Abstract
Background
Patients either with hyperhomocysteinemia or chronic kidney disease (CKD) have an increased risk of cardiovascular disease. Little is known regarding whether hyperhomocysteinemia can increase the risk of CKD in a Chinese middle-aged and elderly population. To help clarify this we conducted a prospective cohort study to measure the association of hyperhomocysteinemia with CKD.
Methods
A total of 5917 adults aged 56.4 ± 9.6 years without CKD at baseline were enrolled. The highest homocysteine quartile (≥15 μmol/L) was defined as hyperhomocysteinemia. CKD was defined as decreased estimated glomerular filtration rate (eGFR < 60 mL/min/1.73 m2) or presence of proteinuria (urine protein ≥ 1+) assessed using a repeated dipstick method.
Results
During 3 years of follow-up, 143 (2.4%) patients developed CKD, 85 (1.4%) patients with proteinuria and 59 (1.0%) patients with decreased eGFR. After adjusted for potential confounders, both homocysteine (per 1 μmol/L increase) and hyperhomocysteinemia were independently associated with increased risk of decreased eGFR [with a fully adjusted OR of 1.07 (95% CI 1.04–1.10) and 3.05 (95% CI 1.71–5.46)] and CKD [with a fully adjusted OR of 1.04 (95% CI 1.02–1.07) and 1.62 (95% CI 1.11–2.35)], respectively. By contrast, neither homocysteine (per 1 μmol/L increase) nor hyperhomocysteinemia were associated with proteinuria in the multivariable logistic regression analysis.
Conclusions
The study revealed that hyperhomocysteinemia increases the risk of decreased eGFR. This suggests that homocysteine could be considered as a useful molecular markers for delaying the development of CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) has become a major public health problem worldwide over the past few decades, because it leads to end-stage renal disease (ESRD) in many people and is associated with an increased occurrence of cardiovascular disease (CVD) [1, 2]. In the USA in 2012, the prevalence of CKD was estimated at 13.6% and 636,905 individuals were treated for ESRD, and the total medicare expenditure for all stages of kidney disease (including ESRD and earlier stages of CKD, but not including prescription medications) was over $87 billion [3]. A recent national survey in China indicates that the prevalence of CKD in China is 10.8%, and the number of patients with CKD is estimated to 119.5 million [4]. Cardiovascular disease (CVD) is the main cause of mortality and morbidity worldwide [5]. A decreased estimated glomerular filtration rate (eGFR) is associated with cardiovascular mortality and morbidity in both high-risk patients [6] and the general population [7]. Microalbuminuria as well as macroalbuminuria are important markers for the progression of renal dysfunction and are currently recognized as predictive factors for CVD [8, 9]. The rapid increase in diabetes and hypertension, both of which are predicted to drive epidemics of CKD. But this increased risk for CKD cannot be fully explained by traditional factors, including age, hypertension, diabetes and dyslipidemia [10]. Methods for screening the risk of CKD is one fundamental strategy for the primary prevention of CKD, but the highest risk patients should be identified to maximize the benefit/cost ratio of treatments.
Previous epidemiological studies have reported associations between homocysteine and atherosclerotic vascular disease [11, 12], and hyperhomocysteinemia has been recognized as an independent factor for CVD [13, 14]. But little is known regarding whether hyperhomocysteinemia can increase the risk of CKD in a Chinese middle-aged and elderly population. To help clarify this we conducted the prospective cohort study to measure the association of hyperhomocysteinemia with CKD.
Methods
Study population
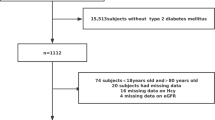
The study population has been described in detail elsewhere [15]. In brief, 5948 adults aged ≥40 years who received homocysteine test at baseline were followed for 3 years. During the follow-up, 17 participants were lost to follow-up. Patients receiving folic acid and/or vitamin B12 were not included in the study. The ethics committee of Qianfoshan Hospital approved the study. All participants gave written informed consent prior to data collection.
Blood biochemistry measurements and biometric parameters at baseline
Blood was collected by means of venipuncture after an overnight fast of at least 10 h. Routine serum and urinary chemistry determinations were performed by standard automated techniques. Total plasma homocysteine was assessed using high-performance liquid chromatography (HPLC) with fluorescence detection. eGFR was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) two-level race equation [16]. Serum creatinine was measured by means of using the Roche enzymatic method on an automatic biochemistry analyzer (Roche P Modular with Roche Creatininase Plus assy, Hoffman-La Roche, Ltd., http://www.roche.com). Protein in urine was measured on a morning urine sample using an immediate semiquantitative urine protein dipstick test and graded as negative, trace, 1+, 2+, 3+ or 4+. Subjects were considered to be proteinuric by repeated measures if their urinalysis showed ≥1+ on both initial and follow-up studies 1 or 2 weeks apart [17, 18]. Participants with pyuria were excluded from the analysis of proteinuria due to concern of urinary tract infection. Women during menstruation were asked to receive urine routine test 3 days after menstruation. The pulse of waveforms of the right carotid and femoral arteries (cfPWV) was assessed using the SphygmoCor device (AtCor Medical LtD., Sydney, Australia) as previously described [15].
Outcomes
The eGFRs were re-evaluated using the same strategy after 3-year follow-up. CKD was defined as decreased eGFR < 60 mL/min/1.73 m2 or presence of proteinuria (urine protein ≥ 1+) assessed using a repeated dipstick method. 14 participants were excluded from analysis due to insufficient blood or urine samples, and 5917 participants were included in the final analysis.
Statistical analysis
Data were presented as proportions for categorical variables and mean ± SD or median [interquartile range (IQR)] for continuous variables. The significance of differences in continuous variables between groups was tested using one-way analysis of variance. The difference in the distribution of categorical variables was tested using Chi-square test. Univariate and multivariate logistic regression analysis was used to estimate the association of hyperhomocysteinemia with CKD. Independent variables included age, sex, hypertension, diabetes, smoking, habitual drinking, BMI, serum uric acid, cfPWV, homocysteine, total cholesterol, triglycerides, LDL cholesterol and HDL cholesterol. The distribution of observed serum homocysteine was right skew. The upper quartile of homocysteine was used as a categorical dependent variable for analyses, in comparison with the lower three quartiles. The 25, 50 and 75 percentile of homocysteine was 9.8, 12.2 and 15.0 μmol/L, respectively. The highest homocysteine quartile (≥15 μmol/L) was defined as hyperhomocysteinemia.
Crude and adjusted odds ratios (ORs) with 95% confidence interval (CI) were reported. All analyses were performed by SPSS statistical package, version 16.0 (SPSS, Inc., Chicago, IL). All P-values are two tailed. A P value of ≤0.05 is considered statistically significant.
Results
Among 5917 participants in the study, the mean age was 56.4 ± 9.6 years (range 40–88 years), and 74.5% of them were males. The mean eGFR was 95.7 ± 12.5 mL/min/1.73 m2. Baseline characteristics of the participants stratified according to the quartiles of homocysteine are shown in Table 1. Among all participants, the percentage of the serum homocysteine levels higher than 15 μmol/L was 25.0%. Participants with increased homocysteine were older and more likely to have higher serum uric acid, higher cfPWV and have higher percentage of hypertension (P < 0.001).
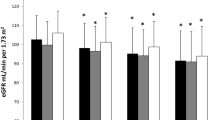
During 3 years of follow-up, the mean change of eGFR was −5.2 mL/min/1.73 m2. Altogether 143 (2.4%) patients developed CKD [85 (1.4%) patients with proteinuria and 59 (1.0%) patients with decreased eGFR], with median change of eGFR of −5.8 mL/min/1.73 m2 (IQR, −18.9–7.5 mL/min/1.73 m2). Comparisons of incidence of kidney damage according to quartiles of homocysteine are shown in Table 2. As a categorical outcome, the prevalence of decreased eGFR and the overall prevalence of CKD in the highest homocysteine quartile group were higher than in the lower three quartiles groups (P < 0.001), but the prevalence of proteinuria among these groups was not significantly different (P = 0.89).
We analyzed the ORs of variables associated with kidney damage. In the univariate logistic regression analysis, homocysteine (per 1 μmol/L increase) was independently associated with increased risk of decreased eGFR and CKD, with a crude OR of 1.08 (95% CI 1.06–1.11) and 1.06 (95% CI 1.04–1.08), respectively. Hyperhomocysteinemia was also independently associated with increased risk of decreased eGFR and CKD, with a crude OR of 4.79 (95% CI 2.83–8.11) and 2.22 (95% CI 1.58–3.11), respectively. After adjusted for potential confounders, including age, sex, hypertension, diabetes, BMI, uric acid, current smoking, habitual drinking, total cholesterol, triglycerides, LDL cholesterol and HDL cholesterol and cfPWV, both homocysteine (per 1 μmol/L increase) and hyperhomocysteinemia were independently associated with increased risk of decreased eGFR [with a fully adjusted OR of 1.07 (95% CI 1.04–1.10) and 3.05 (95% CI 1.71–5.46)] and CKD [with a fully adjusted OR of 1.04 (95% CI 1.02–1.07) and 1.62 (95% CI 1.11–2.35)], respectively. If homocysteine was analyzed in quartiles, a dose–response pattern was observed across quartiles, and the adjusted OR for the top quartile was 5.46 (95% CI 1.76–17.0) for decreased eGFR. By contrast, neither homocysteine (per 1 μmol/L increase) nor hyperhomocysteinemia were associated with proteinuria in the univariate or in the multivariable logistic regression analysis in Table 3.
Discussion
In the current cohort study, by analyzing the data from individuals who underwent general health screening after followed up for 3 years, it was found that hyperhomocysteinemia increases the risk of decreased eGFR. After adjusted for potential confounders, the association of hyperhomocysteinemia with decreased eGFR was still present.
Chronic kidney disease has become an important public health problem in China [4]. The rapid increase in diabetes and hypertension, both of which are predicted to drive epidemics of CKD. But this increased risk for CKD cannot be fully explained by traditional factors, including age, hypertension, diabetes and dyslipidemia. Homocysteine as a thiol-containing amino acid has gained great notoriety, since elevation of its plasma concentrations, a condition known as hyperhomocysteinemia, is correlated with many different diseases, in particular cardiovascular diseases [19, 20] and ESRD [21, 22]. The serum homocysteine levels higher than 15 μmol/L occurs about 25.0% at baseline in the study. Disturbance of homocysteine–methionine cycle by interacting with vitamin B12 and folic acid may cause accumulation of homocysteine, which may play a role in clinical consequences such as vascular calcification, atherothrombosis and cardiovascular disease [23].
To date, little is known regarding whether hyperhomocysteinemia can increase the risk of CKD in a Chinese middle-aged and elderly population. Hyperhomocysteinemia have been shown to be caused by a decrease in eGFR [24], but some studies showed that elevated serum homocysteine do not decrease markedly after dialysis or renal transplantation [25, 26]. These studies suggest that intrinsic derangement is related to the accumulation of homocysteine, not simply to the decline in eGFR. Conversely, our study subjects were participants in a large screening program who had no CKD at baseline. The result showed that hyperhomocysteinemia was independently associated with increased risk of decreased eGFR after adjusted for potential confounders, which revealed that hyperhomocysteinemia increases the risk of CKD. Specifically, it appears in Table 2 that ΔeGFR is lower in quartile 4 (−2.11 ± 10.8 mL/min/1.73 m2) group than in the overall group (−5.2 ± 10.4 mL/min/1.73 m2). This seems to suggest that hyperhomocysteinemia protects against declining GFR. Indeed, by contrast, hyperhomocysteinemia was positively associated with decreased eGFR in the multivariate logistic regression analysis. One possible explanations might be that the proportion of CKD patients was higher in the quartile 4 group than in other three groups, and the CKD-EPI two-level race equation systematic underestimation of measured GFR at higher values (GFR ≥ 90 mL/min/1.73 m2) and overestimation of measured GFR at lower values (GFR < 60 mL/min/1.73 m2) [16]. The underlying mechanisms deserve further investigation.
Recently, evidence is accumulating that hyperhomocysteinemia may directly act on glomerular cells to induce glomerular dysfunction and consequent glomerular sclerosis, leading to ESRD [27, 28]. The mechanisms by which hyperhomocysteinemia may exert adverse effects on the kidney are not well identified. Several important mechanisms mediating the pathogenic action of homocysteine in the glomeruli or in the kidney, such as local oxidative stress, endoplasmic reticulum stress, homocysteinylation and hypomethylation [29]. Homocysteine might lead to endothelial injury through generation of hydrogen peroxide and decrease in NO production [30] and might elicit inflammatory responses and impair endothelial function via activation of transcription factor such as nuclear factor-κB (NF-κB), including oxidative stress and chemokine expression causing monocyte accumulation in the vascular endothelium [31]. Endothelial dysfunction alters endothelial properties and exerts structure and function effects on the target vessel and may therefore enhance inward remodeling. In addition, endothelial dysfunction within the glomerular basement membrane may modify glomerular barrier permeability, thus leading to the decreased eGFR [32]. Han et al. [33] establish a previously unknown function of NOD2 for the regulation of TRPC6 channels, suggesting that TRPC6-dependent Ca2+ signaling is one of the critical signal transduction pathways that links innate immunity mediator NOD2 to podocyte injury in the hyperhomocysteinemia-associated end-stage renal disease. In all, we propose that hyperhomocysteinemia might be a predicator of CKD based on the findings of the studies.
To our knowledge, this is the largest study testing the association of hyperhomocysteinemia and CKD in Chinese middle-aged and elderly population. However, our study has limitations that deserve mention. First, we had only baseline measurement of homocysteine and were unable to longitudinally assess the relationship between changes in homocysteine and CKD. Second, single measurements of serum creatinine at baseline and serum creatinine after 3 years could have resulted in the misclassification of exposure, confounders and outcomes. Also, GFR was not directly measured using the gold standard method of inulin clearance but was instead estimated with a serum creatinine-based equation. This could have over- or underestimated the actual GFR in the population. However, the association observed in the current study could not be attributed to this source of variability because random misclassification of this type would be likely to cause underestimation of study findings and bias results toward the null hypothesis. Third, we could not collect detailed data on the medications that the participants received. Fourth, in our study, 143 subjects developed CKD, including 142 mild-to-moderate CKD patients and only one ESRD patient. We could not evaluate CKD exactly for the lack of formal measurement of urine albumin levels in the dipstick positive patients. These dipsticks usually afford high specificity with few false-positive results. However, they afford low sensitivity, i.e., they may fail to detect some forms of kidney disease during the early stages, when the level of proteinuria is below the sensitivity of the test strip used, which may influence the results of the study on the association between proteinuria and homocysteine levels. Also, the definition of CKD status requires a 3-month duration of low eGFR or kidney damage; this was presumed, not documented, in this study. Finally, since our study was observational, the possibility of residual confounding by some unmeasured covariate exists.
Currently, major strategies to prevent CKD are focused on conventional cardiovascular risk factors, and in this study, we showed that hyperhomocysteinemia increases the risk of decreased eGFR. This suggests that homocysteine could be considered as a useful molecular markers for delaying the development of CKD. A recent randomised clinical trial (RCT) showed that enalapril–folic acid therapy, compared with enalapril alone, can significantly delay the progression of CKD among patients with mild-to-moderate CKD [34], which showed the possibility of nutritional intervention such as folate supplimentation for the possible prevention of CKD. More future RCTs are warranted to evaluate the effect of treatment of hyperhomocysteinemia on the occurrence and progression of CKD.
References
Nugent RA, Fathima SF, Feigl AB, Chyung D (2011) The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract 118:c269–c277
Chen XN, Pan XX, Yu HJ, Shen PY, Zhang QY et al (2011) Analysis of cardiovascular disease in Chinese inpatients with chronic kidney disease. Intern Med 50:1797–1801
Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R et al (2015) US renal data system 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 66:S1–305
Zhang L, Wang F, Wang L, Wang W, Liu B et al (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379:815–822
Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM et al (2014) Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:1005–1070
De Leeuw PW, Thijs L, Birkenhager WH, Voyaki SM, Efstratopoulos AD et al (2002) Prognostic significance of renal function in elderly patients with isolated systolic hypertension: results from the Syst-Eur trial. J Am Soc Nephrol 13:2213–2222
Muntner P, He J, Hamm L, Loria C, Whelton PK (2002) Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13:745–753
Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF et al (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286:421–426
Kong X, Jia X, Wei Y, Cui M, Wang Z et al (2012) Association between microalbuminuria and subclinical atherosclerosis evaluated by carotid artery intima-media in elderly patients with normal renal function. BMC Nephrol 13:37
Chue CD, Townend JN, Steeds RP, Ferro CJ (2010) Arterial stiffness in chronic kidney disease: causes and consequences. Heart 96:817–823
Welch GN, Loscalzo J (1998) Homocysteine and atherothrombosis. N Engl J Med 338:1042–1050
Elias MF, Crichton GE, Abhayaratna WP (2015) Interactions between plasma homocysteine and arterial stiffness in chronic kidney disease in community-dwelling individuals: the Maine-Syracuse Study. J Hum Hypertens 29:726–731
Wald DS, Law M, Morris JK (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 325:1202
Cui R, Moriyama Y, Koike KA, Date C, Kikuchi S et al (2008) Serum total homocysteine concentrations and risk of mortality from stroke and coronary heart disease in Japanese: the JACC study. Atherosclerosis 198:412–418
Kong X, Ma X, Tang L, Wang Z, Li W, Cui M, Xu D (2016) Arterial stiffness evaluated by carotid-femoral pulse wave velocity increases the risk of chronic kidney disease in a Chinese population-based cohort. Nephrology (Carlton). doi:10.1111/nep.12750
Kong X, Ma Y, Chen J, Luo Q, Yu X et al (2013) Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant 28:641–651
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J et al (2005) Definition and classification of chronic kidney disease: a position statement from kidney disease: improving Global Outcomes (KDIGO). Kidney Int 67:2089–2100
Huang JF, Chuang WL, Dai CY, Ho CK, Hwang SJ et al (2006) Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: another chain of link? J Intern Med 260:255–262
Anderson JL, Muhlestein JB, Horne BD, Carlquist JF, Bair TL et al (2000) Plasma homocysteine predicts mortality independently of traditional risk factors and C-reactive protein in patients with angiographically defined coronary artery disease. Circulation 102:1227–1232
Cavalca V, Cighetti G, Bamonti F, Loaldi A, Bortone L et al (2001) Oxidative stress and homocysteine in coronary artery disease. Clin Chem 47:887–892
Perna AF, Ingrosso D, Satta E, Lombardi C, Acanfora F et al (2004) Homocysteine metabolism in renal failure. Curr Opin Clin Nutr Metab Care 7:53–57
Gupta A, Robinson K (1997) Hyperhomocysteinaemia and end stage renal disease. J Nephrol 10:77–84
Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L et al (2011) Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the folic acid for vascular outcome reduction in transplantation trial. Circulation 123:1763–1770
Norlund L, Grubb A, Fex G, Leksell H, Nilsson JE et al (1998) The increase of plasma homocysteine concentrations with age is partly due to the deterioration of renal function as determined by plasma cystatin C. Clin Chem Lab Med 36:175–178
Ducloux D, Motte G, Challier B, Gibey R, Chalopin JM (2000) Serum total homocysteine and cardiovascular disease occurrence in chronic, stable renal transplant recipients: a prospective study. J Am Soc Nephrol 11:134–137
Moustapha A, Gupta A, Robinson K, Arheart K, Jacobsen DW et al (1999) Prevalence and determinants of hyperhomocysteinemia in hemodialysis and peritoneal dialysis. Kidney Int 55:1470–1475
Kumagai H, Katoh S, Hirosawa K, Kimura M, Hishida A et al (2002) Renal tubulointerstitial injury in weanling rats with hyperhomocysteinemia. Kidney Int 62:1219–1228
Li N, Chen YF, Zou AP (2002) Implications of hyperhomocysteinemia in glomerular sclerosis in hypertension. Hypertension 39:443–448
Yi F, Li PL (2008) Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol 28:254–264
Upchurch GR Jr, Welch GN, Fabian AJ, Freedman JE, Johnson JL et al (1997) Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem 272:17012–17017
Zhang F, Siow YL, O K (2004) Hyperhomocysteinemia activates NF-kappaB and inducible nitric oxide synthase in the kidney. Kidney Int 65:1327–1338
Deen WM (2004) What determines glomerular capillary permeability? J Clin Investig 114:1412–1414
Han H, Wang Y, Li X, Wang PA, Wei X et al (2013) Novel role of NOD2 in mediating Ca2+ signaling: evidence from NOD2-regulated podocyte TRPC6 channels in hyperhomocysteinemia. Hypertension 62:506–511
Xu X, Qin X, Li Y, Sun D, Wang J, Investigators of the Renal Substudy of the China Stroke Primary Prevention Trial (CSPPT) et al (2016) Efficacy of folic acid therapy on the progression of chronic kidney disease: the Renal Substudy of the China Stroke Primary Prevention Trial. JAMA Intern Med 176:1443–1450
Acknowledgements
The research for this study was supported by the Key Science and Technology Research Projects from the natural science foundation of Shandong Province (2013GSF11818).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Xianglei Kong and Xiaojing Ma have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kong, X., Ma, X., Zhang, C. et al. Hyperhomocysteinemia increases the risk of chronic kidney disease in a Chinese middle-aged and elderly population-based cohort. Int Urol Nephrol 49, 661–667 (2017). https://doi.org/10.1007/s11255-016-1452-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1452-3