Abstract
This study evaluated the pregnancy rate (PR) after timed artificial insemination (TAI) in water buffalo (Bubalus bubalis) during both non-breeding and breeding season, using either a new or reused intravaginal device (IVD) with two different progesterone concentrations. A total of 247 dairy buffalo cows were randomly assigned using a two-by-three factorial design and four replicates to the following groups: (1) new intravaginal device (IVD-New: DIB®, 1.0 g of P4, n = 51 or CIDR®, 1.38 g of P4, n = 55); (2) intravaginal device previously used once (9 days) (IVD-Used1x: DIB, n = 40 or CIDR, n = 51); or (3) intravaginal device previously used twice (18 days) (IVD-Used2x: DIB, n = 27 or CIDR, n = 23). On day 0, animals received the IVD plus 10.5 μg of buserelin acetate (GnRH) intramuscularly. On day 9, the devices were removed and 25 mg of PGF2α plus 500 IU of eCG was given intramuscularly. On day 11 (48 h after IVD withdrawal), animals received 10.5 μg of GnRH and were artificially inseminated 8–12 h later. Data were analyzed using Proc Logistic of SAS®. Animals that received IVD-New-DIB, had a significantly higher PR (62.7%; P = 0.0193) compared to animals that received IVD-New-CIDR (40%). Pregnancy rate was not negatively affected by reusing both types of IVD. Overall PR (new and reused devices) was higher (P = 0.0055) in the DIB group (62.7%) compared to the CIDR group (45%). In conclusion, PR was higher in buffaloes treated with devices containing 1.0 g of P4 (DIB®) compared to those receiving 1.38 g of P4 (CIDR®). Reusing the intravaginal devices did not affect negatively PR/TAI, suggesting that P4 concentrations within the TAI protocols in water buffaloes could be reduced, without impairing their fertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovulation synchronization and timed artificial insemination (TAI) protocols in water buffalo have the advantage of eliminating the need of heat detection, which is characterized by low efficiency due to poor estrus behavior compared to cattle (Perera 2008). Furthermore, TAI can be used as a tool to improve cyclicity and reproductive performance of buffalo cows, especially during seasonal anestrus. These hormonal protocols are aimed to control follicular development and luteal function, and synchronize the ovulation, allowing TAI without the need of heat detection (Carvalho et al. 2016; Monteiro et al. 2016).
Protocols used during both breeding and non-breeding seasons in buffaloes are based on the use of devices impregnated with progesterone (P4), combined with GnRH (De Rensis et al. 2005) or Estradiol (E2) (Baruselli et al. 2003; Carvalho et al. 2014; Monteiro et al. 2016). Most of these TAI programs have derived from adaptations of the hormonal protocols used in cattle, based on their follicular wave similarities (Baruselli et al. 1997; Perera 2008; Presicce et al. 2005), and hormonal profiles during the estrous cycle (Presicce 2007).
In cows, it has been reported that higher P4 concentrations during TAI protocols could compromise follicular development (Carvalho et al. 2008), ovulation, and pregnancy rates (Dadarwal et al. 2013). Progesterone concentrations during the buffalo cow’s estrous cycle are similar to those in cattle (Presicce 2007). However, lower peak of P4 has been described in buffaloes compared to cows (Perera 2011). This evidence suggests potential negative effects of high P4 concentration in the IVD on the response to ovulation synchronization and TAI in buffaloes. Studies in Mediterranean and Murrah water buffaloes, synchronized with hormonal protocols based on IVDs containing high P4 concentration (PRID®: 1.55 g and CIDR®: 1.38 g), GnRH, and PGF2α, reported lower pregnancy rates (De Rensis et al. 2005; Murugavel et al. 2009; Neglia et al. 2003) than those observed in buffaloes treated with IVDs containing lower (1.0 g) P4 concentrations (Carvalho et al. 2016; Monteiro et al. 2016). Moreover, Carvalho et al. (2014) suggested that lower circulating P4 concentrations (~1 ng/ml) may be adequate to promote normal ovarian follicular growth, ovulation, and pregnancy in buffaloes treated with TAI protocols based on P4 IVD during seasonal anestrus. This suggestion is supported by the fact that buffalo cows treated with IVD containing low P4 concentration (1.0 g) or a reused IVD (once and twice) were able to maintain adequate levels of circulating P4 (~1 ng/ml), without detrimental effects on pregnancy rate (Carvalho et al. 2016; Monteiro et al. 2016).
We hypothesized that higher P4 concentration contained in the IVD could reduce the pregnancy rates in water buffalo cows submitted to ovulation synchronization and TAI protocols. Thus, the aim of the present study was to compare the pregnancy rate after TAI in water buffalo (Bubalus bubalis) mature cows, using new or reused commercial IVD containing two different progesterone concentrations (DIB®: 1.0 g of P4 vs. CIDR®: 1.38 g of P4).
Materials and methods
Animals and management
The study was developed in a commercial water buffalo dairy farm located in the humid rainforest of Venezuela (N: 9° 0′ 35.93″, W: 71° 53′ 25.74″; AMSL: 4 m) during both the non-breeding (from February to June) and breeding seasons (from October to December). A total of 247 multiparous water buffalo cows (1 to 5 calvings), with body condition score between 3 and 3.5 (scale 1 to 5; where 1 = too thin and 5 = too fat; Alapati et al. 2010), were enrolled in this trial in four replicates (two in the non-breeding and two in the breeding season). Animals grazed Tanner grass (Brachiaria arrecta) with free access to water and supplemented with a mineral mix ad libitum. Only animals without apparent anatomical anomalies of the reproductive tract (evaluated by ultrasonography per rectum) were included. Data of days post-partum, body condition score (BCS), and parity were recorded on the first day of the TAI protocol (day 0). All cows were milked twice daily (3:00 AM and 2:00 PM) by an automatic milking machine, with the presence of calves to stimulate milk let down.
Experimental design, synchronization of ovulation, and timed artificial insemination protocol
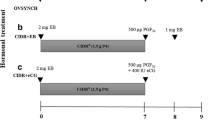
This study was performed using a novel TAI protocol based on intravaginal P4 device, combined with GnRH and PGF2α for synchronization of ovulation. Animals received the hormonal treatments on a random day of their estrus cycle (Fig. 1). The experimental design was based on a factorial two-by-three arrangement. On the first day of treatment, animals were randomly assigned to one of the following groups: (1) new intravaginal device [IVD-New: DIB® (Syntex, Buenos Aires, Argentina), n = 51 or CIDR® (Eazi-Breed CIDR®, Zoetis Animal Health, Parsippany, NJ, USA), n = 55]; (2) intravaginal device previously used once (9 days) (IVD-Used1x-DIB, n = 40 or -CIDR, n = 51); or (3) intravaginal device previously used twice (18 days) (IVD-Used2x-DIB, n = 27 or -CIDR, n = 23). The intravaginal devices DIB and CIDR contained 1.0 and 1.38 g of P4, respectively.
Schematic diagram showing the P4 + GnRH/PGF2α/GnRH-based protocol for synchronization of ovulation and timed artificial insemination (TAI) during both the non-breeding and the breeding season in dairy buffalo cows. IVD-New intravaginal device new. IVD-Used1x a device that had previously been used once for 9 days. IVD-Used2x a device that had previously been used twice (18 days). TAI timed artificial insemination, Dx pregnancy diagnosis, US ultrasonography
On day 0, animals received the IVD for 9 days and a dose of GnRH analogue (10.5 μg of buserelin acetate, Conceptal®, Intervet, MSD Animal Health, Madison, NJ, USA) intramuscularly (im). On day 9, IVDs were removed and 25 mg de PGF2α (Lutalyse®, Zoetis Animal Health) plus 500 IU of eCG (Folligon®, Intervet, MSD Animal Health) was administrated im. On day 11 (48 h after IVD withdrawal), animals received 10.5 μg of GnRH (Conceptal®) im for inducing the ovulation and then they were artificially inseminated by a single AI technician 8–12 h later, using semen from a single Murrah bull of known adequate fertility (Fig. 1).
In order to prevent reproductive tract infections, the previously used IVDs were washed and disinfected for 15 min using a solution of 0.5% lauryl dimethyl benzyl ammonium bromide (Gerdex®, Producciones Rodeneza C.A., Caracas, Venezuela).
Ultrasound examination and pregnancy diagnosis
At the onset of the synchronization protocol (day 0), all cows were examined using ultrasonography per rectum to determine the uterine status and ovarian structures. Animals with any reproductive tract abnormality were not included in this study. Ultrasound examination was performed using a CTS 900V ultrasound scanner equipped with a linear rectal multi-frequency probe (SIUI: Shantou Institute of Ultrasonic Instruments Co., Ltd., Guangdong, China).
Pregnancy diagnosis was performed by ultrasonography per rectum 30–32 days after TAI, through detection of an embryonic vesicle with a viable embryo (presence of heartbeat). Pregnancy rate was defined as the number of pregnant buffalo cows divided by the total number of buffalo cows submitted to TAI in each group.
Statistical analysis
Statistical analysis for pregnancy rate was performed using Proc Logistic of the Statistical Analysis System for Windows (SAS Version 9.3, 2001). Comparisons among treatment groups were performed using chi-square test. Statistical significant differences were considered at P < 0.05.
Results
Season (non-breeding and breeding season), BCS, days in milk (DIM), and parity at the beginning of the protocols did not affect pregnancy rates (P > 0.05; data not shown). Mean for BCS and DIM were 3.15 ± 0.2 (BCS: 3 to 3.5) and 119.9 ± 17.4 days (DIM: 102 to 140), respectively. Median of parity was 3 (parity: 1 to 5).
Pregnancy rate (PR) obtained in each group after TAI, using either a new or reused IVD with two different P4 concentrations, is shown in Fig. 2. The overall pregnancy rate (including new and reused IVD) was higher (P = 0.043) in buffalo cows in the DIB group than those in the CIDR group (62.7%, n = 74/118 vs. 45%, n = 58/129, for DIB and CIDR groups, respectively).
Pregnancy rates in dairy buffalo cows after TAI using new (New) or reused (Used1x; Used2x) intravaginal devices (IVD) with two different progesterone concentrations (1.0 g vs.1.38 g of P4). Superscript letters a and b mean among white or black chart bars with different superscripts differ (P < 0.05). Superscript letters a and b with asterisks mean white or black chart bars with different superscripts differ (P < 0.01). Overall PR overall pregnancy rate, considering new and reused devices
Moreover, animals that received a new DIB device (IVD-New-DIB) had a significantly higher (P = 0.0193) pregnancy rate compared to animals that received a new CIDR device (IVD-New-CIDR; 62.7%, n = 32/51 vs. 40%, n = 22/55, respectively; Fig. 2).
Likewise, logistic regression analysis showed a significant (P = 0.0196) effect of treatment groups on pregnancy rate (Table 1). The odds ratio (OR) for the occurrence of pregnancy indicated that buffalo cows belonging to the IVD-New-DIB and IVD-Used1x-DIB groups were 2.5 and 2.7 times more likely to become pregnant than buffalo cows in the IVD-New-CIDR group. Similarly, the OR for occurrence of pregnancy in buffalo cows in the DIB groups (new and reused DIB) was 2.1 times greater when compared to the CIDR groups (new and reused CIDR).
Discussion
This study showed that the type of P4 IVD (CIDR vs. DIB) within ovulation synchronization and TAI protocols affects pregnancy rate in water buffalo cows. Pregnancy rate was greater in buffalo cows treated with IVD containing 1.0 g of P4 (DIB®), compared to that obtained with IVD containing higher P4 concentration (1.38 g of P4, CIDR®). These differences could be attributed to differences in P4 concentrations, hormone chemical composition, and rate of delivery or/and absorption between intravaginal devices.
It has been reported that high circulating P4 concentration (from endogenous or exogenous sources) during the follicular phase is able to decrease LH pulse frequency (Forde et al. 2011), which may affect ovulation and pregnancy rates in cattle. Moreover, lower P4 environment during growth of the ovulatory follicle at TAI increased the preovulatory follicle size and subsequent corpus luteum (CL) size and function, improving the pregnancy rates in beef cattle (Dadarwal et al. 2013). Despite hormonal profiles (progesterone concentration or LH pulse frequency) and follicular dynamics (including follicle and CL sizes) were not measured in the current experiment, it is possible to speculate that differences in pregnancy rate between the experimental groups could have been influenced by the initial P4 concentration present in the devices (1.38 g of P4 in CIDR® vs. 1.0 g of P4 in DIB®).
Optimization of the dominant follicle size has been an important target in synchronization of ovulation and TAI protocols (Wiltbank et al. 2011; Baruselli et al. 2012). In beef cattle, the presence of larger follicles at the time of the TAI improves the rate of ovulation, which results in greater pregnancy rates (Perry et al. 2007; SáFilho et al. 2010). Increasing plasma progesterone concentrations and ovulation rate in cattle after TAI has been positively correlated with total CL or ovulatory follicle volume, indicating that CL size and function were influenced by the size of the ovulatory follicle (Echternkamp et al. 2009). In buffalo cows, the diameter of the preovulatory follicle has a positive impact on plasma estradiol concentration at estrus, subsequent progesterone profile, and conception rate (Pandey et al. 2011). Likewise, in Bos indicus beef cows (Nellore) treated with previously used twice IVD (1.0 g of P4) produced larger dominant follicles (15.3 ± 0.4 mm; P = 0.06), compared to cows treated with new IVD (13.5 ± 0.8 mm) and previously used once IVD (14.9 ± 0.5 mm) (Sales et al. 2015).
In the present study, the animals treated with DIB had similar pregnancy rates than buffalo cows treated with IVD containing 1.0 g of P4 plus E2 and TAI during the breeding season under tropical conditions (64%; Monteiro et al. 2016), seasonal anestrous (52.7 and 55.9%; Carvalho et al. 2013 and Carvalho et al. 2014). Additionally, the study by Monteiro et al. (2016) showed slightly higher pregnancy rate in animals treated with IVD used once containing 1.0 g of P4 (70.0%), than the values reported in the current experiment in animals treated with IVD used once containing also 1.0 g of P4 (65%). Perhaps, these differences might have been influenced by season. The study by Monteiro et al. (2016) was performed during the breeding season, while the present trial was done in both non-breeding and breeding seasons, during which no seasonal effects were observed on pregnancy rate [68.18% (n = 15/22) vs. 61.11% (n = 11/18) for non-breeding and breeding seasons, respectively].
In contrast, other authors have reported lower pregnancy rates (around 40% in average) in both Mediterranean (De Rensis et al. 2005; Neglia et al. 2003) and Murrah buffaloes (Murugavel et al. 2009), using a combination of P4, GnRH, and PGF2α (PRID®: 1.55 g of P4 and CIDR®: 1.38 g of P4, respectively). It is possible to infer that differences in pregnancy rates observed in different studies using similar P4-based IVD combined with GnRH and PGF2α could be also due to variation in P4 concentration, type of device used, protocol length and device permanence, timing of AI relative to ovulation, semen quality, management, environmental conditions, and breed.
The results of the current study also indicate that reusing the IVD (once and twice) in water buffalo cows do not have negative effects on pregnancy rate. Similar findings were reported by Carvalho et al. (2014), who found that reusing the IVD containing 1.0 g of P4 did not affect negatively the PR in dairy buffalo cows, showing PR values of 55.9, 50.4, and 48.3%, for New, Used1x, and Used2x devices, respectively.
Despite reusing CIDRs (one or two times) that decrease device P4 content (which might be similar to that in new DIB), pregnancy rate was numerically lower (no statistical significance) in the animals treated with used CIDRs compared to those receiving a new DIB (CIDR Used1x: 47%, CIDR Used2x: 52%; DIB New: 62%). In the present study, the sample size was small which could have affected the ability to detect minor differences in pregnancy rate. Other factors different from P4 content in the IVD (i.e., P4 chemical composition, vaginal absorption, hormone lifespan) might have also affected the pregnancy rate in buffalo cows treated with different P4 devices.
Studies evaluating the potential negative effects of high P4 concentration, different chemical composition, or absorption rate using different IVD models on the dominant follicle development and pregnancy rate in water buffalo are warranted. A study performed by Long et al. (2009) in ovariectomized cows to measure plasma P4 concentrations on day 1 after CIDR insertion in animals treated with new CIDR containing 1.9 g of P4, CIDR used once, or CIDR used twice showed a gradual decrease in P4 concentration in all groups. Interestingly, plasma P4 concentrations on the day after insertion were significantly higher (P < 0.05) in cows treated with used-CIDRs than the values at day 7 of the other treatment (Long et al. 2009). These findings could explain the absence of negative effect of reusing CIDR devices on pregnancy rate in the current experiment.
Decreasing the P4 concentration in CIDR prescribed for water buffalo, or reusing the CIDR devices two or three times could enhance the pregnancy rate in water buffalo cows submitted to P4-based ovulation synchronization and TAI protocols. An additional benefit of reusing the IVD is the reduction of the costs of associated with synchronization protocols for TAI programs in water buffalo.
The results obtained in the current trial recapitulate previous studies (Carvalho et al. 2014; Monteiro et al. 2016) and support our hypothesis that higher P4 concentration contained in the IVD could affect the pregnancy rate in water buffalo cows. Based on these results, it is possible to infer that an optimal P4 concentration in IVDs for buffalo cows submitted to TAI protocols could be ≤ 1.0 g. Additional studies in this regard are necessary and should be further investigated.
In conclusion, pregnancy rate was higher in buffaloes treated with the synchronization protocol using 1.0 g of P4 (DIB®) compared to that obtained with 1.38 g of P4 (CIDR®), which could be attributed to differences in P4 concentration, chemical composition, or absorption rate. Likewise, reusing the intravaginal device (two and three times) did not affect negatively the pregnancy rate, suggesting that P4 concentration in protocols for ovulation synchronization and TAI could be adjusted in this species.
References
Alapati, A., Kapa, S.R., Jeepalyam, S., Rangappa, S.M. and Yemireddy, K.R., 2010. Development of the body condition score system in Murrah buffaloes: validation through ultrasonic assessment of body fat reserves, Journal of Veterinary Science, 11(1), 1–8
Baruselli P.S., Mucciolo R.G., Visintin J.A., Viana W.G., Arruda R.P., Madureira E.H., Oliveira C.A., Molero-Filho J.R.., 1997. Ovarian follicular dynamics during the estrous cycle in buffalo (Bubalus bubalis), Theriogenology, 47(8), 1531–1547
Baruselli, P.S., Madureira, E.H., Barnabe, V.H., Barnabe, R.C. and Berber, R.C.A., 2003. Evaluation of synchronization of ovulation for fixed timed insemination in buffalo (Bubalus bubalis), Brazilian Journal of Veterinary Research and Animal Science, 40, 431–442. Accessed online: http://www.scielo.br/pdf/bjvras/v40n6/a07v40n6.pdf
Baruselli, P.S., SáFilho, M.F., Ferreira, R.M., Sales, J.N.S., Gimenes, L.U., Vieira, L.M., Mendanha, M.F. and Bó, G.A., 2012. Manipulation of follicle development to ensure optimal oocyte quality and conception rates in cattle, Reproduction in Domestic Animals, 47 (Suppl. 4), 134–141
Carvalho, J.B.P., Carvalho, N.A.T., Reis, E.L., Nichi, M., Souza, A.H. and Baruselli, P.S., 2008. Effect of early luteolysis in progesterone-based timed AI protocols in Bos indicus, Bos indicus x Bos taurus, and Bos taurus heifers, Theriogenology, 69(2), 167–175
Carvalho, N.A., Soares, J.G., Porto-Filho, R.M., Gimenes, L.U., Souza, D.C., Nichi, M., Sales, J.S. and Baruselli P.S., 2013. Equine chorionic gonadotropin improves the efficacy of a timed artificial insemination protocol in buffalo during the nonbreeding season. Theriogenology, 79(3), 423–428
Carvalho, N.A., Soares, J.G., Souza, D.C., Vannucci, F.S., Amaral, R., Maio, J.R., Sales, J.N., SáFilho, M.F. and Baruselli P.S., 2014. Different circulating progesterone concentrations during synchronization of ovulation protocol did not affect ovarian follicular and pregnancy responses in seasonal anestrous buffalo cows, Theriogenology, 81 (3), 490–495
Carvalho, N.A., Soares, J.G. and Baruselli, P.S., 2016. Strategies to overcome seasonal anestrus in water buffalo, Theriogenology, 86(1), 200–206
Dadarwal, D., Mapletoft, R.J., Adams, G.P., Pfeifer, L.F.M., Creelman, C. and Singh, J., 2013. Effect of progesterone concentration and duration of proestrus on fertility in beef cattle after fixed-time artificial insemination, Theriogenology, 79 (5), 859–866
De Rensis, F., Ronci, G., Guarneri, P., Nguyen, B.X., Presicce, G.A., Huszenicza, G. and Scaramuzzi, R.J., 2005. Conception rate after fixed time insemination following ovsynch protocol with and without progesterone supplementation in cyclic and non-cyclic Mediterranean Italian buffaloes (Bubalus bubalis), Theriogenology, 63(7), 1824–1831
Echternkamp, S.E., Cushman, R.A. and Allan, M.F., 2009. Size of ovulatory follicles in cattle expressing multiple ovulations naturally and its influence on corpus luteum development and fertility, Journal of Animal Science, 87(11), 3556–3568
Federation of Animal Science Societies (FASS). 2010. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. Committees to revise the Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd edition
Forde, N., Beltman, M.E., Lonergan P., Diskin, M., Roche, J.F., and Crowe M.A. 2011. Oestrous cycles in Bos taurus cattle, Animal Reproduction Science, 124(3–4), 163–169
Long, S.T., Yoshida, C., and Nakao, T. 2009. Plasma progesterone profile in ovariectomized beef cows after intra-vaginal insertion of new, once-used or twice-used CIDR. Reproduction in Domestic Animals, 44(1):80–82
Monteiro, B.M., de Souza, D.C., Vasconcellos, G.S., Corrêa, T.B., Vecchio, D., de SáFilho, M.F., Carvalho, N.A. and Baruselli, P.S., 2016. Ovarian responses of dairy buffalo cows to timed artificial insemination protocol, using new or used progesterone devices, during the breeding season (autumn-winter), Animal Science Journal, 87(1), 13–20
Murugavel, K., Antoine, D., Raju, M.S. and López-Gatius, F., 2009. The effect of addition of equine chorionic gonadotropin to a progesterone-based estrous synchronization protocol in buffaloes (Bubalus bubalis) under tropical conditions, Theriogenology, 71(7), 1120–1126
Neglia, G., Gasparrini, B., Di Palo, R., De Rosa, C., Zicarelli, L. and Campanile, G., 2003. Comparison of pregnancy rates with two estrus synchronization protocols in Italian Mediterranean Buffalo cows, Theriogenology, 60(1), 125–133
Pandey, A.K., Dhaliwal, G.S., Ghuman, S.P. and Agarwal, S.K., 2011. Impact of pre-ovulatory follicle diameter on plasma estradiol, subsequent luteal profiles and conception rate in buffalo (Bubalus bubalis), Animal Reproduction Science, 123(3–4), 169–174
Perera, B.M.A.O., 2008. Reproduction in domestic buffalo, Reproduction in Domestic Animals, 43 Suppl 2, 200–206
Perera, B.M.A.O., 2011. Reproductive cycles of buffalo, Animal Reproduction Science, 124(3–4), 194–199
Perry, G.A., Smith, M.F., Roberts, A.J., Macneil, M.D. and Geary, T.W., 2007. Relationship between size of the ovulatory follicle and pregnancy success in beef heifers, Journal of Animal Science, 85(3), 684–689
Presicce, G.A., 2007. Reproduction in the water buffalo, Reproduction in Domestic Animals, 42 Suppl 2, 24–32, Review.
Presicce, G.A., Bella, A., Terzano, G.M., De Santis, G. and Senatore, E.M., 2005. Postpartum ovarian follicular dynamics in primiparous and pluriparous Mediterranean Italian buffaloes (Bubalus bubalis), Theriogenology, 63(5), 1430–1439
SáFilho, M.F., Crespilho, A.M., Santos, J.E., Perry, G.A. and Baruselli, P.S., 2010. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows, Animal Reproduction Science, 120(1–4), 23–30
Sales, J.N.S., Carvalho, J.B., Crepaldi, G.A., Soares, J.G., Girotto, R.W., Maio, J.R., Souza, J.C. and Baruselli, P.S., 2015. Effect of circulating progesterone concentration during synchronization for fixed-time artificial insemination on ovulation and fertility in Bos indicus (Nelore) beef cows, Theriogenology, 83(6), 1093–100
SAS, SAS Institute, Inc., Cary, NC, 2001
Wiltbank, M.C., Sartori, R., Herlihy, M.M., Vasconcelos, J.L.M., Nascimento, A.B., Souza, A.H., Ayres, H., Cunha, A.P., Keskin, A., Guenther, J.N. and Gumen, A., 2011. Managing the dominant follicle in lactating dairy cows, Theriogenology, 76(9), 1568–1582
Acknowledgments
The authors are grateful to “Búfalos del Sur, C.A.” (BUFASUR) for providing all financial support and for allowing the use of their animals and facilities for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of animal rights
All animals were cared for in accordance with acceptable practices as for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (FASS 2010).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gutiérrez-Añez, J.C., Palomares, R.A., Jiménez-Pineda, J.R. et al. Pregnancy rate in water buffalo following fixed-time artificial insemination using new or used intravaginal devices with two progesterone concentrations. Trop Anim Health Prod 50, 629–634 (2018). https://doi.org/10.1007/s11250-017-1479-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-017-1479-1