Abstract
Background
The coexistence of crescents and membranous glomerulonephritis (MGN) is a special characteristic in lupus nephritis. In the absence of the characteristic histological features of lupus nephritis, MGN with crescents should raise the possibility of two other histopathological entities: anti-GBM disease and necrotizing and crescentic glomerulonephritis. The last one includes patients with positive ANCA serology or not.
Results and conclusions
Here, we describe a case of a male patient who presented with extrarenal vasculitis symptoms, acute renal failure, hematuria and nephrotic-range proteinuria. ANCA serology was positive, and the biopsy revealed crescentic vasculitis plus membranous nephropathy. Reviewing the whole literature about similar histological cases, we included 38 cases with ANCA-positive serology and 30 ones with no ANCA in serum. It seems that in the first category vasculitis symptoms predominate, while in the second one these symptoms are absent. Their histological features have no major differences. In any case, the clinical course of these patients is serious, and in most cases, immunosuppression is essential in order to avoid end-stage renal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pauci-immune necrotizing and crescentic glomerulonephritis (NCGN) is characterized by glomerular necrosis and crescent formation with a paucity of glomerular immune complex deposits. The majority of patients have circulating antineutrophil cytoplasmic antibodies (ANCAs), while all of them present rapidly progressive glomerulonephritis and active urine sediment. On the contrary, membranous glomerulonephritis (MGN) is characterized by the formation of subepithelial and intramembranous immune complex deposits which results in effacement of the podocyte foot processes. In immunofluorescence, granular depositions of IgG are the predominant ones in idiopathic MGN. Ιn most cases, patients with MGN present with nephrotic syndrome and one-third of them usually progress to ESRD [1]. M-type phospholipase A2 receptor (PLA2R) is the target antigen in podocytes, and antibodies against this antigen are present in 75 % of patients with idiopathic MGN [2].
The coexistence of crescents and MGN is a special characteristic in ISN/RPS lupus nephritis class III and V or IV and V. In the absence of the characteristic histological features of lupus nephritis, MGN with crescents should raise the possibility of two other histopathological entities: anti-GBM disease and NCGN. The last one includes patients with positive ANCA serology or not. Here, we present a case of a patient with both the pathological entities of MGN and ANCA-associated NCGN. We also reviewed the literature including all cases with both pathological lesions (MGN and NCGN).
Case report
A 58-year-old white male, farmer and builder, was admitted to our clinic due to acute renal injury and abnormal urine findings. The patient visited his family doctor due to persisted myalgias and arthralgias, low-grade fever and nonproductive cough a month ago. His physician ordered a chest X-ray 2 weeks before his admission. It was normal, and the patient was prescribed cefuroxime and clarithromycin. Due to persistence of the symptoms and the appearance of dark-colored urine, the patient was transferred to our hospital.
The patient had a known history of coronary heart disease. On admission, the temperature was normal, blood pressure 140/80 mmHg, pulse rate 75/min and BMI 27.43 kg/m2. The clinical examination revealed no specific pathological findings such as rush, edema or abnormal pulmonary sounds. The laboratory evaluation showed creatinine 7.39 mg/dl, CRP 18 mg/L (normal values 0–5 mg/L), hematocrit 40.1 % and white blood count 9900/mm3, while the urine analysis showed +3 protein, +4 hemoglobin, 14–16 white blood cells, plenty of erythrocytes (30 % of them were dysmorphic) and some granular casts. The proteinuria was 8 g/24 h. One year ago, creatinine was 0.96 mg/dl and urine sediment was normal. Blood and urine cultures were negative. The titers for antinuclear antibodies, anti-GBM, anti-dsDNA and c- ANCA were negative, while titers of complement (C3 and C4), rheumatoid factor and immunoglobulins G and A were within normal limits. The titers of p-ANCA antibodies were 1/160. CT scan of the lung was inconclusive (paratracheal nodes up to 2.2 cm, an aortopulmonary lymph node of 1 cm and “honey comb” lung). The kidney ultrasound showed kidneys of normal size, parenchyma and echogenicity. A kidney biopsy was performed.
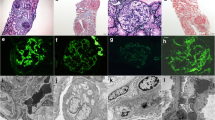
In light microscopy, 24 glomeruli were identified, 2 of them were globally sclerosed. Ten out of 24 (42 %) glomeruli showed destruction of some segments by cellular crescents of variable size. Adjacent mesangial segments were unremarkable. One glomerulus revealed fibrinoid necrosis. Glomerular capillary walls were slightly thickened as demonstrated by PAS and Masson histochemical stains. A reticular pattern was segmentally seen in glomerular capillaries, using periodic acid–silver methenamine stain (Figs. 1, 2). No vasculitic changes in vessel walls were seen. Interstitium demonstrated acute tubular injury with tubular dilatation and flattening of tubular epithelium. In addition, red blood cells casts were noted.
In indirect immunofluorescence examination (in a scale intensity 1–4+), granular deposits for IgG of 3+ were found along capillary walls in a subepithelial location. IgA showed 1+ in a similar pattern with IgG, while IgM was negative. C3 showed 2+ , and C1q was negative, while κ and λ light chains were evaluated 1+ and 2+, respectively. No linear stain for IgG was noticed. In electron microscopy examination, electron-dense deposits along capillary walls were seen in a membranous pattern (Fig. 3). The final histological diagnosis was crescentic glomerulonephritis in association with membranous glomerulopathy stage II.
The second day of his admission, treatment was initiated with 1 g of methylprednisolone intravenously daily, for 3 days (followed by 48 mg of methylprednisolone per os daily). Hemodialysis sessions started due to increasing creatinine values and metabolic acidosis. We also prescribed 1 g of cyclophosphamide IV, while the serious acute kidney injury led to the decision of prescribing 7 sessions of plasmapheresis according to Walsh et al. [3]. The patient was discharged 20 days after his admission, with creatinine level of 3 mg/dl. Five more monthly intravenous cyclophosphamide doses were programmed to follow. On the last re-evaluation, 3 months after the first visit, the patient referred no symptoms. Creatinine was 1.51 mg/dl, the 24-h urine protein was 1.725 g, and urine sediment had no erythrocytes or casts.
Discussion
Coexistence of MGN and crescents is an unusual pattern in kidney biopsies. The most common underlying histopathology includes lupus nephritis and antiglomerular basement membrane (GBM) disease. In lupus nephritis, crescents are usually accompanied with endocapillary proliferation and immune complex depositions in subendothelial space. On the other hand, in anti-GBM disease, there is a prominent linear staining of the glomerular basement membrane (anti-IgG and anti-C3) and positive titers of anti-GBM antibodies in serum [4]. Exceptionally rarely, pauci-immune crescentic glomerulonephritis coexists with MGN. In that case, the patient may have serum titers of ANCA antibodies and/or clinical manifestation of vasculitis. There are also rare reports of concomitant MGN and crescentic glomerulonephritis in which there is no serological or clinical evidence of vasculitis. Herein, we review the literature including all cases with concomitant MGN and crescentic glomerulonephritis. However, the cases are discussed and compared separately in two categories: positive ANCA serology and negative ANCA in serum.
In the first category (16 patients positive for MPO, 8 for cANCA, 11 for pANCA), there are 38 cases (39 included ours) reported in the literature [5–18], which comprise 17 men and 21 women with a mean age of 54.4 years (range 30–79) (Table 1). The largest series was published by Nasr [15], where these biopsies represented 1.2 % of all MGN cases of their hospital. On the whole, in 2 patients MGN preceded, while in other two cases proteinuria had been known for months prior the biopsy. In the remaining patients (90 %), MGN and ANCA-associated NCGN were diagnosed simultaneously. Twelve cases (31 %) showed extrarenal manifestations of vasculitis, while all cases presented with hematuria and proteinuria (0.56–14gr/day). At presentation, the majority of patients had renal insufficiency (range of serum creatinine levels 0.9–13.78 mg/dl). Induction therapy consisted of prednisone and cyclophosphamide (iv or per os) in 29 out of 36 patients (Table 1). Only 4 patients were treated with plasmapheresis. Ten out of 38 (26 %) patients ended in ESRD.
The second category (30 cases) included patients with MGN, crescentic glomerulonephritis and negative ANCA serology [17, 19–24] (Table 1, in italics). In this group, none patient had extrarenal manifestation of vasculitis. Twenty-one patients were men, and mean age was 50 years (range 5–86). Fifteen percentage of patients had an established diagnosis of MGN before the presentation of the crescentic GN. All patients had proteinuria (0.4–22 g/day), and most presented with decline of GFR (range of serum creatinine 0.73–19.8 mg/dl). Sixteen patients were treated with cortisone and cyclophosphamide, while plasmapheresis was not performed. Six in 30 (20 %) progressed to ESRD. The largest group was presented by Rodriguez et al. [22] (19 cases), where those cases represented 0.4 % of all the MGN cases in their hospital. Generally, regarding the clinical course of these patients, there are no major differences with the first category, apart from the presence of extrarenal manifestation of vasculitis in some patients of the first group.
Generally, the review of the histological findings of all cases is inconclusive due to insufficient published data and heterogeneity of the histopathological reports. However, crescents and MGN features are common in all cases. The mean percentage of glomeruli with crescents were 30–69 % in the first group and 2–100 % in the second. Evident MGN histology was obvious on light microscopy in all cases of the second group, while only 15 out of 38 cases had apparent MGN histology in the first group. Nevertheless, on electron microscopy, MGN-stage percentages present small differences (e.g., stages II–III or III are present in 14.8 % of the second group and 22.8 % of the first). Fibrinoid necrosis and/or endocapillary fibrin formation is most common in the first group (17/18 cases) compared with the second one (2/20). Immunofluorescence showed fine granular staining for IgG and C3 in most cases in both groups. The most common subclasses for IgG—when available—were 1 and 4. Our case has all the main characteristics of the first group; extrarenal manifestation of vasculitis, acute renal failure, hematuria and proteinuria, as well as characteristic crescentic glomerulonephritis and MGN with fibrinoid necrosis in the kidney biopsy.
According to Nasr et al. [15], cases with MGN and crescent formation just represent “an unusual morphological expression of MGN.” On the opposite, one could argue that these cases may represent a variant of MGN with superimposed pauci-immune negative ANCA glomerulonephritis. It is well established in the literature that 10–30 % of patients with NCGN lack ANCA in serum [25]. Nevertheless, the absence of fibrinoid necrosis, endo- or extracapillary fibrin (only 2 patients in 30) in the second group and lack of extrarenal vasculitis symptoms support the view that these cases represent a special MGN variant, more severe than typical MGN, perhaps due to serious glomerular damage related to immune complex deposition.
Is the coexistence of MGN and ANCA-positive NCGN coincidental? Anti-MPO-IgG is proved to cause crescentic glomerulonephritis in rat models [26], while these depositions along the GBM are evident only at the early stage and disappear later [27]. This may explain the “pauci-immune” characteristic of NCGN, although rare immune complex deposits (mainly IgM and C3) may be seen in glomeruli (50 % of biopsies on electron microscopy) [28]. These depositions may potentiate the effect of ANCA in the development of NCGN and seem to produce a more severe form of glomerulonephritis. Matsumoto et al. [14] demonstrated MPO-IgG depositions in a case of crescentic and MGN glomerulonephritis. Moreover, Hanamura et al. [29] proved partial colocalization of myeloperoxidase and IgG within the GBM and mesangium in 35 % of biopsies of NCGN and membranous cases. Do these depositions trigger a pattern of membranous nephropathy superimposed on an ANCA-positive NCGN? Probably, the answer is yes, but there are no explanations why this occurs only in a small number of NCGN cases.
Conclusions
Clinicians and pathologists should be aware of the rare case of coexistence of NCGN and membranous glomerulonephritis. It seems that there are two subcategories in this entity, ANCA serology positive or negative. In the first group, clinical and pathological course is basically a vasculitis-like NCGN, while in the second one, MGN characteristics seem to predominate.
References
Beck LH Jr, Salant DJ (2014) Membranous nephropathy: from models to man. J Clin Invest 124(6):2307–2314
Beck LH Jr, Salant DJ (2010) Membranous nephropathy: recent travels and new roads ahead. Kidney Int 77(9):765–770
Walsh M et al (2011) Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis 57(4):566–574
Basford AW et al (2011) Membranous nephropathy with crescents. J Am Soc Nephrol 22(10):1804–1808
Surindran S et al (2012) Coexistence of ANCA-associated glomerulonephritis and anti-phospholipase A(2) receptor antibody-positive membranous nephropathy. Clin Kidney J 5(2):162–165
Watanabe S et al (2011) Case of MPO-ANCA-associated vasculitis with membranous nephropathy. Nihon Jinzo Gakkai Shi 53(1):46–52
Kanahara K et al (1997) Myeloperoxidase-antineutrophil cytoplasmic antibody-associated glomerulonephritis with membranous nephropathy in remission. Intern Med 36(11):841–846
Barrett CM et al (2014) Membranous glomerulonephritis with crescents. Int Urol Nephrol 46(5):963–971
Shimada M et al (2013) A case of myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA)-associated glomerulonephritis and concurrent membranous nephropathy. BMC Nephrol 14:73
Hu ZJ et al (2014) A case of membranous nephropathy and myeloperoxidase anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Exp Ther Med 8(4):1170–1172
Dwyer KM et al (2001) Membranous nephropathy and anti-neutrophil cytoplasmic antibody-associated glomerulonephritis: a report of 2 cases. Clin Nephrol 56(5):394–397
Gaber LW, Wall BM, Cooke CR (1993) Coexistence of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis and membranous glomerulopathy. Am J Clin Pathol 99(2):211–215
Taniguchi Y et al (1999) Myeloperoxidase antineutrophil cytoplasmic antibody-positive necrotizing crescentic glomerulonephritis and membranous glomerulonephropathy. Clin Nephrol 52(4):253–255
Matsumoto K (2009) MPO-ANCA crescentic glomerulonephritis complicated by membranous nephropathy: MPO demonstrated in epimembranous deposits. NDT Plus 2:461–465
Nasr SH et al (2009) Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol 4(2):299–308
Thajudeen B et al (2014) Membranous nephropathy with crescents in a patient with Hashimoto’s thyroiditis: a case report. Medicine (Baltimore) 93(8):e63
Tse WY et al (1997) Association of vasculitic glomerulonephritis with membranous nephropathy: a report of 10 cases. Nephrol Dial Transplant 12(5):1017–1027
Suwabe T et al (2005) Membranous glomerulopathy induced by myeloperoxidase-anti-neutrophil cytoplasmic antibody-related crescentic glomerulonephritis. Intern Med 44(8):853–858
Kwan JT et al (1991) Crescentic transformation in primary membranous glomerulonephritis. Postgrad Med J 67(788):574–576
Unver S et al (2008) A rare complication of idiopathic membranous nephropathy: crescentic transformation. Ren Fail 30(5):573–575
Gadonski G et al (2010) Rapidly deteriorating kidney function in a young man previously diagnosed with membranous nephropathy. Nephron Clin Pract 115(2):c100–c106
Rodriguez EF et al (2014) Membranous nephropathy with crescents: a series of 19 cases. Am J Kidney Dis 64(1):66–73
James SH et al (1995) Acute renal failure in membranous glomerulonephropathy: a result of superimposed crescentic glomerulonephritis. J Am Soc Nephrol 6(6):1541–1546
Hall AM et al (2006) Crescentic transformation of membranous glomerulopathy: a reversible condition. Nephrol Dial Transplant 21(4):1136–1137
Chen M, Kallenberg CG, Zhao MH (2009) ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol 5(6):313–318
Xiao H et al (2002) Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110(7):955–963
Kobayashi K, Shibata T, Sugisaki T (1995) Aggravation of rat nephrotoxic serum nephritis by anti-myeloperoxidase antibodies. Kidney Int 47(2):454–463
Haas M, Eustace JA (2004) Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int 65(6):2145–2152
Hanamura K et al (2011) Detection of myeloperoxidase in membranous nephropathy-like deposits in patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Hum Pathol 42(5):649–658
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balafa, O., Kalaitzidis, R., Liapis, G. et al. Crescentic glomerulonephritis and membranous nephropathy: a rare coexistence. Int Urol Nephrol 47, 1373–1377 (2015). https://doi.org/10.1007/s11255-015-1031-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1031-z