Abstract
As the kidneys age, gradual changes in the structures and functions of mitochondria occur. Dietary restriction (DR) can play a protective role in ageing-associated renal decline, however the exact mechanisms involved are still unclear. This study aims to clarify the beneficial effects of long-term DR on renal ageing and to explore the potential mechanisms of mitochondrial homeostasis. Eight-week-old C57BL/6 male mice (n = 30) were randomly divided into three groups, Young-AL (AL, ad libitum), Aged-AL, and Aged-DR (60% intake of AL). Mice were sacrificed at age of 7 months (Young) or 22 months (Aged). Heavier body and kidney weights were associated with ageing, but DR reduced these increases in aged mice. Ageing caused extensive tubulointerstitial fibrosis and glomerulosclerosis in the kidney. Giant mitochondria with looser and irregular crista were observed in Aged-AL kidneys. DR retarded these morphological alterations in aged kidneys. In addition, DR reversed the increase of MDA caused by ageing. Renal ATP level was elevated by DR treatment. Mitochondrial-related proteins were analysed to elucidate this association. Ageing downregulated the renal levels of VDAC, FOXO1, SOD2, LC3I and II, and upregulated the renal levels of MFN2 and PINK1. In contrast, DR elevated the levels of VDAC, FOXO1, and LC3I and reduced the ratio of LC3II to LC3I in aged kidneys. To conclude, impaired mitochondria, increased oxidative stress, and severe fibrosis were noticed in the aged kidneys, and DR improved these changes by increasing functional mitochondria and promoting autophagic clearance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing is a natural phenomenon of functional and structural decline that happens in organs. Ageing kidneys display some functional alterations, such as increased renal vascular resistance, reduced renal plasma flow and increased filtration fraction (Rosner et al. 2018). Glomerular lesions, thickening of basement membrane and tubular dilatation are observed in the kidney of aged animals and humans (Rosner et al. 2018; Singh and Krishan 2019). These ageing-related changes retard the normal response of the kidneys to stressors, leading to severe damage and an impaired ability to repair the damage, sequentially causing renal failure (Rosner et al. 2018).
The potential molecular mechanisms involved in ageing have been studied extensively, and mitochondrial dysfunction plays a critical role and has thus attracted researchers’ attention (Duann and Lin 2017). The major functions of the mitochondria include energy supply, calcium homeostasis and programmed cell death. Continued mitochondrial health is thus necessary to maintain normal functions and cell survival. There are several processes in charge of maintaining mitochondrial homeostasis (Duann and Lin 2017). Mitochondrial biogenesis and bioenergetics are required for energy supply, while a powerful antioxidant system in mitochondria is essential to decrease oxidative stress during energy production. Mitochondria undergo fusion or fission to strengthen these functions or remove malfunctioning components. For malfunctioning or damaged mitochondria, mitophagy and autophagy are responsible for clearance. Qualified coordination and integration of these actions make mitochondrial function efficiently and thereby ensure cell survival.
Dietary restriction (DR) refers to the reduction of nutrient intake without malnutrition, and exists a beneficial role on lifespan (Rattan 2008). DR is also a promising hormetin for renal protection against various kidney diseases (Singh and Krishan 2019; Xu et al. 2015). A meta-analysis on the effect of DR on chronic kidney diseases (CKD) in the rodent model showed that DR exhibits a protective role against CKD, such as restoring kidney functions, decreasing the incidence of CKD and increasing the survival rate (Xu et al. 2015). As a promising approach for renal protection, various DR regimens have been used to reduce ageing-associated renal decline (Andrianova et al. 2021). However, the efficacy of DR depends on several factors, such as degree, timing of initiation and duration of DR (Xu et al. 2015). The possible mechanisms involved in DR protection are still not clear. Moreover, sex and strains of rodent model has been demonstrated as critical regulators for the contribution of DR (Mitchell et al. 2016). This study aims to clarify whether long-term DR delays renal ageing of male mice, and to explore potential mechanisms of mitochondrial homeostasis.
Materials and methods
Animals and experimental diets
The Institutional Animal Care and Use Committee of the National Taiwan University College of Medicine and College of Public Health approved all animal care procedures used in this study (IACUC no: 20170321). C57BL/6 male mice were obtained from the National Taiwan University College of Medicine Laboratory Animal Centre. Eight-week-old C57BL/6 mice (n = 30) were randomly divided into three groups: Young-AL, Aged-AL, and Aged-DR. The AL groups were fed the breeding diet (LabDiet 5058) ad libitum (3.454 g/day), and DR mice were fed a restricted dietary treatment (60% intake of ad libitum, 2.072 g/day) as described by our previous study (Li et al. 2022). Water was available ad libitum, and body weight was monitored monthly. Mice were maintained in an animal room with a controlled temperature of 22–24 °C and humidity at 50–55% under a 12-h light/dark cycle. Mice were sacrificed with CO2 at the age of 7 months (Young) or 22 months (Aged). Left kidneys were excised, frozen in liquid nitrogen and stored at –80 °C until analysis.
Histological analysis
Segments of the right kidney were fixed in 100 g/ kg neutral formalin solution for a week, embedded in paraffin and sectioned at 5 μm. The sections were then stained with haematoxylin and eosin (H&E). Masson's trichrome stain was used to determine the degree of renal fibrosis.
Transmission electron microscopy (TEM)
The sample preparation for ultrastructure observation by TEM was done as described by our previous study (Li et al. 2022). The cortex of right kidney was flushed with saline solution and dissected into 1 mm3 cubes before fixation. Kidney tissue pieces were preserved in a fixative solution (4% paraformaldehyde and 2.5% glutaraldehyde in 1× PBS; pH 7.4) at 4 °C. Ultrathin sections (70–90 nm) were mounted on nickel grids and stained using saturated aqueous uranyl acetate. The ultra-structures of kidney samples were detected by using a FEI Tecnai G2 F20 S-Twin transmission electron microscope (FEI, Hillsboro, OR, USA) at the Academia Sinica Biological Electron Microscopy Core Facility.
ATP production
The amount of renal ATP was estimated using ATP Colorimetric Assay (Biovision, Waltham, MA, USA) according to manufacturer instructions. Initially, renal tissue (10 mg) was lysed in ATP assay buffer and centrifuged at 15,000×g for 2 min to collect supernatant afterwards. Deproteinized tissue homogenate was centrifuged at 14,000×g for 15 min at 4 °C to collect the filtrate using 10 kDa spin columns (ab93349). The filtrate was collected, the final volume was brought to 50 μL with the ATP assay buffer, and then 50 μL of the ATP reaction mix was added to a 96-well plate. After 30 min of incubation at 37 °C in darkness, absorbance of the sample was measured at 570 nm in a spectrophotometer (CLARIOstar, BMG Labtech, Ortenberg, Germany). The absorbance of the sample was incorporated into the standard curve to calculate ATP concentration.
Malondialdehyde (MDA) analysis
Because MDA is the most often examined marker for lipid peroxidation and the most significant measure of oxidative stress in CKD patients (Vodosek Hojs et al. 2020), MDA was analysed by measurement of thiobarbituric acid reactive substances (TBARS) to present the degree of lipid peroxidation by oxidative stress. TBARS of the left kidney were extracted using radioimmunoprecipitation (RIPA) lysis buffer (20–188, Millipore, Burlington, MA, USA) and centrifuged at 14,000×g for 10 min at 4 °C. The supernatant fraction was mixed with a thiobarbituric acid solution and then mixed with trichloroacetic acid–HCl reagent. After boiling for 30 min, the solution was cooled to room temperature and then centrifuged (5,000×g, 4 °C for 3 min), the absorbance of the supernatant fraction was measured at 535 nm.
Western blotting
The sample of the left kidney was homogenized in RIPA assay buffer and centrifuged at 14,000×g for 10 min at 4 °C. Twenty μg proteins from the tissue lysate were applied to a sodium dodecyl sulfate/polyacrylamide gel (10 or 15% acrylamide) for electrophoresis. Following electrophoresis, proteins were transferred to a PVDF membrane. After that, the membrane was used for hybridization with target protein antibodies. The membrane was incubated for 1 h at room temperature with primary antibodies against 5’ AMP-activated protein kinase (AMPK) (#2532) (Cell Signaling Technology, Danvers, MA, USA), phospho-AMPK (pAMPK) Thr172 (#2535), Forkhead box protein O1 (FOXO1) (#2880), mitofusin-2 (MFN2) (#9482), mitochondrial transcription factor A (TFAM) (gwb-22c6c2) (GenWay Biotech, San Diego, CA, USA), Complex II subunit 30 kDa (SDHB) (ab14714)(Abcam, Cambridge, UK), Complex IV subunit II (MTCOI)(ab110258), Optic Atrophy 1 (OPA1) (ab423634), mitochondrial fission 1 protein (FIS1) (sc-98900) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), superoxidase dismutase 2 (SOD2)(sc-137254), PTEN-induced putative kinase 1 (PINK1) (sc-33796), Parkin (sc-30130), Light Chain 3 (LC3B) (#2775), and voltage-dependent anion channel (VDAC) (#4866) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (#2118). The abundance of proteins was determined by the enhanced chemiluminescence (ECL) Western blotting detection system kit (Amersham Pharmacia Biotech, Amersham, Buckinghamshire, UK). The immunoblotting was visualized and quantified using the ChemiDoc Touch Image System (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data were expressed as mean ± standard error (SE). The results were analysed by one-way ANOVA followed by Dunnett’s multiple comparison test. Statistical analyses were performed using GraphPad Prism 7.0. p < 0.05 was considered a significant difference.
Results
Ageing was associated with kidney damage and long-term DR retarded the damage
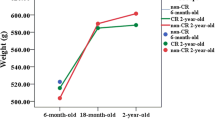
This study aimed to clarify the potential effect of long-term DR during renal ageing. Compared with young mice, Aged-AL mice had greater body and kidney weight. In contrast, a smaller relative kidney weight was observed in Aged-AL mice (Fig. 1a–c). DR contributed to a lower body and kidney weight in aged mice, but significantly contributed to maintaining normal relative kidney weight during ageing.
Body weight (a), kidney weight (b), relative kidney weight (c) and renal morphology (d) of mice. All results are expressed as mean ± SEM. n = 10. *, p < 0.05. AL = ad libitum, DR = dietary restriction. Red arrow = glomerulus, blue arrow = casting accumulation. Scale bar: 50 μm for H & E and Masson trichrome staining
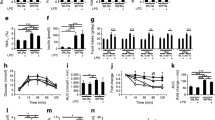
Kidney morphology was visualized to investigate the effect of ageing (Fig. 1d). A greater size of glomeruli with the loss of capillaries (red arrow) and more casting accumulation (blue arrow) in the renal tubules was observed in aged kidneys. Ageing caused extensive tubulointerstitial fibrosis (Masson trichrome stained collagen fibres) and glomerulosclerosis, whereas DR significantly normalized the morphological alterations during renal ageing. These structural changes are direct evidence of the renal damage during ageing, and the amelioration of this damage associated with DR.
Mitochondrial dysfunctions were involved in renal ageing and retarded by long-term DR
Kidney ultrastructure was analysed by TEM (Fig. 2). Aged-AL kidneys exhibited abnormalities in mitochondria morphology (red arrow), including looser and irregular crista, giant size and rounder shapes. The Aged-DR kidneys exhibited a smaller size of mitochondria with a spherical or elongated ovoid shape and intensive crista. Aged-AL kidneys exhibited fewer lysosomes (brown arrow) and more autophagosomes (blue arrow) than Aged-DR kidneys. All these cellular structural changes indicate the impairments of mitochondrial functions and autophagy in aged kidney, and DR reversed these changes.
MDA was measured to clarify whether ageing caused lipid peroxidation induced by oxidative stress. Ageing induced a higher level of MDA while DR reduced it (Fig. 3a). Because energy supply is the major function of mitochondria, ATP level was analysed to monitor mitochondrial functions. Ageing did not change renal ATP levels (mmole/g kidney), however, DR elevated ATP level in aged kidney (Fig. 3b).
Expressions of mitochondria-related proteins
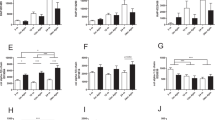
Because ageing caused mitochondrial dysfunctions, mitochondria-related proteins were further analysed to explore the potential mechanisms involved in mitochondrial homeostasis. FOXO1 and SOD2, markers for antioxidant systems, were monitored to investigate their roles in DR regulation (Fig. 4a). Ageing caused a lower level of FOXO1, while DR elevated FOXO1 level. SOD2 was suppressed during ageing and was not regulated by DR. These results suggest that FOXO1, but not SOD2, is the critical regulator for DR’s protection against oxidative stress.
Expressions of mitochondrial-related proteins in kidney of mice. Antioxidant (a), biogenesis (b), respiratory chain complexes (c), mitochondrial dynamics (d), mitophagy (e), and autophagy (f). All results are expressed as mean ± SEM. n = 4–5. *, p < 0.05. AL = ad libitum, DR = dietary restriction, SDHB = Complex II subunit, MTCO1 = Complex IV subunit, TFAM = mitochondrial transcription factor A, MFN2 = Mitofusin 2, Pink1 = PTEN-induced putative kinase 1, LC3 = Light Chain 3, VDAC = voltage-dependent anion channel, GAPDH = glyceraldehyde 3-phosphate dehydrogenase
A greater level of ATP caused by DR during renal ageing might be due to a larger mitochondrial mass, increased mitochondrial biogenesis or better efficiency of mitochondrial bioenergetics. To elucidate these possibilities, protein markers related to mitochondrial mass (VDAC), biogenesis (TFAM and AMPK) and bioenergetics (SDHB and MITO1) were measured by western blot (Fig. 4b, c). Ageing significantly reduced VDAC level, whereas DR elevated VDAC level. Similar levels of TFAM, AMPK and pAMPK were observed among all groups. Ageing did not regulate bioenergetic proteins, while DR inhibited their expressions during ageing. These results suggest that ageing reduces mitochondrial mass, while DR increases mitochondrial mass, thus affecting ATP production in the aged kidney.
Remarkably, large numbers of giant mitochondria were observed in Aged-AL kidneys, suggesting the involvement of mitochondrial dynamics. Proteins related to mitochondrial fusion (OPA1 and MFN2) and fission (FIS1) were detected (Fig. 4d). There were no differences in OPA1 and FIS1 among treatments. Ageing significantly upregulated MFN2, and DR further increased its level.
Since autophagosomes were noticed in the Aged-AL kidneys, indicating dysfunction of mitochondrial clearance, we further analysed protein expressions related to mitophagy (Parkin and PINK1) and autophagy (LC3I/II) (Fig. 4e, f). Ageing increased the PINK1 level, and DR did not affect it during ageing. Neither ageing nor DR regulated Parkin expression. A lower level of LC3I and LC3II was observed in the Aged-AL kidneys compared with Young-AL kidneys. DR elevated LC3I without changing LC3II in the aged kidneys and eventually decreased the ratio of LC3II/LC3I.
These protein analysis results provide evidence that ageing induced mitochondrial fusion and impaired autophagy, thus causing an accumulation of giant mitochondria, while DR improved clearance efficiency. Consequently, DR kidneys displayed smaller mitochondria and fewer autophagosomes and maintained greater efficiency of energy supply.
Discussion
For glomerular filtration to be efficient, the kidney requires a significant amount of energy supply. As a high-energy demand organ, its health is dependent on mitochondrial homeostasis, and manipulating mitochondrial functions ameliorates kidney ageing (Andrianova et al. 2021). Hormesis is an adaptive mechanism of cellular response to mild stress, a potential approach for anti-ageing via the actions of the antioxidant system, autophagy, mitochondrial and energy metabolism (Mattson 2008; Rattan 2008). The present study demonstrated that ageing caused mitochondrial abnormalities, autophagosome accumulation, and severe fibrosis in the kidney. DR displayed a hormetic effect on renal ageing, including autophagy and mitochondrial homeostasis, thus reducing renal damage.
Ageing in kidneys is accompanied by lower autophagic activity (Bao et al. 2019), however, inconsistent patterns in protein expressions of LC3I and II were observed in the kidney of aged animals. Cui and his colleagues (2012) showed that ageing caused a decrease in LC3I but not LC3II, which ended in an increased ratio of LC3II to LC3I, whereas, Zhang et al. (2019) found that ageing led to a decrease in LC3II and the ratio of LC3II to I without altering LC3I expression. There was no difference in the expressions of autophagic related proteins (LC3II, beclin, and p62) between aged and young kidney in the study of Diao et al. (2019). Our study demonstrated a different pattern, i.e., ageing caused a reduction in both LC3I and II expressions, and resulted in an unchanged ratio of LC3II to I. Nevertheless, large numbers of giant mitochondria and autophagosomes found in TEM images of aged mice reveal defective autophagy in aged kidneys. Long-term DR maintained relatively normal structures of mitochondria in TEM, supporting a positive effect of DR on autophagic clearance during ageing. Mitophagy might not be involved in the beneficial contribution of DR to aged kidneys.
Manipulating autophagy is a promising approach to strengthen the ability to delay ageing (Bao et al. 2019; Cui et al. 2013). However, the protective capability declines in an age-dependent manner. Four weeks of DR was found to be sufficient to activate autophagy in young but not in old rats (Andrianova et al. 2020), whereas 20 month of DR significantly increased autophagic flux and mitophagy and restored kidney functions (Cui et al. 2013), indicating that the timing of DR intervention manipulates autophagy efficiency, which further affects renal ageing.
Ageing accompanies with the loss of functioned nephron and an increase in nephrosclerosis, the degree and extent of nephron loss determine the percentage of renal function left (Elsherbiny et al. 2014; Geraci et al. 2017). Meanwhile, the remaining nephrons undergo with compensatory hypertrophy to counteract the reduction of renal functions (Geraci et al. 2017). Ageing did not alter the renal ATP level in this study could be due to the hypertrophic growth of remaining nephrons. Nevertheless, DR elevated ATP production without causing any abnormal structures in aged kidney, and these ATPs produced could partially delay the function reduction in the following ageing.
The fusion of two mitochondria (mitofusion) is a mechanism that can increase the efficiency of energy supply in kidneys when facing stressors. A decline in mitofusion and greater richness of fragmented mitochondria are noticed in impaired kidneys, acute kidney injury and CKD (Bhatia et al. 2020). Interestingly, a higher level of fusion-related protein (MFN2) was found in both Aged-AL and Aged-DR kidneys in the present study. MFN2, located on the outer mitochondrial membrane, participates in the process of mitochondrial fusion and autophagy (Sebastian et al. 2012). It also serves as a tether to connect mitochondria and endoplasmic reticulum, and normalizes glucose homeostasis. MFN2 deletion in the liver causes mitochondrial fragmentation, oxidative stress, insulin intolerance and endoplasmic reticulum stress (Sebastian et al. 2012). Increased MFN2 in aged chondrocytes promotes mitochondrial respiration. MFN2 knockdown leads to metabolic changes (increased glucose uptake, lower ATP production and mitochondrial ROS) and contributes to the development of osteoarthritis (Xu et al. 2020). Upregulating MFN2 in podocytes reserves the structure and functions of mitochondria-associated ER membranes, thus exerting an anti-apoptotic effect on diabetic nephropathy (Cao et al. 2021). Because MFN2 plays a role in multiple cellular functions in various organs, an increase in MFN2 expression originating from different stressors might represent different regulation patterns.
AMPK is the master regulator for energy sensing, metabolic balance, and autophagy initiation in cells. There are two regulation pathways for AMPK activation, phosphorylation and allosteric activation (Garcia and Shaw 2017). Phosphorylation at Thr172 of α subunit, as the major route for AMPK activation, was determined to be the marker for AMPK activity in the present study. Some compounds, such as small molecules, directly bind and activate AMPK, independent of upstream kinase signalling (Garcia and Shaw 2017; Scott et al. 2014). In addition, we found that ageing caused a decrease in FOXO1 level, which is downstream of AMPK signalling. Because the AMPK-FOXO1 pathway plays a critical role in DR-induced longevity (Greer et al. 2009), it is reasonable to postulate that ageing might regulate AMPK via allosteric action or phosphorylation independently; whereas DR increased AMPK activity, this elevated its downstream-FOXO1 level.
DR significantly increased FOXO1 level in aged kidneys, implying that the antioxidative function of FOXO1 is involved in the renal ageing protection afforded by DR. FOXO1 participates in several cellular mechanisms such cell cycle, anti-oxidation and anti-inflammation, and thus serves as a potential therapeutic target for diseases and ageing (Kim et al. 2021; Xing et al. 2018). Animal models demonstrate the protective role of FOXO1 in ageing kidneys. A decrease in FOXO1 expression is present in aged rats. Chemical and genetic manipulation of FOXO1 mitigates oxidative stress and inflammation, thus enhancing the survival of kidney cells (Kim et al. 2019), whereas suppressing FOXO1 accelerates the death of kidney cells (Cheng et al. 2019; Kim et al. 2019). Similarly, our study found that FOXO1 functions as the antioxidation regulator affected by DR in the context of kidney ageing. Recent evidence supports the conclusion that FOXOs mediate mitochondrial homeostasis in organs (Cheng 2022). Activating FOXO1 reverses the imbalance of mitochondrial dynamics and ameliorates diabetic nephropathy (Wu et al. 2015). Our results also suggest a potential link between FOXO1 and mitochondrial homeostasis during ageing. The question of whether DR accelerated mitochondrial homeostasis via FOXO1 signalling requires further research.
The long-term DR use in mice in the present study showed remarkable benefits during renal ageing, demonstrating the importance of DR duration and starting age in delaying renal ageing. The associated parameters such as duration, degree and timing of the DR intervention determine its effectiveness (Xu et al. 2015). Podkowka-Sieczka et al. (2009) found that a 6-month DR introduced later in life yielded similar benefits for the structures and functions of the kidney as a 12-month DR immediately after puberty did. Similarly, adult-onset DR remains beneficial in a duration-dependent manner. A reduction in glomerulosclerosis and tubular atrophy was found after a 6-month DR (McKiernan et al. 2007). When extending the duration to 12 months, further benefits (such as decreased interstitial fibrosis formation,) were observed, and an 18-month DR even reduced vascular wall thickening. A DR treatment’s reduction percentage is another important factor. Andrianova et al. (2018) investigated the differences between four weeks of 25% or 35% DR on renal protection under acute kidney injury, and found that while both regimens were effective, the 35% DR treatment had better outcomes. However, this dietary regimen did not work in old rats with acute kidney injury unless the period was extended to eight weeks (Andrianova et al. 2020). For lowering the incidence of CKD, a 40% DR exhibited greater effectiveness than a DR of lower reduction percentage (Xu et al. 2015). Consequently, it would not be expected that the progression of renal ageing could be fully mitigated by DR unless factors such as duration or initiation age were optimized.
To conclude, this study demonstrated that renal fibrosis accompanies the ageing process. Impaired mitochondria, increased oxidative stress and severe fibrosis were noticed in aged kidneys. We found that long-term DR ameliorated such damages. The actions of DR during ageing are potentially mediated by the increasing of functional mitochondrion mass, elevation of autophagic clearance and suppression of oxidative stress. A limitation of this study was that the systemic effect of DR on the lipid metabolism was not taken into account. The increased visceral adiposity and ectopic lipid accumulation that accompanies ageing cause systemic inflammation; DR-mediated decreases in visceral adiposity might change the blood adipokine profile and normalize the systemic lipid metabolism, thereby contributing to the hormetic effect of DR on age-related renal fibrosis. The related mechanisms are in need of further investigation.
Data availability
The datasets generated and analysed during the current study are available in the Mendeley Data, https://doi.org/10.17632/ysv6jgnhrx.2.
Abbreviations
- AL:
-
Ad libitum
- CKD:
-
Chronic kidney diseases
- DR:
-
Dietary restriction
- DRP1:
-
Dynamin-related protein 1
- FIS1:
-
Mitochondrial fission 1 protein
- FOXO1:
-
Forkhead box protein O1
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- LC3:
-
Light chain 3
- MFN2:
-
Mitofusin-2
- MTCOI:
-
Complex IV subunit II
- OPA1:
-
Optic Atrophy 1
- PINK1:
-
PTEN-induced putative kinase 1
- ROS:
-
Reactive oxygen species
- SDHB:
-
Complex II subunit 30 kDa
- SOD2:
-
Superoxidase dismutase 2
- TBARS:
-
Thiobarbituric acid reactive substances
- TEM:
-
Transmission electron microscopy
- TFAM:
-
Mitochondrial transcription factor A
- VDAC:
-
Voltage-dependent anion channel
References
Andrianova NV, Jankauskas SS, Zorova LD, Pevzner IB, Popkov VA, Silachev DN et al (2018) Mechanisms of age-dependent loss of dietary restriction protective effects in acute kidney injury. Cells 7:178. https://doi.org/10.3390/cells7100178
Andrianova NV, Zorova LD, Pevzner IB, Popkov VA, Chernikov VP, Silachev DN et al (2020) Resemblance and differences in dietary restriction nephroprotective mechanisms in young and old rats. Aging 12:18693–18715. https://doi.org/10.18632/aging.103960
Andrianova NV, Buyan MI, Bolikhova AK, Zorov DB, Plotnikov EY (2021) Dietary restriction for kidney protection: decline in nephroprotective mechanisms during aging. Front Physiol 12:699490. https://doi.org/10.3389/fphys.2021.699490
Bao C, Yang Z, Cai Q, Li Q, Li H, Shu B (2019) Incremental load training improves renal fibrosis by regulating the TGFbeta1/TAK1/MKK3/p38MAPK signaling pathway and inducing the activation of autophagy in aged mice. Int J Mol Med 44:1677–1686. https://doi.org/10.3892/ijmm.2019.4344
Bhatia D, Capili A, Choi ME (2020) Mitochondrial dysfunction in kidney injury, inflammation, and disease: potential therapeutic approaches. Kidney Res Clin Prac 39:244–258. https://doi.org/10.23876/j.krcp.20.082
Cao Y, Chen ZW, Hu JJ, Feng J, Zhu ZJ, Fan YQ et al (2021) Mfn2 regulates high glucose-induced MAMs dysfunction and apoptosis in podocytes via PERK pathway. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2021.769213
Cheng ZY (2022) FoxO transcription factors in mitochondrial homeostasis. Biochem J 479:525–536. https://doi.org/10.1042/Bcj20210777
Cheng FY, Lee YH, Hsu YH, Chiu IJ, Chiu YJ, Lin YF et al (2019) Promising therapeutic effect of thapsigargin nanoparticles on chronic kidney disease through the activation of Nrf2 and FoxO1. Aging 11:9875–9892. https://doi.org/10.18632/aging.102437
Cui J, Bai XY, Shi S, Cui S, Hong Q, Cai G et al (2012) Age-related changes in the function of autophagy in rat kidneys. Age 34:329–339. https://doi.org/10.1007/s11357-011-9237-1
Cui J, Shi SZ, Sun XF, Cai GY, Cui SY, Hong Q et al (2013) Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS ONE 8:e69720. https://doi.org/10.1371/journal.pone.0069720
Diao C, Wang L, Liu H, Du Y, Liu X (2019) Aged kidneys are refractory to autophagy activation in a rat model of renal ischemia-reperfusion injury. Clin Interv Aging 14:525–534. https://doi.org/10.2147/CIA.S197444
Duann P, Lin PH (2017) Mitochondria damage and kidney disease. Adv Exp Med Biol 982:529–551. https://doi.org/10.1007/978-3-319-55330-6_27
Elsherbiny HE, Alexander MP, Kremers WK, Park WD, Poggio ED, Prieto M et al (2014) Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol 9:1892–1902. https://doi.org/10.2215/Cjn.02560314
Garcia D, Shaw RJ (2017) AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell 66:789–800. https://doi.org/10.1016/j.molcel.2017.05.032
Geraci S, Chacon-Caldera J, Cullen-McEwen L, Schad LR, Sticht C, Puelles VG et al (2017) Combining new tools to assess renal function and morphology: a holistic approach to study the effects of aging and a congenital nephron deficit. Am J Physiol Renal Physiol 313:F576–F584. https://doi.org/10.1152/ajprenal.00329.2015
Greer EL, Banko MR, Brunet A (2009) AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Ann N Y Acad Sci 1170:688–692. https://doi.org/10.1111/j.1749-6632.2009.04019.x
Kim DH, Park MH, Ha S, Bang EJ, Lee Y, Lee AK et al (2019) Anti-inflammatory action of beta-hydroxybutyrate via modulation of PGC-1alpha and FoxO1, mimicking calorie restriction. Aging 11:1283–1304. https://doi.org/10.18632/aging.101838
Kim DH, Bang E, Ha S, Jung HJ, Choi YJ, Yu BP et al (2021) Organ-differential roles of Akt/FoxOs axis as a key metabolic modulator during aging. Aging Dis 12:1713–1728. https://doi.org/10.14336/Ad.2021.0225
Li SJ, Lin YH, Chiang CH, Wang PY, Chen CY (2022) Early-onset dietary restriction maintains mitochondrial health, autophagy and ER function in the left ventricle during aging. J Nutr Biochem 101:108944. https://doi.org/10.1016/j.jnutbio.2022.108944
Mattson MP (2008) Dietary factors, hormesis and health. Ageing Res Rev 7:43–48. https://doi.org/10.1016/j.arr.2007.08.004
McKiernan SH, Tuen VC, Baldwin K, Wanagat J, Djamali A, Aiken JM (2007) Adult-onset calorie restriction delays the accumulation of mitochondrial enzyme abnormalities in aging rat kidney tubular epithelial cells. Am J Physiol Renal Physiol 292:F1751-1760. https://doi.org/10.1152/ajprenal.00307.2006
Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA et al (2016) Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 23:1093–1112. https://doi.org/10.1016/j.cmet.2016.05.027
Podkowka-Sieczka R, Wieczorowska-Tobis K, Niemir ZI, Styszynski A, Breborowicz A, Oreopoulos DG (2009) The effect on renal structure and function of late-life-introduced caloric restriction (CR) in rats. Int Urol Nephrol 41:211–217. https://doi.org/10.1007/s11255-008-9499-4
Rattan SI (2008) Hormesis in aging. Ageing Res Rev 7:63–78. https://doi.org/10.1016/j.arr.2007.03.002
Rosner MH, La Manna G, Ronco C (2018) Acute kidney injury in the geriatric population. Contrib Nephrol 193:149–160. https://doi.org/10.1159/000484971
Scott JW, Ling NM, Issa SMA, Dite TA, O’Brien MT, Chen ZP et al (2014) Small molecule drug A-769662 and AMP synergistically activate naive AMPK independent of upstream kinase signaling. Chem Biol 21:619–627. https://doi.org/10.1016/j.chembiol.2014.03.006
Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D et al (2012) Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA 109:5523–5528. https://doi.org/10.1073/pnas.1108220109
Singh G, Krishan P (2019) Dietary restriction regimens for fighting kidney disease: insights from rodent studies. Exp Gerontol 128:110738. https://doi.org/10.1016/j.exger.2019.110738
Vodosek Hojs N, Bevc S, Ekart R, Hojs R (2020) Oxidative stress markers in chronic kidney disease with emphasis on diabetic nephropathy. Antioxidants. https://doi.org/10.3390/antiox9100925
Wu L, Wang Q, Guo F, Zhou Y, Ji H, Liu F et al (2015) Activation of FoxO1/ PGC-1alpha prevents mitochondrial dysfunction and ameliorates mesangial cell injury in diabetic rats. Mol Cell Endocrinol 413:1–12. https://doi.org/10.1016/j.mce.2015.06.007
Xing YQ, Li A, Yang Y, Li XX, Zhang LN, Guo HC (2018) The regulation of FOXO1 and its role in disease progression. Life Sci 193:124–131. https://doi.org/10.1016/j.lfs.2017.11.030
Xu XM, Cai GY, Bu R, Wang WJ, Bai XY, Sun XF et al (2015) Beneficial effects of caloric restriction on chronic kidney disease in rodent models: a meta-analysis and systematic review. PLoS ONE 10:e0144442. https://doi.org/10.1371/journal.pone.0144442
Xu L, Wu Z, He Y, Chen Z, Xu K, Yu W et al (2020) MFN2 contributes to metabolic disorders and inflammation in the aging of rat chondrocytes and osteoarthritis. Osteoarthritis Cartil 28:1079–1091. https://doi.org/10.1016/j.joca.2019.11.011
Zhang L, Zhou F, Yu X, Zhu Y, Zhou Y, Liu J et al (2019) C/EBPalpha deficiency in podocytes aggravates podocyte senescence and kidney injury in aging mice. Cell Death Dis 10:684. https://doi.org/10.1038/s41419-019-1933-2
Funding
This work was supported by the Research Grant MOST 110-2320-B-002-039-MY3 (from Ministry of Science and Technology, Taiwan).
Author information
Authors and Affiliations
Contributions
C-HC: methodology, investigation, data curation, formal analysis, validation, writing—original draft; S-JL: methodology, data curation, investigation, formal analysis, validation; T-RZ: data curation, visualization; C-YC: conceptualization, funding acquisition, resource, supervision, writing-review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiang, CH., Li, SJ., Zhang, TR. et al. Long-term dietary restriction ameliorates ageing-related renal fibrosis in male mice by normalizing mitochondrial functions and autophagy. Biogerontology 23, 731–740 (2022). https://doi.org/10.1007/s10522-022-09993-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-022-09993-8