgen (2002) Prevalence and socio-economic aspects of chronic kidney disease. Nephrol Dial Transplant 17:8–12
Brattstrom L, Lindgren A, Israelsson B, Anderson A, Hultberg B (1994) Homocysteine and cysteine: determinants of plasma levels in middle-aged to elderly subjects. J Intern Med 236:633–641
Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH (2001) The kidney and homocysteine metabolism. J Am Soc Nephrol 12:2181–2189
Ferechide D, Radulescu D (2009) Hyperhomocysteinemia in renal diseases. J Med Life 2:53–59
Boushey CJ, Beresford SA, Omenn GS, Motulsky AG (1995) A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. probable benefits of benefits of increasing folic acid intakes. JAMA 274:1049–1057
Robinson Killian (2004) Renal disease, homocysteine, and cardiovascular complications. Circulation 109:294–295
Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Tanaka K, Okubo K, Nakamura H, Hata J, Oishi Y, Kato I, Hirakata H, Iida M (2004) Hyperhomocysteinemia and the development of chronic kidney disease in a general population: the Hisayama study. Am J Kidney Dis 44:437–445
Levi A, Cohen E, Levi M, Goldberg E, Garty M, Krause I (2014) Elevated serum homocysteine is a predictor of accelerated decline in renal function and chronic kidney disease: a historical prospective study. Eur J Intern Med 25:951–955
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351:1296–1305
Kielstein JT, Salpeter SR, Buckley NS, Cooke JP, Fliser D (2008) Two cardiovascular risk factors in one? Homocysteine and its relation to glomerular filtration rate. A meta-analysis of 41 studies with 27,000 participants. Kidney Blood Press Res 31:259–267
Sakuta H, Suzuki T (2005) Alcohol consumption and plasma homocysteine. Alcohol 37:73–77
Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I (1997) Association between leisure time physical activity and 10-year body mass change among working-aged men and women. Int J Obes Relat Metab Disord 21:288–296
Homocysteine Studies Collaboration (2002) Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 288:2015–2022
Wald DS, Law M, Morris JK (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 325:1202–1208
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Dietin Renal Disease Study Group. Ann Intern Med 130:461–470
Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C (2005) Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352:2049–2060
Menon V, Wang X, Greene T, Beck GJ, Kusek JW, Selhub J, Levey AS, Sarnak MJ (2005) Homocysteine in chronic kidney disease: effect of low protein diet and repletion with B vitamins. Kidney Int 67:1539–1546
Shankar A, Wang JJ, Chua B, Rochtchina E, Flood V, Mitchell P (2008) Positive association between plasma homocysteine level and chronic kidney disease. Kidney Blood Press Res 31:55–62
Bostom AG, Kronenberg F, Jacques PF, Kuen E, Ritz E, Konig P, Kraatz G, Lhotta K, Mann JF, Muller GA, Neyer U, Riegel W, Schwenger V, Riegler P, Selhub J (2001) Proteinuria and plasma total homocysteine levels in chronic renal disease patients with a normal range serum creatinine: critical impact of true glomerular filtration rate. Atherosclerosis 159:219–223
Sarnak MJ, Levey AS (2000) Cardiovascular disease and chronic renal disease: a new paradigm. Am J Kidney Dis 35:117–131
Bhalodia YS, Sheth NR, Vaghasiya JD, Jivani NP (2011) Homocysteine-dependent endothelial dysfunction induced by renal ischemia/reperfusion injury. J Nephrol 24:631–635
Francis ME, Eggers PW, Hostetter TH, Briggs JP (2004) Association between serum homocysteine and markers of impaired kidney function in adults in the United States. Kidney Int 66:303–312
Henry RM, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ et al (2002) Mild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn Study. Kidney Int 62:1402–1407
Stam F, van Guldener C, Becker A, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD (2006) Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol 17:537–545
Wollesen F, Brattström L, Refsum H, Ueland PM, Berglund L, Berne C (1999) Plasma total homocysteine and cysteine in relation to glomerular filtration rate in diabetes mellitus. Kidney Int 55:1028–1035
Lewerin C, Ljungman S, Nilsson-Ehle H (2007) Glomerular filtration rate as measured by serum cystatin C is an important determinant of plasma homocysteine and serum methylmalonic acid in the elderly. J Intern Med 261:65–73
Hucks D, Thuraisingham RC, Raftery MJ, Yaqoob MM (2004) Homocysteine induced impairment of nitric oxidedependent vasorelaxation is reversible by the superoxide dismutase mimetic TEMPOL. Nephrol Dial Transplant 19:1999–2005
Ganji V, Kafai MR (2003) Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 77:826–833
Jonasson T, Ohlin H, Andersson A, Arnadottir M, Hultberg B (2002) Renal function exerts only a minor influence on high plasma homocysteine concentrations in patients with acute coronary syndromes. Clin Chem Lab Med 40:137–142
Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO (1995) Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47:312–318
Bostom AG, Gohh RY, Bausserman L, Hakas D, Jacques PF, Selhub J, Dworkin L, Rosenberg IH (1999) Serum cystatin C as a determinant of fasting total homocysteine levels in renal transplant recipients with a normal serum creatinine. J Am Soc Nephrol 10:164–166
Ramel A, Jonsson PV, Bjornsson S, Thorsdottir I (2007) Total plasma homocysteine in hospitalized elderly: associations with vitamin status and renal function. Ann Nutr Metab 51:527–532
Gibson A, Woodside JV, Young IS, Sharpe PC, Mercer C, Patterson CC, Mckinley MC, Kluijtmans LAJ, Whitehead AS, Evans A (2008) Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers—a randomized, crossover intervention study. QJM 101:881–887
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. E-2014153).
Rights and permissions
About this article
Cite this article
Tak, Y.J., Jeong, D.W., Kim, Y.J. et al. Hyperhomocysteinaemia as a potential marker of early renal function decline in middle-aged Asian people without chronic kidney disease. Int Urol Nephrol 48, 239–248 (2016). https://doi.org/10.1007/s11255-015-1180-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1180-0