Abstract
Previous studies have shown that life-long caloric restriction in rats protects the kidneys from age-dependent injury. In this study, we analyzed whether late-life-introduced caloric restriction has a similar effect. The study lasted 12 months. Three groups of animals were analyzed: rats fed “ad libitum” (AD, n = 9), rats on 60% caloric restriction (CR, n = 9), and rats fed “ad libitum” for the first six months of their life then switched to 60% caloric restriction thereafter (LCR, n = 9). At the end of the study kidney function was assessed and kidney samples were analyzed histologically. Serum creatinine and urine albumin were higher in AD than in both CR and LCR (P < 0.001). Creatinine clearance (Clcr) corrected for body weight was lowest in AD and comparable in CR and LCR. Similarly Clcr corrected for kidney weight was lower in AD than in both CR and LCR (P < 0.05). Severe albuminuria was observed only in AD. In CR and LCR the amount of albumin excreted was comparable (AD vs. CR, P < 0.0001; AD vs. LCR, P < 0.001). In morphometric analysis, the mean size of the glomeruli was higher in AD than in both CR and LCR (P < 0.01). Similar results were found for the mesangial area (AD vs. CR, P < 0.001; AD vs. LCR, P < 0.01) and for mesangial cell counts (AD vs. CR, P < 0.001; AD vs. LCR, P < 0.05). No difference was found between CR and LCR in morphometry. In conclusion, our study indicates that late-life introduction of caloric restriction reverses most of the structural and functional changes observed in the kidneys of “ad libitum”-fed rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with degenerative changes in many organs including the kidney [1]. As the kidney has a pivotal role in the maintaining body health and homeostasis, age-related nephropathy has been studied in detail on the basis of many different models, including rat models. Despite the inconsistency of the results obtained from different rat strains, there is a general belief that aging affects both morphology and function of the kidney and that chronic progressive nephropathy in a common spontaneous disease in senescent rats (reviewed in [2]).
It had already been shown by Owen et al. [3] in the late 1980s that in male Sprague–Dawley rats the incidence of chronic progressive kidney damage increased from 12% to 81% between the first and second years of age. Haley and Bugler [4] had shown that in twelve-month-old male animals of this strain GFR expressed per 100 g body weight was lower than for younger animals. According to Baylis [5], in male Wistar rats both GFR and RPF were significantly lower in nineteen-month-old than in eight-month-old animals. Remuzzi et al. [6] found massive proteinuria of glomerular origin in twenty-one-month-old male Wistar rats. In comparison with seven-month old animals protein excretion was a factor of six lower. Wiggins et al. [7] had shown that in male Fischer 344 rats age-dependent proteinuria was accompanied by an enlarged glomerular volume with increased glomerular total cell count and also percentage of sclerotic glomeruli.
Diet has been, and is, a major tool in experimental biology. McCay discovered that caloric restriction retards the aging process, increases longevity, and reduces age-dependent pathology without causing malnutrition in the rat (reviewed in [8]). It was shown that in “ad libitum”-fed rats kidney function declined faster than in caloric-restricted animals [9–11]. Studies conducted by Wiggins et al. on male Fischer rats had shown that 60% caloric restriction for two years had a beneficial effect not only on glomerular proteinuria but also on glomerular hypertrophy and glomerulosclerosis [7].
Caloric intervention introduced in rats up to six months from birth has been shown to have a similar effect on longevity to whole-life-long caloric restriction [12]. Data on the effect of late-life introduction of caloric restriction on kidney structure and function are very scarce.
McKiernan et al. [13] studied eighteen-month old male Fischer–Brown Norway rats (up to this point all animals were fed “ad libitum”). For the next six months the control group was fed “ad libitum” and the other was put on 40% caloric restriction diet. They found that dietary intervention reduced glomerular sclerosis and tubular atrophy. However, in that study no animals were kept on the diet for their whole life. Hence, the question whether late-life introduction of caloric restriction is as effective as a life-long low caloric diet is still open. This seems to be very important, as late-life introduction of the diet is more realistic for humans.
Thus, the aim of this study was to compare the structure and function of the kidney in rats fed “ad libitum” for the first 12 months of their life with those which started caloric restriction after six months of age.
Material and methods
Animals and diet strategy
The experiment was conducted on 30 male Wistar rats. During the study the rats were housed at a constant room temperature (22–25°C) with 50–65% humidity and were exposed to a 12-h lights-on/12-h lights-off cycle with free access to water.
When the animals were seven-weeks old they were randomly divided into three groups:
-
group AD (n = 10)—animals fed “ad libitum”;
-
group CR (n = 10)—diet-restricted rats: total caloric intake equaled 60% of that of the AD-fed controls; and
-
group LCR (n = 10)—rats were fed “ad libitum” for the first six months of their life and switched to 60% caloric restriction thereafter.
According to the protocol, after twelve months, animals from every group finished the experiment. However, one rat from the AD group died during the study period. Therefore, only nine animals ended the study in this group. In the other two groups ten animals completed the study, but because of technical problems with blood sampling (clot formation) in one rat from the CD group and one from the LCR group, eventually only nine rats in each group were included in the statistical analysis.
Study design
At twelve months, before the end of the study, rats were placed in metabolic cages for the last 24 h, for urine collection. Thereafter, rats were sacrificed by bleeding under ether anesthesia and blood samples were collected. Hemoglobin concentration (Hb) and hematocrit (Ht) were measured immediately in blood samples (hematologic blood analyzer Sysmec K-1000). It was assumed that in malnourished animals, because of low iron level, Hb may be low. To exclude the effect of dehydration Ht also was measured.
Next, both serum samples (after separation and rinsing) and urine samples were frozen (–80°C) and kept for further analyses.
Immediately after the animals’ death, the kidneys were removed, weighed, and samples from them were fixed in 4% buffered formaldehyde (pH 7.3) and embedded in paraffin. For further analyses, 5-μm thick slides were used.
Biochemical and morphological methods
In each serum sample, albumin (SALB—colorimetric method/Hitachi 904/Roche) and creatinine (Scr—Jaffe method/Hitachi 904/Roche) concentration were measured. The same compounds were analyzed in urine samples (Ucr, UALB—colorimetric method/Hitachi 904/Roche). Additionally, creatinine clearance (Clcr) was calculated and corrected for body weight and for kidney weight.
For morphometric analyses, slides stained with periodic acid–Schiff (PAS staining) were assessed by light microscopy (200× magnification; Nikon, Eclipse E 400) supported with digital image-analyzing equipment (Screen Measurement, 4.21; Laboratory Imaging, Prague, Czech Republic). In each kidney, an area of 25 consecutive glomeruli was measured and then the mean size of glomeruli was calculated.
Similarly, in ten consecutive glomeruli, the area covered by the mesangium was measured and the number of mesangial cells was assessed for calculation of the mean mesangial area and the mean number of mesangial cells per glomerulus.
Statistical analyses
Data are expressed as mean ± SD. The statistical analysis between the studied groups was performed using ANOVA with the post-hoc test by Kruskall–Wallis. The frequency of positive results within the groups was compared by the Fisher test. A P-value <0.05 was defined as statistically significant.
Results
After twelve months, the mean body weight of AD rats was statistically significantly higher than that of CR animals (562 ± 52 g vs. 333 ± 10 g; P < 0.0001). In the LCR group, the mean body weight was lower than that of AD rats (385 ± 9 g, P < 0.05 vs. AD) but did not differ significantly from that of CR animals.
There was no difference in Hb levels and Ht values between the groups examined (Table 1). SALB was lower in AD rats (32.2 ± 3.8 g/l) than in both diet-restricted groups of animals, however (CR: 48.5 ± 5.5 g/l, P < 0.0001; LCR: 45.7 ± 3.5 g/l, P < 0.001). Mean SALB were similar in the CR and LRC groups.
Mean kidney weight was higher in AD than in CR rats (3.40 ± 0.32 g vs. 2.17 ± 0.10 g; P < 0.001). In this respect, the difference between the LCR (2.74 ± 0.30 g) and CR rats also reached statistical significance (P < 0.05). Interestingly, when kidney weight was factored for body weight, only the ratio between the LCR and AD animals differed significantly (respectively: 0.0072 ± 0.0007 and 0.0060 ± 0.0003; P < 0.001).
Scr was higher in AD animals than in both CR and LCR (P < 0.001—Table 1; Fig. 1a). Clcr corrected for body weight was the lowest in AD rats (P < 0.05 vs. both CR and LCR) and comparable in the CR and LCR groups (Table 1; Fig. 1b). Similarly Clcr corrected for kidney weight was statistically significantly lower in AD rats (0.54 ± 0.13 ml/min/1 g) than in both diet-restricted groups (P < 0.05; CR: 0.71 ± 0.13 ml/min/1 g; LCR: 0.72 ± 0.13 ml/min/1 g) (Fig. 1c).
Detailed results characterizing kidney function in the studied rats (AD, animals fed “ad libitum”; CR, restricted rats—total caloric intake equaled 60% of that of the AD-fed controls; LCR, rats fed “ad libitum” for the first six months of their life and switched to 60% caloric restriction thereafter)
Urine volume was higher in AD rats than in CR rats (15.60 ± 6.02 ml vs. 8.16 ± 2.31 ml, P < 0.01). Urine volume for LCR animals was comparable with that for AD rats and higher than for CR rats (14.78 ± 4.02 ml, P < 0.01). In CR and LCR animals albumin excretion was comparable (CR: 5 ± 2 mg/24 h; LCR: 9 ± 14 mg/24 h). More severe albuminuria was observed in animals from the AD group (AD: 190 ± 161 mg/24 h; P < 0.0001 vs. CR and P < 0.001 vs. LCR) (Fig. 1d).
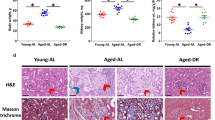
In morphometric analysis, the mean size of the glomeruli, mesangial area, and mesangial cell counts were statistically significantly higher in the AD rats than in both CR and LCR groups (detailed data are presented in Table 2 and Fig. 2). No difference was found between the CR and LCR animals in this respect. Representative glomeruli from each of the three groups are presented in Fig. 3.
Detailed results from morphometric analysis of kidney samples from the studied rats (AD, animals fed “ad libitum”; CR, restricted rats—total caloric intake equaled 60% of that of the AD-fed controls; LCR, rats fed “ad libitum” for the first six months of their life and switched to 60% caloric restriction thereafter)
Discussion
Regression of kidney damage may retard progression of chronic kidney disease (CKD) [14]. In humans with CKD, increase in GFR and reduction in proteinuria serves as indirect evidence of regression. However, it is difficult to document morphological changes in the human kidney, because kidney biopsy cannot be repeated for the same subject several times [15]. Thus, to study mechanisms of kidney damage an animal model is necessary. As age-related changes in the kidney are relatively severe in male Wistar rats, we decided to conduct our experiment on this strain of rats.
Our study showed the beneficial effect of caloric restriction on functional and morphological aspects of kidney damage. These results are in agreement with those demonstrating that life-long 40% caloric restriction slows kidney damage [9–11]. We also used 40% caloric restriction for the studied rats. Because Hb levels and Ht values were comparable for all groups of animals studied, it is unlikely the results were affected by the undernourishment. Furthermore, serum albumin levels were the lowest in the AD group. This not only excludes dehydration of the studied animals but also argues against malnutrition. In the AD group, severe albuminuria was observed. In aging rats, proteinuria is the most striking characteristic change in renal function [2].
McKiernan et al. [13], who introduced 40% caloric restriction in eighteen-month-old Fischer–Brown Norway rats, showed that already after six months of diet there were fewer morphological changes in the kidneys compared with “ad libitum”-fed animals. Our observations support these findings, because we also found that after six months of diet all analyzed aspects of kidney morphology were much better. Moreover, in our study six months of diet introduced in rats that were six months old reversed most of the structural alterations observed in the “ad libitum”-fed rats. The study of McKiernan et al. [13] did not encompass rats on long-life restricted diet. This would be necessary to answer the question concerning the reversibility of renal injury.
In female obese Zucker rats, Maddox et al. [16] observed that food restriction introduced at six or twelve weeks of age prevented almost completely the development of proteinuria and glomerulosclerosis. When the introduction of caloric restriction was delayed until twenty-six weeks of age, it was less effective. After thirty-two weeks of diet the severity of proteinuria and glomerular injury was lower than in “ad libitum”-fed animals but still higher than in lean female Zucker rats. The obese Zucker rat is an animal model of genetically inherited obesity and lean rats are regarded as controls. In the study of Maddox et al. [16] obese rats switched to a diet were given the amount of food defined as normal intake for lean female Zucker rats. It must be pointed that our experiment started when the animals were seven weeks old and the LCR was introduced six months later. Thus, it was later than in the study of Maddox et al. Different effects may be related to different animal strain, sex, and diet regimen.
In LCR animals, caloric restriction was introduced in adulthood. After six months, the mean body weight in this group was as low as in CR rats with comparable HBG and SALB. Interestingly, kidney weight was still higher in the LCR rats than in CR rats after six months of the diet, and when expressed per 100 g body weight it was even higher in LCR than in AD animals. Possibly, because of kidney hypertrophy, LCR rats had higher urine volume than CR rats. In this context, a combination of the lowest values of serum creatinine with the highest values of creatinine clearance in LCR rats can further indicate early stages of renal damage. Thus, it seems likely that six months of diet is too short a time to repair kidney injury completely. In conclusion, our study indicates that late-life introduction of caloric restriction reverses most of the structural and functional changes observed in the “ad libitum”-fed rats. Nevertheless, it is to be expected that with earlier introduction of the diet even better results may be achieved.
References
Zhou XJ, Saxena R, Liu Z, Vaziri ND, Silva FG (2008) Renal senescence in 2008: progress and challenges. Int Urol Nephrol 40:823–839. doi:10.1007/s11255-008-9405-0
Goldstein RS, Tarloff JB, Hook JB (1988) Age-related nephropathy in laboratory rats. FASEB J 2:2241–2251
Owen RA, Heywood R (1986) Age-related variations in renal structure and unction in Sprague-Dawley rats. Toxicol Pathol 14:158–167
Haley DP, Bulger RE (1983) The aging male rat: structure and function of the kidney. Am J Anat 167:1–13. doi:10.1002/aja.1001670102
Baylis C (1994) Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest 94:1823–1829. doi:10.1172/JCI117531
Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G (1988) Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int 34:481–486. doi:10.1038/ki.1988.206
Wiggins JE, Goyal M, Sanden SK et al (2005) Podocyte hypertrophy, “adaptation”, and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol 16:2953–2966. doi:10.1681/ASN.2005050488
Masoro EJ (2005) Overview of caloric restriction and ageing. Mech Ageing Dev 126:913–922. doi:10.1016/j.mad.2005.03.012
Everitt AV, Porter BD, Wyndham JR (1982) Effects of caloric intake and dietary composition on the development of proteinuria, age-associated renal disease and longevity in the male rat. Gerontology 28:168–172
Fujita Y, Ichikawa M, Kurimoto F, Rikimaru T (1984) Effects of feed restriction and switching the diet on proteinuria in male Wistar rats. J Gerontol 39:531–537
Macconi D, Laurens W, Paris S et al (1997) Selective dietary restriction of protein and calorie intakes prevents spontaneous proteinuria in male MWF rats. Exp Nephrol 5:404–413
Merry BJ (2005) Dietary restriction in rodent—delayed or retard ageing? Mech Ageing Dev 126:951–959. doi:10.1016/j.mad.2005.03.015
McKiernan SH (2007) Adult-onset calorie restriction delays the accumulation of mitochondrial enzyme abnormalities in aging rat kidney tubular epithelial cells. Am J Physiol Renal Physiol 292:F1751–F1760. doi:10.1152/ajprenal.00307.2006
Abbate M, Remuzzi G (2003) Can we really lesion kidney damage to the point that the loss of renal function of progressive nephropathy may revert? J Am Soc Nephrol 14:1411–1414. doi:10.1097/01.ASN.0000067478.61759.42
Kohli HS, Jairam A, Bhat A et al (2006) Safety of kidney biopsy in elderly. Int Urol Nephrol 38:815–820. doi:10.1007/s11255-006-0088-0
Maddox DA, Alavi FK, Santella RN, Zawada ET Jr (2002) Prevention of obesity-linked renal disease: age-dependent effects of dietary food restriction. Kidney Int 62:208–219. doi:10.1046/j.1523-1755.2002.00412.x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Podkowka-Sieczka, R., Wieczorowska-Tobis, K., Niemir, Z.I. et al. The effect on renal structure and function of late-life-introduced caloric restriction (CR) in rats. Int Urol Nephrol 41, 211–217 (2009). https://doi.org/10.1007/s11255-008-9499-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-008-9499-4