Abstract
It is often argued that biodiversity and ecosystem functioning are linked by both habitat and species composition, and that this relationship is particularly critical for mobile ecosystem service providers. This may be especially true for pollinators, which are essential for the reproduction of the majority of flowering plant species, are highly mobile, and can exhibit dramatically different foraging behaviors across ecosystems. Understanding how habitat and community composition impact pollination is especially relevant in urban environments where pollinators can promote food security. We examined the relationships between local resource density, landscape composition, pollinator abundance and richness, and pollination services in an urban agricultural system spanning >125 km of the California central coast. We used a replicated, experimental approach to evaluate the reproductive success of jalapeño peppers across urban gardens and conducted a greenhouse experiment to evaluate the benefits of insect-mediated pollination to pepper reproduction. In the greenhouse, we found that jalapeño fruit weight and seed number was significantly greater with insect-mediated pollination than without. In the field, we found that jalapeño seed number increased significantly with herbaceous (weed, crop, and ornamental) plant richness and the number of perennial trees and shrubs at the site level, but decreased with the amount of natural landscape cover. We also found that higher pollinator richness enhanced seed number in floral-dense gardens, likely due to the greater functional complementarity of a more diverse pollinator community. Furthermore, there was a positive relationship between pollinator abundance and seed number, but it weakened in gardens with more flowers, likely through lower per-plant pollinator visitation in the presence of competing floral resources. As in past studies, we found that mulch had a negative impact on pollinator abundance, highlighting that abiotic factors commonly managed by gardeners can directly impact ecosystem service providers. This study demonstrates that local conditions can significantly influence ecosystem service provision and that urban gardeners need to optimize for both pollinator richness and floral resource availability to achieve optimal pollination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiversity is rapidly declining as a result of numerous global change drivers (Butchart et al. 2010; Tittensor et al. 2014), threatening ecosystem functioning and services that are essential for human well-being (Loreau et al. 2001; Hooper et al. 2005; Balvanera et al. 2006; Naeem et al. 2009; Cardinale et al. 2012). This relationship (biodiversity-ecosystem-function, or BEF) has been the focus of many studies within rural agricultural systems that strive to understand food security, pest suppression, and other ecosystem services. Though these studies have largely found strong, positive relationships between biodiversity and ecosystem functioning (Larsen et al. 2005, Tscharntke et al. 2005, Tscharntke et al. 2012, but see Cardinale et al. 2006), the mechanisms underlying this relationship are less understood. Further, while ecosystem functioning is often mediated by biotic and abiotic factors at multiple spatial scales (Maire et al. 2012), and by the foraging patterns of mobile ecosystem service providers (Kremen et al. 2007; Hoehn et al. 2008; Albrecht et al. 2016), the relative role of local resource levels in shaping animal foraging and resulting ecosystem services is not typically evaluated. Specifically, the diversity and abundance of food resources may serve to either potentially concentrate (Williams et al. 2015; Xie et al. 2019) or dilute (Veddeler et al. 2006) foraging organisms at resource patches, but this phenomenon is rarely quantified outside of rural agricultural systems (but see Jha and Vandermeer 2009).
The relationship between local resources, landscape composition, and biodiversity may be particularly important for pollinators, whose movement patterns directly impact ecosystem service provisioning (Kremen et al. 2007). This is because pollinators, which are essential for the reproduction of more than 80% of flowering plant species (Ollerton et al. 2011), are both highly mobile and exhibit tremendous variation in community characteristics such as plant visitation behaviors (Burkle and Alarcon 2011). While pollination service indices, such as plant reproduction, are often a function of the richness and abundance of the wild pollinator community (e.g. Gómez et al. 2007; Lowenstein et al. 2015), the ability to deliver pollination services may also be contingent on local and landscape habitat factors. In rural agricultural systems, pollinator abundance and richness (e.g. Kennedy et al. 2013; Goulson et al. 2015), as well as pollination services (e.g. Potts et al. 2010; Cusser et al. 2016), are often mediated by landscape drivers such as agricultural intensification and natural habitat cover (e.g. Kim et al. 2006; Kennedy et al. 2013; Blaauw and Isaacs 2014a).
In addition to landscape factors, local floral diversity, abundance, and composition can mediate pollination through impacts on pollinator densities (Steffan-Dewenter and Westphal 2008; Williams et al. 2015), foraging dynamics (Kunin and Iwasa 1996; Kunin 1993), visitation rates (Van Nuland et al. 2013, Veddeler et al. 2006), and pollen deposition (Lortie and Aarssen 1999; Evans et al. 2017). Classic research in foraging biology has shown that, when a limited number of foragers spread out in a high resource patch, it can reduce per-plant visitation, something called the “dilution effect” (Root and Kareiva 1984; Yamamura 1999). In contrast, when high resource patches recruit more foragers to a site, increasing per-plant visitation, it is known a “concentration effect” (Sih and Baltus 1987; Kunin 1993; Totland and Matthews 1998). Dilution and concentration effects, respectively, reduce or enhance forager visitation rates, with implications for plant reproduction. Dilution may also occur when, at high floral densities, co-flowering hetereospecific plants result in inter-specific competition for pollinators, reducing plant visitation (Ghazoul 2006). Furthermore, floral fidelity may be relaxed in communities with high plant diversity, increasing inter-specific pollen transfer (Fontaine et al. 2008) and inhibiting pollination (Wilcock and Neiland 2002; Holland and Chamberlain 2007).
In agricultural systems, both foraging dilution and concentration responses to floral resources are sensitive to scale (Veddeler et al. 2006, Jha and Vandermeer 2009, Riedinger et al. 2013, Holzshuh et al. 2016) and pollinator species composition (Jha and Vandermeer 2009), likely because pollinators vary in foraging behavior and foraging distance (Greenleaf et al. 2007; Pisanty et al. 2015). Indeed, beyond pollinators, there is a strong link between species trait diversity and ecosystem function that has been documented across taxa and ecological systems (Naeem and Wright 2003; Cardinale et al. 2004; Cardinale et al. 2006; Hooper et al. 2005; Schleuning et al. 2014). For pollinators, communities exhibiting a diversity of functional traits provide greater pollination resources (Hoehn et al. 2008; Cadotte et al. 2011; Gagic et al. 2015), but the relationship between pollinator diversity and pollination may be dependent on local floral conditions (Tylianakis et al. 2008). In this study, we experimentally evaluate how pollination services are mediated by both landscape context and the interactions between local floral resources and pollinator abundance and richness in an understudied system, urban agriculture.
Urban systems are compelling habitats in which to investigate these interactions. Urban gardens are important sites for food production (McCormack et al. 2010) and serve as important refuges for pollinator communities within cities (Fetridge et al. 2008; Matteson et al. 2008; Frankie et al. 2009; Matteson and Langellotto 2010; Pardee and Philpott 2014; Burr et al. 2016; Quistberg et al. 2016). A number of studies have found that local habitat features, like the availability of bare ground, can positively impact pollinator abundance and richness in gardens, especially for ground-nesting bees (Quistberg et al. 2016; Ballare et al. 2019). Bare, unpaved ground can also impact crop fruit set (Bennett and Lovell 2019). While some urban studies have found that increased floral abundance at local spatial scales positively impacts pollinator richness (Pardee and Philpott 2014; Quistberg et al. 2016), pollinator abundance (Bennett and Lovell 2019) and pollination (Potter and LeBuhn 2015; Lowenstein et al. 2015), others have found no beneficial impact of increased floral resources (Glaum et al. 2017); this variation may be a result of the dynamic relationship between floral resources and pollinator foraging, in which the composition, diversity, and abundance of floral resources is theorized to either concentrate or dilute pollinators across a landscape (Veddeler et al. 2006). Despite the importance of crop production within urban systems, the impact of the interaction between floral resources and pollinator abundance and richness on pollination services has not been quantitatively evaluated.
In this study, we use a replicated experimental approach to evaluate the reproductive success of jalapeño peppers in gardens spanning more than 125 km of urban habitat to quantify the links between pollinators and pollination function across heterogeneous landscapes. Urban gardens are important places for the evaluation of pollination services given their ecological, cultural, and economic value to humans (Bellows et al. 2003; Baker 2004; Freeman et al. 2012; Goddard et al. 2013). We used jalapeño peppers as a model system because they are commonly cultivated in urban gardens and may benefit from insect visitation (Delaplane and Mayer 2000), though the benefit of this visitation has not been investigated across heterogeneous field conditions. While past experiments in urban garden studies have focused on bee-pollinators, non-bee pollinators are important contributors to crop production (Rader et al. 2009), so we include both bee and non-bee pollinators in our study. Non-bees include flies (Diptera), which are often generalists that visit multiple plant species (Kearns 2001). Although butterflies (Lepidoptera) and wasps (Hymenoptera) are often considered inefficient pollinators, visitation rates to crop plants by pollinators are often mediated by complex, indirect interactions between species (Primack and Inouye 1993). The pollination success of insect-pollinated plant species is therefore usually not dependent on single, highly specialized pollinator species, but rather on a diverse community of pollinators (Greenleaf and Kremen 2006, Steffan-Dewenter and Westphal 2008, Albrecht et al. 2012 Garibaldi et al. 2016).
In this study, we evaluate three hypotheses: 1) successful reproduction of jalapeño peppers requires insect-mediated pollination, 2) local floral resources and bare ground cover drive pollinator richness and abundance, 3) and jalapeño pollination success is positively related to the interactions between local floral resource availability and pollinator abundance and richness.
Methods
Field study: Characterization of the study sites
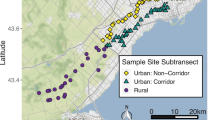
The field study was conducted in July 2016 in 21 urban community gardens, 16 of which have been investigated for urban ecological studies for multiple years (e.g. Otoshi et al. 2015; Quistberg et al. 2016; Egerer et al. 2017). The 21 gardens are separated by a minimum of 21 km and range in size from from 444 m2 to 15,525 m2 (mean 4419.4 ± SD 3884.5 m2) and are distributed across three counties (Monterey, Santa Clara, and Santa Cruz) in the California central coast (Fig. 1). Each garden is a community garden managed in allotments or collectively and each garden contained vegetable and fruit crops and had been actively cultivated between 5 and 49 years. We evaluated both local and landscape characteristics of all the gardens. At the local (within-garden) scale, we measured habitat characteristics within a 20 × 20 m plot placed at the center of all 21 gardens between July 8th and July 13th of 2016. Specifically, we measured garden size and canopy cover with a convex spherical densiometer at the center of the plot, and 10 m to the N, S, E, and W. We also counted and identified all trees and shrubs as the number of woody plants in the plot. In each plot, we randomly selected eight 1 × 1 m quadrats within which we identified all herbaceous flowering plants (forbs) to morphospecies, measured height of the tallest non-woody vegetation, counted the total number of flowers, and assessed percent ground cover from bare soil, grass, herbaceous plants (crops, weeds, and ornamentals), leaf litter, rocks, and mulch. When counting the number of flowers, we counted flowers inside inflorescences as individual flowers regardless of size.
At the landscape scale, we classified land cover types within 2 km buffers surrounding each garden with data from the 2011 National Land Cover Database (NLCD, 30 m resolution) (Homer et al. 2015). We selected 2 km buffer zones given that bee abundance responds significantly to habitat composition at a 2 km scale (as Kremen et al. 2004). Based on known bee nesting preferences and classification systems used in past studies (e.g., Ritchie et al. 2016; Quistberg et al. 2016; Cohen et al. 2017), we created four land-use categories: 1) natural habitat (including deciduous, evergreen, mixed forests, dwarf scrub, shrub/scrub, and grassland/herbaceous), 2) open habitat (including lawn grass, parks, and golf courses), 3) urban habitat (including low, medium, and high intensity developed land), and 4) agriculture habitat (including pasture/hay and cultivated crop). Other land cover types that covered <5% of the total area were not included. We assessed land cover with ArcGIS v.10.1.
Field study: Pollinator survey
To compare pollinator richness and abundance across 21 sites with different local and landscape features, we conducted visual pollinator surveys in July 2016 along four 4 × 20 m transects that ran E-W in each plot, starting at 0, 5, 10, and 15 m from the southern edge at all sites. Surveys were conducted by one observer and occurred for 30 min between 0:900 and 16:00 on sunny days with less than 50% cloud cover. During each survey we walked the four transects in the 20 × 20 m plot, at a pace of approximately 3.5 min per transect. Because pollination experiments were running at the time of the survey (see below) we did not collect pollinators. Given that active sampling and observational data for pollinator sampling are often highly correlated (e.g. Mandelik et al. 2012), and that observational data is useful for reflecting community-level changes in abundance over space (Kremen et al. 2011), we visually recorded all pollinating insects visiting any flower in the garden, including bees, flies, wasps, and butterflies, and identified each individual to the finest resolution possible (family, tribe, genus, and species). To guide in-field identifications, we brought a box of representative physical specimens with us to the field that were previously collected in the same system (see Quistberg et al. 2016); in addition, we consulted online resources (Ascher and Pickering 2017), image databases (e.g. Packer and Ratti 2007), books (Michener 2007), and dichotomous keys (Michener et al. 1994; Gibbs 2010) to create a visual field reference. In-field identifications were conducted by researchers trained in bee identification and systematics according to Michener et al. (1994) at the 2014 Bee Course (American Museum of Natural History). Because visual counts may be subject to observer bias and sampling effort, they were conducted by one individual.
Field study: Pollination experiment
We examined pollination of C. annuum jalapeño peppers in a subset of 15 gardens (Fig. 1). We used the ‘Jalapeño’ cultivar of the species Capsicum annuum (Solanaceae) as a focal plant because it is an annual crop commonly grown in urban gardens, it has multiple hermaphroditic flowers per plant opening from June-Aug, and because it is attractive to a wide variety of pollinators, including bees (Dialictus spp., Augochlora spp., Exomalopsis spp., Bombus spp., Halictus spp., Dialictus spp., Hylaeus spp. (Raw 2000). C. annum cultivars are also pollinated by flies in the syrphid (Jarland et al. 1997) and tephritid (Vargas and Mitchell 1987) families, as well as by wasps (Bosland and Votava 2000).
We planted C. annum from organic seed in standard soil mixes and grew them in one-gallon pots for 105 days under standard growing and irrigation conditions in a covered outdoor greenhouse at the University of California, Santa Cruz, located within the same geographic region as the study. Upon budding, we placed mesh insect exclusion bags on all peppers until they were utilized for lab or field experiments. Every evening, we tied colored strings on all unopened flowers of all plants in order to record and identify flowers that had opened in the previous 12 h (hereafter ‘recently opened’) at the time of the experiment.
At each garden site we temporarily installed six plants. We placed the six bagged, potted-plants all together as a cluster in random location within the 20 × 20 m plot. We did not place pots in beds, but adjacent to them. The six pepper pots were clustered together in two rows of 3 pots per row, and at a distance of ~20 cm apart from one another. Upon placement in gardens, we randomly assigned three plants into one of three treatments: open (O), open outcross (OO), or a closed (C) treatment to evaluate the importance of outcross pollen to seed and fruit production (Cusser et al. 2016). The remaining plants had bags removed and served as pollen donor plants.
In the open (O) treatment, the bag was opened and one recently opened, virgin flower was randomly selected from the plant and marked, and the entire pepper plant was left unbagged in the field for 48 h to allow visitation by insect pollinators. In the closed (C) treatment, the bag was opened, one recently opened, virgin flower was randomly selected from the plant and marked, and then immediately rebagged for use as a ‘control’ to evaluate the impact of no insect visitation and the experimental manipulation of the bags. The open outcross (OO) treatment received the “maximum” pollination service, a combination of insect visitation and hand-pollination, so that we could evaluate relative pollination limitation in open treatments across the sites (Cusser et al. 2016). In this treatment, the bag was opened, and we randomly selected one recently opened flower and hand-pollinated using flowers from donor plants. Specifically, for the donor plants, we randomly selected four anthers from two randomly selected pollen donor plants (2 anthers per pollen donor). Anthers were removed from the donor using forceps and manually rubbed in a circular motion on the receptive stigma of the closed outcross pollination treatment flower for 2 s each. Plants in this treatment were maintained unbagged in the field for 48 h to allow for insect visitation. After 48 h in the field, plants were picked up, rebagged, and returned to standard sterile indoor greenhouse growing conditions, and then after another 48 h (to ensure that all experimental flowers were completely closed), bags were removed and plants were maintained in greenhouse conditions, free from insect pollinators. Each plant was allowed to grow for 69 d, when we measured fruit weight (g) and seed number for the fruits in each treatment.
Greenhouse study: Pollination experiment
The greenhouse pollination study was conducted in June–July 2016. Because C. annuum may be self-compatible (Pickersgill 1997), we wanted to evaluate the impact of outcross pollination seed pollination (Parker 1997, Benjamin et al. 2014). We therefore evaluated the impact of closed self-cross (CS) and closed outcross (CO) treatments on fruit weight and seed number in the greenhouse, in tandem with the field experiments. We randomly selected 24 jalapeño plants for a closed self-cross (CS) and closed outcross (CO) experiment in the greenhouse (12 plants per treatment). In this experiment, closed refers to the fact that insect pollinators were excluded from the plants with mesh bags prior to the treatment and we measured the role of self-pollination on pepper reproduction. In the closed self-cross (CS) treatment, the bag was opened and then one recently opened flower was randomly selected from the plant and hand-pollinated using a sterile q-tip that was rubbed in a circular motion against the anthers of the flower for 8 s, then rubbed on the stigma of the same flower in a circular motion for 8 s (same duration as the closed outcross treatment), and then the bag was closed immediately after the treatment. In the closed outcross (CO) treatment, the bag was opened and then one recently opened flower was randomly selected from the plant and hand-pollinated for 8 s using flowers from donor plants. Specifically, we randomly selected four anthers from two randomly selected pollen donor plants (2 pollen-laden anthers per pollen donor, of the six anthers available per flower). Anthers were removed from the donor flowers using forceps and manually rubbed in a circular motion on the receptive stigma of the closed outcross pollination treatment flower for 2 s each, and then the bag was closed immediately after the treatment. After 48 h the plants were unbagged to allow for unhindered fruit growth after the period of pistil receptivity had passed (Aleemullah et al. 2000; Ofosu-Anim et al. 2006). To further safeguard against any potential insect vitiation that might bias our treatments, we maintained the plants in a sterile indoor insect-free greenhouse space for 69 d. We then measured fruit weight (g) and seed number for the fruits in each treatment.
Data analysis
We first evaluated the potential role of local and landscape habitat factors on pollinator abundance and richness. Then, using just the open treatment of the field experiments (those which experienced ambient insect pollination), we evaluated the potential relationship between pollinator abundance and richness, as well as local and landscape habitat factors, on fruit weight and seed number. Finally, we compared the fruit weight and seed number of the different pollination treatments for the greenhouse and field experiments.
Impacts of local and landscape features on pollinator abundance & richness
We used linear models with the lm function in R to examine relationships between local and landscape variables on both number of pollinators (abundance) and pollinator richness, as both pollinator indices met assumptions of normality. Richness for the pollinator communities was calculated using the Chao1 estimator (Chao 1984; Chao 2006) in the vegan R package (Oksanen et al. 2016). Chao1 is an extrapolated measure of species richness which accounts for undersampling by estimating higher species richness for samples with more rare taxa present (Chao 1984). We felt this approach was appropriate for our study given substantial variation in pollinator abundance across urban landscapes (as see in Ballare et al. 2019). Because many local and landscape variables were correlated, we prioritized variables previously found to be ecologically meaningful in describing pollinator richness in the same field sites (Quistberg et al. 2016). Using this process, our full model contained four local vegetation predictor variables – mulch, herbaceous plant richness, the number of woody plants, and the number of flowers, as well as one landscape predictor variable – proportion natural habitat cover (in a 2 km radius). The herbaceous plant diversity index included crop, weed, and ornamental species in the garden and was calculated using the Chao estimator. We then ran tests to identify collinearity of predictors in all of our linear models by calculating a variance inflation factor (VIF) for each model set using the car package in R (Fox and Weisberg 2018) and ensured that all variables met a VIF cutoff score of 3. We ran model selection using the MuMIn package (Barton 2018), an information-theoretic selection process that operates by subsetting the model. A set of models is generated with all possible combinations of the five predictor variables listed above (base model). We selected the top model based on the AICc values. For models where the AICc for top models was within 2 points of the next best model, we calculated model averages (Table S1).
Local, landscape, and pollinator impacts on pollination
Next, we evaluated the impact of these same local and landscape variables on fruit weight and seed number for the open pollination treatments. Because we were interested in evaluating the impact of the interaction between pollinators and their food resources, we included an interaction between the number of pollinators and the number of flowers (number of pollinators*number of flowers) and the richness of pollinators and number of flowers (richness of pollinators*number of flowers). Our models contained the following predictor variables: number of pollinators*number of flowers, richness of pollinators*number of flowers, mulch, number of woody plants, diversity of herbaceous plant species, and proportion of natural habitat (2 km) as fixed effects. We checked that VIF scores were still below 3 and used a linear model for the fruit weight data using lm in the stats R package, as fruit weight data met assumptions of normality, and a generalized linear model with a Poisson distribution for the seed number data using glm in the MASS R package (Ripley et al. 2013), because seed number is count based and was underdispersed for a Negative Binomial distribution. We again ran model selection using the MuMIn package (Barton 2013) and selected the top model based on the AICc values. For models where the AICc for top models was within 2 points of the next best model, we calculated model averages. We also ran the same models using bee richness and bee abundance instead of pollinator richness and abundance, and found similar patterns (Table S2). To determine the goodness-of-fit of all best models, we calculated a pseudo-R2 value as [(null deviance - residual deviance)/ null deviance] (Dobson & Barnett 2008).
Greenhouse and field experiments
In the greenhouse experiments we examined if pepper plants are self-compatible and/or enhanced by cross-pollination. To examine how fruit weight was influenced by these two treatment types, we ran a linear model using the lm function in the stats R package. To examine how seed set was influenced by these treatment two types, we used a generalized linear model with a Poisson distribution using the glm function in the stats R package. We also compared the means via a post-hoc Tukey test. For the field experiments, we examined if there was a significant difference between the closed treatment (C), open treatment (O), and outcrossed-open (OO) treatment for fruit weight and seed number. Specifically, to examine how fruit weight was influenced by these three treatment types, we ran a linear mixed effect model using the lmer function in R with garden as a random effect. For seed number, we used a generalized linear mixed effect model with a Poisson distribution using the glmer function also with garden as a random effect. Both analyses were conducted with the lme4 R package in R (Bates et al. 2015). We then compared means via a posthoc Tukey test.
Results
Local and landscape impacts on pollinator abundance & richness
The taxonomic identities of pollinators observed in this study are described in the supplemental information (Table S3). We observed 2583 total pollinators visiting flowers. We observed 22 distinct taxonomic groups and an average abundance of 132.83 (± 11.51 SE) individuals and average richness of 9.54 (± 0.59 SE) morphospecies per site. Pollinator abundance was lower where mulch cover was higher (p < 0.05, Fig. 3). After averaging the top models (AICc<2), mulch was the only significant predictor of pollinator abundance (Table 1b). Pollinator richness (Chao estimator) was not significantly predicted by any local or landscape habitat factor (Table S4a & b).
Local, landscape, and pollinator impacts on pollination
Fruit weight was not predicted by any local or landscape habitat factor or pollinator factor. Seed number increased with number of woody plants (p < 0.0001) and herbaceous plant species (p < 0.0001), decreased with the proportion of semi-natural habitat in a 2 km radius (p < 0.0001), and was unaffected by mulch (p = 0.834). We found a positive interaction between pollinator richness and flower number (p < 0.0001). When there were more flowers in the garden, the positive relationship between pollinator richness and seed number was steeper (Fig. 4). The model also documents a negative effect of the interaction of number of pollinators and number of flowers (p < 0.0001), where the higher the pollinator abundance, the more negative the relationship between number of flowers and seed number (p < 0.0001)(Fig. 5). After averaging the top models (AIC <2), the same factors remained significant predictors of seed number (Table 2). We found similar patterns when bee richness and bee abundance were substituted for pollinator richness and abundance in the analysis (Table S2).
Greenhouse and field experiments
Our greenhouse experiment confirmed that jalapeños benefit from outcross pollination. Mean fruit weight was higher in closed outcross (CO) plants (19.69 g ± 1.12 SE) than for closed self-cross (CS) plants (13.39 g ± 1.29 SE, p = 0.0015). Likewise, mean seed number was higher in closed outcross (CO) plants (83.50 g ± 11.71 SE) than for closed self-cross (CS) plants (34.60 g ± 9.28 SE) (p < 0.0001).
For plants placed in the field, mean fruit weight was 8.28 g (± 1.67 SE), 12.28 g (± 2.04 SE), and 16.92 g (± 1.32 SE), for the closed treatment (C), open treatment (O), and outcrossed-open (OO) treatment, respectively (Fig. 2). Fruit weight was significantly greater in the OO treatment compared to the C treatment (p = 0.0005). Fruit weight was not significantly greater between the O and OO treatment (p = 0.078), and not significantly different between the O and C treatments (p = 0.144). For these same plants, the mean seed number was 23.84 (± 6.60 SE), 39.42 (± 12.11 SE), and 48.36 (± 8.71 SE) for the closed treatment (C), open treatment (O), and outcrossed-open (OO) treatment, respectively. Seed number was significantly different between all treatment comparisons (p < 0.0001).
Box and whisker plots of hand pollination field experiments with resulting (a) Fruit weight and (b) Seed number, where the box is bounded by the 25th and 75th percentile (50th percentile indicated by central horizontal line), the whiskers represent 1.5 x the interquartile range, and the points represent outliers. Boxes with the same letter are not significantly different, while those with no common letters are significantly different (p < 0.001)
Impact of the interaction between richness of pollinators and number of flowers on seed number from the glm (Poisson) model. In order to visualize the interaction, the panels represent a split in the data at the average median floral abundance cover across sampling periods and gardens (41 flowers). The left panel (low floral abundance) represents gardens with less-than or equal to the median number of flowers, the right panel (high floral abundance) represents gardens with more the median number of flowers. The shaded area represents standard errors
Impact of the interaction between number of pollinators and number of flowers on seed number from the glm (Poisson) model. In order to visualize the interaction, the panels represent a split in the data at the average median floral abundance cover across all of the gardens (41 flowers). The left panel (low floral abundance) represents gardens with less-than or equal to the median number of flowers, the right panel (high floral abundance) represents gardens with more the median number of flowers. The shaded area represents standard errors
Discussion
Our findings highlight a critical interaction between floral abundance and pollinator community features, with consequences for pollination services in urban landscapes. First, we found that pollinator abundance was significantly higher in urban gardens with less mulch, but was unaffected by other local and landscape factors. Second, we found that jalapeño seed number was significantly and positively impacted by the interaction between pollinator richness and the number of flowers, where the more flowers there are in the garden, the more positive the relationship between pollinator richness and seed number, likely through increased pollinator functional complementarity. In contrast, we found that gardens with high floral abundance had lower seed number when they supported high pollinator abundance, likely through forager dilution effects on plant reproduction. Finally, we observed that jalapeño plant reproduction was significantly higher for insect-mediated treatments (open and open-outcross treatments) compared to no pollination treatments, and highest in the outcross-pollination treatments.
Local and landscape impacts on pollinator abundance & richness
Previous studies have found that garden size, annual floral resources, and woody perennial plants are associated with bee abundance and richness (Quistberg et al. 2016; Matteson and Langellotto 2009). We did not find that floral abundance (which was colinear with garden size in this system) impacted pollinator abundance and richness, possibly because many commonly grown flowers in urban garden systems are not used by pollinators (Lowenstein et al. 2019). However, local groundcover management, specifically, the proportion of soil covered with mulch, was significantly and negatively correlated with pollinator abundance. Mulch was also negatively correlated with pollinator richness, although not significantly. Mulch is negatively associated with the availability of bare soil in many urban gardens (e.g. Quistberg et al. 2016; Ballare et al. 2019), and many bee and non-bee pollinators use bare soil for their nest sites. Previously, in the same study system (Quistberg et al. 2016), a large fraction of the bee community in the study region was comprised of below-ground nesting species (61% of morphospecies), and the abundance of these species was positively correlated with bare ground availability. Our results resonate with past research within this system and in other systems that also find a positive correlation between bare ground availability and urban bee abundance (Frankie et al. 2009; Pardee and Philpott 2014; Ballare et al. 2019), indicating that nesting resource availability can be a critical factor structuring urban pollinator communities. In other words, our results suggest that urban gardeners may be able to promote pollinator abundance by reserving undisturbed patches of bare soils in their urban gardens.
Local, landscape, and pollinator impacts on pollination
For our open-pollination treatments, pollinator richness was associated with increased seed set, a relationship that is well-documented in previous literature. A diverse community may enhance ecosystem services through functional complementarity (Loreau and Hector 2001, Finke and Snyder 2008, Cardinale et al. 2011), which has been found for pollinator diversity and plant reproduction in rural (Fründ et al. 2013a; Mallinger and Gratton 2014; Martins et al. 2015) and urban (Lowenstein et al. 2015) agricultural systems. This is because diverse pollinator communities exhibit species-specific spatial and temporal variation in floral foraging traits and behavior that likely influence plant reproduction (Hoehn et al. 2008; Pisanty et al. 2015). From the plant’s viewpoint, temporal and environmental variation in pollinator species’ morphology (Larsen et al. 2005; Greenleaf et al. 2007) and behavior (Blüthgen and Klein 2011; Rader et al. 2011; Martins et al. 2015) contributes to complementary effects on pollination. The importance of functional complementary has been documented for pollination in urban systems comprised of specialist and generalist pollinators (Cane et al. 2006; Pauw 2007).
Consistent with previous studies in natural (Blaauw and Isaacs 2014b) and urban systems (Potter and LeBuhn 2015; Davis et al. 2017), our study indicates that pollination function is strongly impacted by floral resources in the habitat. We found that the relationship between pollinator richness and seed set became steeper in gardens with high floral abundance. This may be due to impacts of floral resource availability on pollinator foraging behavior. Floral quantity, structure, and diversity at local scales can concentrate pollinator activity (Veddeler et al. 2006) and can influence plant constancy (Kunin and Iwasa 1996), preference (Hambäck 2001), and pollen deposition (Lortie and Aarssen 1999). One methodological limitation to this study is that we relied on visual counts to sample pollinators, which may alter our ability to compare our results with other collection-based pollination studies. For example, Quistberg et al. 2016 collected pollinators with pan traps and netting and reported 55 unique species in this system, whereas we only identified 22 morphospecies, likely because visual surveys often miss rare species (Bosch et al. 2009). However, past studies have found that visual surveys are representative of the pollinator community and can be useful methods when destructive sampling is not possible (Mandelik et al. 2012). Further, past studies have often focused on bees as key pollinators, and while pepper flowers are visited by a wide range of insects, they do benefit from buzz pollination provided by bees (Raw 2000). We therefore modeled the impact of bees on crop seed number and found similar patterns (Table S2). Another caveat to our findings is that study does not distinguish between floral visitors and pollination visits (King et al. 2013), as we do not measure pollen loads or pollen deposition. This may be important because landscape composition and agricultural management can impact pollinator foraging behavior (Moreirra et al. 2015) and may impact resulting pollen deposition (Werrell et al. 2009). However, the benefit of observing all floral visitors, regardless of pollen transfer, is that the diversity of these interaction webs is known to enhance the persistence of plant communities (Fontaine et al. 2005) and inter-specific, indirect interactions have been shown to promote pollination of crops (Greenleaf and Kremen 2006).
While we found that the impact of pollinator richness on seed set becomes stronger in floral-abundant habitats, we found that the impact of pollinator abundance on seed set becomes weaker. This may indicate a per-plant dilution effect in which pollinator densities at flowers decrease in resource-dense patches. This has been previously observed in a prairie system when flower density was increased (Wenninger et al. 2016), possibly due to competition between pollinator individuals in abundant communities of pollinators. The underlying mechanism for why high pollinator abundance without species richness leads to greater competition is likely the same mechanism that promotes co-existence within all communities: intraspecific competition is assumed to be greater than interspecific competition (reviewed in Chesson 2000). Competition might translate into negative effects on plant reproduction if the energetic costs of foraging are minimized by avoiding competitive interactions at floral sites (Charnov 1976). Additionally, high resource density patches might provide fewer resources for abundant foragers due to saturation effects (Totland and Matthews 1998; Andersson et al. 2013), wherein floral resources attract an abundance of pollinators but there are not enough resources if they all rely on the same pollen and nectar types. Thus, effects to pollination should be more pronounced in low-diversity pollinator communities. While some research has suggested that the presence of dilution effects depends on pollinator species identity and their behavior (Jha and Vandermeer 2009), further research is required to examine how species-specific pollinator foraging traits differentially mediate pollination outcomes across a gradient of resource landscapes.
Our finding that increasing floral resources only mediates plant reproductive success when pollinator richness is high is pertinent because urbanization is associated with increased homogenization of pollinator communities (Deguines et al. 2016; Harrison et al. 2018; Fitch et al. 2019; Wilson and Jamieson 2019). In rural agricultural systems, Kleijn et al. found that a few abundant species provide the bulk of pollination services (Kleijn et al. 2015), but our findings suggest that the importance of pollinator richness likely varies between urban systems and rural agricultural systems, possibly because resource pulses in urban systems are not predictably constrained to seasonable mass-bloom events. Within urban gardens, past studies have shown that pollinator species richness is mostly driven by floral diversity (Plascencia and Philpott 2017), but we did not find this. More research is needed on which species of flowers are most important for bees; Lowenstein et al. found that the composition and identity of flowering resources may be more important than floral abundance for predicting plant-pollinator interactions (2019). Mechanistically, many pollinators require pollen and nectar for calories (Tepedino and Parker 1982) and a greater diversity of pollen has been linked to enhanced pollinator growth rates (Tasei and Aupinel 2008) and pollinator diversity (Petanidou and Vokou 1990). Taken together, these studies indicate that efforts to provision for pollinators with floral plantings will have the greatest impacts on seed set if they also promote species-rich pollinator communities (rather than being dominated by a few, abundant species).
We observed a positive influence of woody plant abundance on pollination function. Many trees and shrubs planted in urban gardens are fruit-producing with blooms that provide nectar for a diversity of flies, wasps, and bees (Somme et al. 2016). Furthermore, trees and shrubs have been shown to influence bee abundance and richness in past studies (Jha and Vandermeer 2010; Pardee and Philpott 2014), and their abundance in an agroecosystem is often predictive of crop reproductive success (Garibaldi et al. 2013). We therefore were surprised not to find a relationship between woody plants and pollinator richness or abundance. There may be other indirect mechanisms by which trees and shrubs positively impact pollination, for example, by modifying local microclimate (Kilkenny and Galloway 2007). In this system, woody plant availability was colinear with garden size, which Werrell et al. previously found to be important for con-specific pollen deposition on experimental cucumber crops in urban gardens (2009). The availability of woody plants may also influence pollinator behavior (Klein et al. 2004; Williams 2011) and increase overall pollen collection (Dyer et al. 2011). Overall, our study highlights the importance of local habitat conditions when managing for optimal ecosystem function. One possible application of our findings is the suggestion that gardeners plan for a combination of perennial woody plants and annual herbaceous plant species in the garden.
We found that the amount of natural habitat surrounding a garden had a negative effect on seed number. While we had hypothesized that natural cover would enhance beneficial insect movement between crop and non-crop habitat, as seen in many rural landscapes (e.g., Chaplin-Kramer et al. 2011; Klein et al. 2012; Cusser et al. 2016), we did not find this pattern, likely because of our crop selection and the urban landscape. First, our focal crop, the C. annuum pepper, is not found in natural habitat fragments, so pollinators recently visiting natural habitat are not likely to carry conspecific pollen. Second, we posit that pollinators in urban landscapes may not always be moving from natural habitat into gardens. Third, given the challenges of traversing private property surrounding the gardens, we could not ground-truth to verify the landcover data for the region. Indeed, past studies in our study system have found that natural habitat cover does not have the expected positive impact on pollinator abundance and richness (Quistberg et al. 2016) or pest-control provision (Egerer et al. 2017). These findings corroborate those of Gaines-Day and Gratton (2016), which indicate that wild bees may prefer to remain locally when surrounded by less hospitable landscapes. In another study of urban gardens in New York City, Matteson & Langellotto found that 45% of marked bumble bee individuals were later collected in the gardens where they had initially been documented, indicating that bumble bees, otherwise long-distance foragers, may largely forage within a single garden in urban areas (Matteson and Langellotto 2009). Based on the findings and other similar intensely managed landscapes, it is possible that within-garden habitat features may play a disproportionally large role in mediating pollinator foraging and nesting patterns in urban landscapes.
Greenhouse and field pollination experiments
For both the greenhouse and field-based experiments, jalapeño seed number was significantly higher in open-outcross treatments than in open treatments or closed treatments. This finding highlights the importance of insect pollinators for enhancing the reproduction of crops like jalapeño peppers, however, gardeners likely benefit more from increased fruit weight than seed number. We found that fruit weight followed similar patterns but was not significantly different between treatments, possibly because fruit is largely comprised of maternal tissue and can reach maximum weight in peppers even when seed number is low (Marcelis 1997). Furthermore, to asses fruit weight we measured wet weight, not dry weight. While plants were watered in a standardized manner under homogenous greenhouse conditions, it is possible that slight differences in watering could influence fruit weight data. We found that the fruit weight and seed number of plants grown in greenhouse conditions (across all treatments) were higher than those grown in the field, perhaps due to the optimal climate conditions provided by greenhouses for peppers (Bakker 1989; Shaked et al. 2004; Pagamas and Nawata 2008).
Conclusions
Animal-mediated pollination is critical for the majority of flowering wild plant species (Ollerton et al. 2011) and within agricultural systems, where more than 75% of domesticated crops show increases to fruit or seed set (Klein et al. 2006). In this study, we add to the body of literature suggesting that insect pollination is an essential part of successful plant reproduction in urban systems (Verboven et al. 2014, Leong et al. 2014, Lowenstein et al. 2015, Potter and LeBuhn 2015). Furthermore, while resource-driven foraging interactions have been documented to impact pollinator visitation in simulated models (Essenberg 2012), grasslands (Totland and Matthews 1998floodplains (Ebeling et al. 2008), and rural agricultural systems (Veddeler et al. 2006; Jha and Vandermeer 2009; Evans et al. 2017; Xie et al. 2019), our study is the first to document this interaction mediating plant reproduction within urban landscapes. Specifically, we show that when urban garden floral density is high, increasing pollinator richness (and not pollinator abundance) increases crop reproductive success. In other words, our study indicates that local pollinator foraging interactions can significantly influence ecosystem service provision. To achieve optimal pollination services in their urban gardens, more research is needed to identify which practices optimize for both pollinator richness and floral resource availability.
References
Albrecht M, Ramis MR, Traveset A (2016) Pollinator-mediated impacts of alien invasive plants on the pollination of native plants: the role of spatial scale and distinct behaviour among pollinator guilds. Biol Invasions 18:1801–1812. https://doi.org/10.1007/s10530-016-1121-6
Albrecht M, Schmid B, Hautier Y, Müller CB (2012) Diverse pollinator communities enhance plant reproductive success. P Roy Soc B: Biol Sci 279:4845–4852
Aleemullah M, Haigh AM, Holford P (2000) Anthesis, anther dehiscence, pistil receptivity and fruit development in the Longum group of Capsicum annuum. Australian J Exp Ag 40:755–762
Andersson P, Lӧfstedt C, Hämback PA (2013) Insect density relationships: a modified view of insect responses to resource concentrations. Oecologia 173:1333–1344. https://doi.org/10.1007/s00442-013-2737-1
Ascher JS, Pickering J (2017) Discover life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila)
Baker LE (2004) Tending cultural landscapes and food citizenship in Toronto’s community gardens. Geogr Rev 94:305–325
Bakker JC (1989) The effects of temperature on flowering, fruit set and fruit development of glasshouse sweet pepper (Capsicum annuum L.). J Hortic Sci 64:313–320. https://doi.org/10.1080/14620316.1989.11515959
Ballare KM, Neff JL, Ruppel R, Jha S (2019) Multi-scalar drivers of biodiversity: local management mediates wild bee community response to regional urbanization. Ecol Appl 29:e01869. https://doi.org/10.1002/eap.1869
Balvanera P, Pfisterer AB, Buchmann N et al (2006) Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett 9:1146–1156. https://doi.org/10.1111/j.1461-0248.2006.00963.x
Bates D, Maechler M, Bolker B, et al (2015) Package ‘lme4’. Convergence 12
Bellows AC, Brown K, Smit J, et. al (2003) Health benefits of urban agriculture. Community Food 1–8
Benjamin F, Reilly RJ, Winfree R (2014) Pollinator body size mediates the scale at which land use drives crop pollination services. J Appl Ecol 51:440–449 10.1111.1365-2665.12198
Bennett AB, Lovell S (2019) Landscape and local site variables differentially influence pollinators and pollination services in urban agriculture sites. PLoS One 14(2). https://doi.org/10.1371/journal.pone/0212034
Blaauw BR, Isaacs R (2014a) Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J Appl Ecol 51:890–898. https://doi.org/10.1111/1365-2664.12257
Blaauw BR, Isaacs R (2014b) Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl Ecol 15:701–711
Blüthgen N, Klein A-M (2011) Functional complementarity and specialisation: the role of biodiversity in plant--pollinator interactions. Basic Appl Ecol 12:282–291
Bosch J, Martín González AM, Rodrigo A, Navarro D (2009) Plant–pollinator networks: adding the pollinator’s perspective. Ecol letters 12(5):409–419. https://doi.org/10.1111/j.1461-0248.2009.01296.x
Bosland PW, Votava EJ (2000) Taxonomy, pod types and genetic resources. Peppers: vegetable and spice capsicums Crop Production Sci Hort 14–38
Burkle LA, Alarcon R (2011) The future of plant-pollinator diversity: understanding interaction networks across time, space, and global change. Am J Bot 98:528–538. https://doi.org/10.3732/ajb.1000391
Burr A, Schaeg N, Muñiz P et al (2016) Wild bees in the city: reimagining urban spaces for native bee health. Consilience:106–131
Butchart SHM, Walpole M, Collen B et al (2010) Global biodiversity: indicators of recent declines. Science (80- ) 328:1164–1168. https://doi.org/10.1126/science.1187512
Cadotte MW, Carscadden K, Mirotchnick N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48:1079–1087
Cane JH, Minckley RL, Kervin LJ, Roulston TAH, Williams NM (2006) Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Eco App 16: 632–644. 10/1098/rspb.2006.3721
Cardinale BJ, Matulich KL, Hooper DU et al. (2011) The functional role of producer diversity in ecosystems. Am J Bot 98:572–592. https://doi.org/10.3732/ajb.1000364
Cardinale BJ, Duffy JE, Gonzalez A et al (2012) Biodiversity loss and its impact on humanity. Nature 486:59–67. https://doi.org/10.1038/nature11148
Cardinale BJ, Ives AR, Inchausti P (2004) Effects of species diversity on the primary productivity of ecosystems: extending our spatial and temporal scales of inference. Oikos 104:437–450
Cardinale BJ, Srivastava DS, Emmett Duffy J et al (2006) Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443:989–992
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scandinavian J Stat 11:265–270
Chao A (2006) Species estimation and applications. Encycl Stat Sci. https://doi.org/10.1002/0471667196.ess5051
Chaplin-Kramer R, Tuxen-Bettman K, Kremen C (2011) Value of Wildland habitat for supplying pollination services to Californian agriculture. Rangelands 33:33–41
Charnov EL (1976) Optimal foraging, the marginal value theorem. Theor Popul Biol 9:129–136
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366. https://doi.org/10.1146/annurev/ecolsys.31.343
Cohen H, Quistberg RD, Philpott SM (2017) Vegetation management and host density influence bee--parasite interactions in urban gardens. Environ Entomol 46:1313–1321. https://doi.org/10.1093/ee/nvx155
Cusser S, Neff JL, Jha S (2016) Natural land cover drives pollinator abundance and richness, leading to reductions in pollen limitation in cotton agroecosystems. Agric Ecosyst Environ 226:33–42. https://doi.org/10.1016/j.agee.2016.04.020
Davis AY, Lonsdorf EV, Shierk CR et al (2017) Enhancing pollination supply in an urban ecosystem through landscape modifications. Landsc Urban Plan 162:157–166. https://doi.org/10.1016/j.landurbplan.2017.02.011
Deguines N, Julliard R, de Flores M, Fontaine C (2016) Functional homogenization of flower visitor communities with urbanization. Ecol Evol 6:1967–1976
Delaplane KS, Mayer DF (2000) Crop pollination by bees. Cabi Publishing
Dobson AJ, Barnett A (2008) An introduction to generalized linear models. CRC press
Dyer RJ, Chan DM, Gardiakos VA, Meadows CA (2011) Pollination graphs: quantifying pollen pool covariance networks and the influence of intervening landscape on genetic connectivity in the north American understory tree, Cornus florida L. Landsc Ecol 27:239–251. https://doi.org/10.1007/s10980-011-9696-x
Ebeling A, Klein A-M, Schumacher J et al (2008) How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 117:1808–1815. https://doi.org/10.1093/jue/jux001
Egerer MH, Arel C, Otoshi MD et al (2017) Urban arthropods respond variably to changes in landscape context and spatial scale. J Urban Ecol 3
Essenberg CJ (2012) Explaining variation in the effect of floral density on pollinator visitation. Am Nat 180:153–166
Evans TM, Cavers S, Ennos R et al (2017) Florally rich habitats reduce insect pollination and the reproductive success of isolated plants. Ecol Evol 7:6507–6518. https://doi.org/10.1002/ece3.3186
Fetridge ED, Ascher JS, Langellotto GA (2008) The bee fauna of residential gardens in a suburb of new York City (Hymenoptera: Apoidea). Ann Entomol Soc Am 101:1067–1077. https://doi.org/10.1603/0013-8746-101.6.1067
Finke DL, Snyder WE (2008) Niche partitioning increases resource exploitation by diverse communities. Science (80) 321:1488–1490. https://doi.org/10.1126/science.1160854
Fitch G, Wilson CJ, Glaum P, Vaidya C, Simao MC, Jamieson MA (2019) Does urbanization favour exotic bee species? Implications for the conservation of native bees in cities. Biol Lett 15(12):20190574. https://doi.org/10.1098/rsbl.2019.0574
Fontaine C, Collin CL, Dajoz I (2008) Generalist foraging of pollinators: diet expansion at high density. J Ecol 96:1002–1010. https://doi.org/10.1111/j.1365-2745.2008.01405.x
Fontaine C, Dajoz I, Merguet J, Loreau M (2005) Functional diversity of plant-pollinator interaction webs enhance the persistence of plant communities. PLoS Biol 4. https://doi.org/10.1371/journal.pbio.00400001
Fox J, Weisberg S (2018) Using car functions in other functions. Cran R
Frankie GW, Thorp RW, Hernandez J et al (2009) Native bees are a rich natural resource in urban California gardens. Calif Agric 63:113–120. https://doi.org/10.3733/ca.v063n03p113
Freeman C, Dickinson KJM, Porter S, van Heezik Y (2012) “my garden is an expression of me”: exploring householders’ relationships with their gardens. J Environ Psychol 32:135–143. https://doi.org/10.1016/j.jenvp.2012.01.005
Fründ J, Dormann CF, Holzschuh A, Tscharntke T (2013b) Bee diversity effects on pollination depend on functional complementarity and niche shifts. Ecology 94:2042–2054. https://doi.org/10.1890/12-1620.1
Gaines-Day HR, Gratton C (2016) Crop yield is correlated with honey bee hive density but not in high-woodland landscapes. Agric Ecosyst Environ 218:53–57. https://doi.org/10.1016/j.agee.2015.11.001
Gagic V, Bartomeus I, Jonsson T et al (2015) Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc R Soc B Biol Sci 282:20142620. https://doi.org/10.1098/rspb.2014.2620
Garibaldi LA, Carvalheiro LG, Vaissière BE, Gemmill-Herren B, Hipólito J, Freitas BM, Ngo HT, Azzu N, Sáez A, Åström J (2016) Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms. Science 6271:388–391 10.1126.science.aac7287
Garibaldi LA, Steffan-Dewenter I, Winfree R et al (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339:1608–1611. https://doi.org/10.1126/science.1230200
Ghazoul J (2006) Floral diversity and the facilitation of pollination. J Ecol 94:295–304
Gibbs J (2010) Revision of the metallic species of Lasioglossum (Dialictus) in Canada (Hymenoptera, Halictidae, Halictini). Zootaxa. 2591(1):1–382
Glaum P, Simao MC, Vaidya C, Fitch G, Iulinao B (2017) Big city Bombus: using natural history and land-use history to find significant environmental drivers in bumble-bee declines in urban development. Royal Soc Open Sci 4(5):170156. https://doi.org/10.1098/rsos/170156
Goddard MA, Dougill AJ, Benton TG (2013) Why garden for wildlife? Social and ecological drivers, motivations and barriers for biodiversity management in residential landscapes. Ecol Econ 86:258–273. https://doi.org/10.1016/j.ecolecon.2012.07.016
Gómez JM, Bosch J, Perfectti F et al (2007) Pollinator diversity affects plant reproduction and recruitment: the tradeoffs of generalization. Oecologia 153:597–605. https://doi.org/10.1007/s00442-007-0758-3
Goulson D, Nicholls E, Botias C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science (80- ) 347:1255957. https://doi.org/10.1126/science.1255957
Greenleaf SS, Kremen C (2006) Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc Natl Acad Sci 103:13890–13895. https://doi.org/10.1073/pnas.0600929103
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153:589–596
Hambäck PA (2001) Direct and indirect effects of herbivory: feeding by spittlebugs affects pollinator visitation rates and seedset of Rudbeckia hirta. Écoscience 8:45–50
Harrison T, Gibbs J, Winfree R (2018) Phylogenetic homogenization of bee communities across ecoregions. Global Ecol and Biogeog 27:1457–1466 10.1111.geb.12822
Hoehn P, Tscharntke T, Tylianakis JM, Steffan-Dewenter I (2008) Functional group diversity of bee pollinators increases crop yield. Proc R Soc B Biol Sci 275:2283–2291. https://doi.org/10.1098/rspb.2008.0405
Holland JN, Chamberlain SA (2007) Ecological and evolutionary mechanisms for low seed: ovule rations: need for a pluralistic approach? Ecology 88:706–715. https://doi.org/10.1890/06-1283
Homer C, Dewitz J, Yang L et al (2015) Completion of the 2011 National Land Cover Database for the conterminous United States--representing a decade of land cover change information. Photogramm Eng Remote Sens 81:345–354
Hooper DU, Chapin FS, Ewel JJ et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35. https://doi.org/10.1890/04-0922
Jarland A, De Oliveira D, Gringas J (1997) Pollination by Eristali tenax (Diptera: Syrphidae) and seed set of greenhouse sweet pepper. J Econ Ent 6:1646–1649. https://doi.org/10.1093/jee/90.6.1646
Jha S, Vandermeer JH (2009) Contrasting bee foraging in response to resource scale and local habitat management. Oikos 118:1174–1180. https://doi.org/10.1111/j.1600-0706.2009.17523.x
Jha S, Vandermeer JH (2010) Impacts of coffee agroforestry management on tropical bee communities. Biol Conserv 143:1423–1431. https://doi.org/10.1016/j.biocon.2010.03.017
Kearns CA (2001) North American dipteran pollinators: assessing their value and conservation status. Cons Eco 5(1)
Kennedy CM, Lonsdorf E, Neel MC et al (2013) A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol Lett 16:584–599. https://doi.org/10.1111/ele.120
Kilkenny FF, Galloway LF (2007) Reproductive success in varying light environments: direct and indirect effects of light on plants and pollinators. Oecologia 155:247–255. https://doi.org/10.1007/s00442-007-0903-z
Kim J, Williams N, Kremen C (2006) Effects of cultivation and proximity to natural habitat on ground-nesting bative bees in California sunflower fields. J Kansas Entomol Soc 79:309–320. https://doi.org/10.2317/0507.11.1
King C, Ballantyne G, Willmer PG (2013) Why flower visitation is a poor proxy for pollination: measuring single-visit pollen deposition, with implications for pollination networks and conservation. Methods Ecol Evo 4(9):811-818. Xf
Kleijn D, Winfree R, Bartomeus I et al (2015) Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat Commun 6. https://doi.org/10.1038/ncomms8414
Klein A-M, Brittain C, Hendrix SD et al (2012) Wild pollination services to California almond rely on semi-natural habitat. J Appl Ecol. https://doi.org/10.1111/j.1365-2664.2012.02144.x
Klein A-M, Steffan-Dewenter I, Tscharntke T (2004) Foraging trip duration and density of megachilid bees, eumenid wasps and pompilid wasps in tropical agroforesty systems. J Anim Ecol 73:517–525
Klein A-M, Vaissière BE, Cane JH et al (2006) Importance of pollinators in changing landscapes for world crops. Proc R Soc B Biol Sci 274:303–313. https://doi.org/10.1098/rspb.2006.3721
Kremen C, Ullman KS, Thorp RW (2011) Evaluating the quality of citizen-scientist data on pollinator communities. Conserv Biol 25:607–617. https://doi.org/10.1111/j.1523-1739.2011.01657.x
Kremen C, Williams NM, Aizen MA et al (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314. https://doi.org/10.1111/j.1461-0248.2007.01018.x
Kremen C, Williams NM, Bugg RL et al (2004) The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecol Lett 7:1109–1119. https://doi.org/10.1111/j.1461-0248.2004.00662.x
Kunin WE (1993) Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology 74:2145–2160. https://doi.org/10.2307/1940859
Kunin W, Iwasa Y (1996) Pollinator foraging strategies in mixed floral arrays: density effects and floral Constancy. Theor Popul Biol 49:232–263. https://doi.org/10.1006/tpbi.1996.0013
Larsen TH, Williams NM, Kremen C (2005) Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol Lett 8:538–547. https://doi.org/10.1111/j/1461-0248.2005.00749.x
Leong M, Kremen C, Roderick GJ (2014) Pollinator interacitons with yellow starthistle (Centaurea solstitialis) across urban, agricultural, and natural landscapes. PLoS One 9:e86357. https://doi.org/10.1371/journal/pone.0086357
Loreau M, Hector A (2001) Partitioning selection and complementarity in biodiversity experiments. Nature 412:72–76. https://doi.org/10.1038/3509712
Loreau M, Naeem S, Inchausti P, et al (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science (80- ) 294:804–808. https://doi.org/10.1126/science.1064088
Lortie CJ, Aarssen LW (1999) The advantage of being tall: higher flowers receive more pollen in Verbascum thapsus L. (Scrophulariaceae). Écoscience 6:68–71
Lowenstein DM, Matteson KC, Minor ES (2015) Diversity of wild bees supports pollination services in an urbanized landscape. Oecologia 179:811–821. https://doi.org/10.1007/s00442-015-3389-0
Lowenstein DM, Matteson KC, Minor ES (2019) Evaluating the dependence of urban pollinators on ornamental, non-native, and “weedy” floral resources. Urban Ecosyst 22:293–302. https://doi.org/10.1007/s11252-018-0817-z
Maire V, Gross N, Börger L et al (2012) Habitat filtering and niche differentiation jointly explain species relative abundance within grassland communities along fertility and disturbance gradients. New Phytol 196:497–509. https://doi.org/10.1111/j.1469-8137.2012.04287.x
Mallinger RE, Gratton C (2014) Species richness of wild bees, but not the use of managed honeybees, increases fruit set of a pollinator-dependent crop. J Appl Ecol 52:323–330. https://doi.org/10.1111/1365-2664.12377
Mandelik Y, Dayan T, Chikatunov V, Kravchenko V (2012) The relative performance of taxonomic vs. environmental indicators for local biodiversity assessment: a comparative study. Ecol Indic 15:171–180. https://doi.org/10.1016/j.ecolind.2011.09.033
Marcelis L (1997) Effects of seed number on competition and dominance among fruits in Capsicum annuum L. Ann Bot 79:687–693. https://doi.org/10.1006/anbo.1997.0398
Martins KT, Gonzalez A, Lechowicz MJ (2015) Pollination services are mediated by bee functional diversity and landscape context. Agric Ecosyst Environ 200:12–20. https://doi.org/10.1016/j.agee.2014.10.018
Matteson KC, Ascher JS, Langellotto GA (2008) Bee richness and abundance in new York City urban gardens. Ann Entomol Soc Am 101:140–150. 10.1603/0013-8746(2008)101[140:braain]2.0.co;2
Matteson KC, Langellotto GA (2009) Bumble bee abundance in New York community gardns: implications for urban agriculture. CATE 2:5
Matteson KC, Langellotto GA (2010) Determinates of inner city butterfly and bee species richness. Urban Ecosyst 13:333–347. https://doi.org/10.1007/s11252-010-0122-y
McCormack LA, Laska MN, Larson NI, Story M (2010) Review of the nutritional implications of farmers’ markets and community gardens: a call for evaluation and research efforts. J Am Diet Assoc 110:399–408
Michener CD (2007) The bees of the world
Michener CD, McGinley RJ, Danforth BN (1994) The bee genera of north and Central America (Hymenoptera: Apoidea). Smithsonian Institution Press
Moreirra EF, Boscolo D, Viana BF (2015) Spatial heterogeneity regulates plant-pollinator networks across multiple landscape scales. PLoS One 10(4). https://doi.org/10.1371/journal/pone.0123628
Naeem S, Bunker DE, Hector A, et al (2009) Biodiversity, ecosystem functioning, and human wellbeing: an ecological and economic perspective. Oxford University Press
Naeem S, Wright JP (2003) Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecol Lett 6:567–579. https://doi.org/10.1046/j.1461-0248.2003.00471.x
Ofosu-Anim J, Offei Sk, Yamaki S (2006) Pistil receptivity, pollen tube growth and gene xpressoin during early fruit development in sweet pepper (Capsicum annuum). Intl J Ag Bio
Oksanen J, Blanchet FG, Kindt R et al (2016) Vegan: community ecology package. R package version:24–21
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326. https://doi.org/10.1111/j.1600-0706.2010.18644.x
Otoshi MD, Bichier P, Philpott SM (2015) Local and landscape correlates of spider activity density and species richness in urban gardens. Environ Entomol 44:1043–1051. https://doi.org/10.1093/ee/nvv098
Pagamas P, Nawata E (2008) Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Sci Hortic (Amsterdam) 117:21–25. https://doi.org/10.1016/j.scienta.2008.03.017
Pardee GL, Philpott SM (2014) Native plants are the bee’s knees: local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosyst 17:641–659. https://doi.org/10.1007/s11252-014-0349-0
Pauw A (2007) Collapse of a pollination web in small conservation areas. Ecology 88:1759–1769. https://doi.org/10.1890/06-1383.1
Petanidou T, Vokou D (1990) Pollination and pollen energetics in mediterranean ecosystems. Am J Bot 77:986–992. https://doi.org/10.1002/j.1537-2197.1990.tb13593.x
Pickersgill B (1997) Genetic resources and breeding of Capsicum spp. Euphytica 96:129–133
Pisanty G, Afik O, Wajnberg E, Mandelik Y (2015) Watermelon pollinators exhibit complementarity in both visitation rate and single-visit pollination efficiency. J Appl Ecol 53:360–370 10.1111.1365-2664.12574
Plascencia M, Philpott SM (2017) Floral abundance, richness, and spatial distribution drive urban garden bee communities. Bull Ent Res 107:658–667. https://doi.org/10.1017/S0007485417000153
Potter A, LeBuhn G (2015) Pollination service to urban agriculture in San Francisco, CA. Urban Ecosyst 18:885–893. https://doi.org/10.1007/s11252-015-0435-y
Potts SG, Biesmeijer JC, Kremen C et al (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Primack RB, Inouye DW (1993) Factors affecting pollinator visitation rates: a biogeographic comparison. Curren Sci 10:257–262
Quistberg RD, Bichier P, Philpott SM (2016) Landscape and local correlates of bee abundance and species richness in urban gardens. Environ Entomol 45:592–601
Rader R, Howlett BG, Cunningham SA et al. (2009) Alternative pollinator taxa are equally efficient but not as effective as honey bees in a mass flowering crop. J Appl Ecol 46:1080–1087. https://doi.org/10.1111/j.1365-2664.2009.01700.x
Rader R, Howlett BG, Cunningham SA et al (2011) Spatial and temporal variation in pollinator effectiveness: do unmanaged insects provide consistent pollination services to mass flowering crops? J Appl Ecol 49:126–134 https://doi.org/10.1111/j.1365-2664.2011.02066.x
Raw A (2000) Foraging behaviour of wild bees at hot pepper flowers (Capsicum annuum) and its possible influence on cross pollination. Ann Bot 85:487–492. https://doi.org/10.1006/anbo.1999.1090
Riedinger V, Renner M, Rundlöf M et al (2013) Early mass-flowering crops mitigate pollinator dilution in late-flowering crops. Landsc Ecol 29:425–435. https://doi.org/10.1007/s10980-013-9973
Ripley B, Venables B, Bates DM, et al (2013) Package ‘mass’. Cran R
Ritchie AD, Ruppel R, Jha S (2016) Generalist behavior describes pollen foraging for perceived oligolectic and polylectic bees. Environ Entomol 45:909–919. https://doi.org/10.1093/ee/nvw032
Root RB, Kareiva PM (1984) The search for resources by cabbage butterflies (Pieris Rapae): ecological consequences and adaptive significance of Markovian movements in a patchy environment. Ecology 65:147–165
Schleuning M, Fründ J, García D (2014) Predicting ecosystem functions from biodiversity and mutualistic networks: an extension of trait-based concepts to plant-animal interactions. Ecography 38:380–392
Shaked R, Rosenfeld K, Pressman E (2004) The effect of low night temperatures on carbohydrates metabolism in developing pollen grains of pepper in relation to their number and functioning. Sci Hortic (Amsterdam) 102:29–36. https://doi.org/10.1016/j.scienta.2003.12.007
Sih A, Baltus M-S (1987) Patch size, pollinator behavior, and pollinator limitation in catnip. Ecology 68:1679–1690
Somme L, Moquet L, Quinet M et al (2016) Food in a row: urban trees offer valuable floral resources to pollinating insects. Urban Ecosyst 19:1149–1161. https://doi.org/10.1007/s11252-016-0555-z
Steffan-Dewenter J, Westphal C (2008) The interplay of pollinator diversity, pollination services and landscape change. J App Eco 45(3):737–741 10.1111.j.1365-2664.2008.01483.x
Tasei JN, Aupinel P (2008) Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumble bee works (Bombus terrestris, Hymenoptera: Apidae) Apidologi 39:397-409. https://doi.org/10.1051/apido:200817
Tepedino VJ, Parker FD (1982) Interspecific differences in the relative importance of pollen and nectar to bee species foraging on sunflowers. Environ Entomol 11:246–250. https://doi.org/10.1093/ee/11.1.246
Tittensor DP, Walpole M, Hill SLL, et al (2014) A mid-term analysis of progress toward international biodiversity targets. Science (80- ) 346:241–244. https://doi.org/10.1126/science.1257484
Totland Ø, Matthews I (1998) Determinants of pollinator activity and flower preference in the early spring blooming Crocus vernus. Acta Oecol 19:155–165
Tscharntke T, Klein AM, Kruess A et al (2005) Landscape perspectives on agricultural intensification and biodiversity -- ecosystem service management. Ecol Lett 8:857–874. https://doi.org/10.1111/j.1461-0248.2005.00782.x
Tscharntke T, Tylianakis JM, Rand TA et al (2012) Landscape moderation of biodiversity patterns and processes - eight hypotheses. Biol Rev 87:661–685. https://doi.org/10.1111/j.1469-185X.2011.00216.x
Tylianakis JM, Rand TA, Kahmen A et al (2008) Resource heterogeneity moderates the biodiversity-function relationship in real world ecosystems. PLoS Biol 6:e122
Van Nuland ME, Haag EN, Bryant JA, Read QD, Klein RN, Douglas MJ, Gorman CE, Greenwell TD, Busby MW, Collins J, LeRoy JT (2013) Fire promotes pollinator visitation: implications for ameliorating declines of pollination services. PLoS One 12:e79853
Vargas RI, Mitchell S (1987) Two artificial larval diets for rearing Dacus latifrons (Diptera: Tephritidae). J Econ Entomol 80:1337–1339. https://doi.org/10.1111/j.0030-1299.2006.14111.x
Veddeler D, Klein A-M, Tscharntke T (2006) Contrasting responses of bee communities to coffee flowering at different spatial scales. Oikos 112:594–601
Verboven HAF, Wim A, Brys R, Hermy M (2014) Pollinatino and seed set of an obligatory outcrossing plant in an urban-peri-urban gradient. Perspect Plan Ecol 16:121–131. https://doi.org/10.1016/j.ppees.2014.03.002
Werrell PA, Langellotto GA, Morath SU, Matteson K (2009) The influence of garden size and floral cover on pollen deposition in urban community gardens. CATE 2(1):6. https://doi.org/10.15365/cate.2162009
Wenninger A, Kim TA, Spiesman BJ, Gratton C (2016) Contrasting foraging patterns: testing resource-concentration and dilution effects with pollinators and seed predators. Insects 72:23. https://doi.org/10.3390/insects7020023
Wilcock C, Neiland R (2002) Pollination failure in plants: why it happens and when it matters. Trends Plant Sci 7:270–277
Williams NM (2011) Restoration of nontarget species: bee communities and pollination function in riparian forests. Restor Ecol 19:450–459. https://doi.org/10.1111/j.1526-100X.2010.00707.x
Williams NM, Ward KL, Pope N et al (2015) Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol Appl 25:2119–2131. https://doi.org/10.1890/14-1748.1
Wilson CJ, Jamieson MA (2019) The effects of urbanization on bee communities depends on floral resource availability and bee functional traits. PLoS One 14(12). https://doi.org/10.1371/journal.pone.0225852
Xie Z, Wang J, Pan D, An J (2019) Landscape-modified concentration effect and waylaying effect of bees and their consequences on pollination of mass-flowering plants in agricultural ecosystems. Agric Ecosyst Environ 280:24–34. https://doi.org/10.1016/j.agee.2019.04.023
Yamamura K (1999) Relation between plant density and arthropod density in cabbage fields. Popul Ecol 41:177–182
Acknowledgements
P. Bichier, Z. Jordon, M. Macdonald, C. Kirk, D. Hafalia-Yackel, A. Rubio, and M. Egerer participated in logistics, greenhouse work, and field data collection. Thank you to J. Velzy, M. Dillingham and other greenhouse staff at University of California, Santa Cruz for support with choosing plant varieties, plant growing, and equipment. Special thank you to the community gardens for allowing us to conduct research: Goodwill Community Garden, Obama Way Garden, Pacific Grove Garden, Mid-County Senior Center in Capitola, Green Thumb Garden, Aptos Community Garden, Beach Flats Community Garden, Berryessa Community Garden, Center for Agroecology and Sustainable Food Systems, Chinatown Community Garden, Coyote Creek Community Garden, El Jardín at Emma Prusch Park, The Forge at Santa Clara University, Giving Garden at Faith Lutheran Church, Homeless Garden Project, La Colina Community Garden, Laguna Seca Community Garden, The Live Oak Grange, MEarth at Carmel Valley Middle School, Mi Jardín Verde at All Saints’ Episcopal Church, Our Green Thumb Garden at Monterey Institute for International Studies, Salinas Community Garden at St. George’s Episcopal Church, Trescony Community Garden.
Funding
Funding for this project came from the National Institute of Food and Agriculture, United States Department of Agriculture [grant 2016–67,019-25,185] to SMP, SJ, HL, and BBL.
Author information
Authors and Affiliations
Contributions
HC participated in study design, participated in fieldwork, conducted data analysis, and coordinated manuscript writing and publication. SMP acquired funding for the project, participated in study design, fieldwork logistics, and manuscript writing. BBL and HL acquired funding for the project, participated in study design, and manuscript writing. SJ acquired funding for the project, coordinated the study and participated in study design, field work, data analysis, and manuscript writing and publication. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Declaration of interest
none.
Electronic supplementary material
ESM 1
(DOCX 90 kb)
Rights and permissions
About this article
Cite this article
Cohen, H., Philpott, S.M., Liere, H. et al. The relationship between pollinator community and pollination services is mediated by floral abundance in urban landscapes. Urban Ecosyst 24, 275–290 (2021). https://doi.org/10.1007/s11252-020-01024-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-01024-z