Abstract
Mirafiori lettuce virus (MiLV), a plant RNA virus belonging to the genus Ophiovirus, is considered to be a causal agent of lettuce big-vein disease. In this study, inverted repeats of a fragment of the coat protein (CP) gene of MiLV in a binary vector pBI121 were transferred via Agrobacterium tumefaciens-mediated transformation into lettuce (Lactuca sativa L.) in order to generate MiLV-resistant lettuce. Forty T1 lines were analyzed for resistance to MiLV by detecting MiLV in leaves, and two lines (lines 408 and 495) were selected as resistant to MiLV. Both lines were susceptible to Lettuce big-vein associated virus (LBVaV), and line 495 showed higher resistance to MiLV than line 408. Further analysis indicated that line 495 showed resistance to big-vein symptoms expression. Small interfering RNA (siRNA) molecules derived from the transgene were detected in plants of line 495. MiLV was detected in roots but not in leaves of line 495 plants after MiLV inoculation, suggesting that resistance to MiLV is less effective in roots than in leaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lettuce big-vein disease is a soil-borne viral disease in major lettuce production areas worldwide. Infected lettuce plants show vein banding, leaf ruffling and distortion, which results in poor quality plants with reduced market value. The infection also causes delayed head formation, decreased head size, and a reduced proportion of harvestable plants.
Two viruses, Lettuce big-vein associated virus (LBVaV) and Mirafiori lettuce virus (MiLV), are associated with lettuce big-vein disease. Both LBVaV and MiLV are transmitted by the fungus Olpidium brassicae and belong to the genera Varicosavirus and Ophiovirus, respectively (Fauquet et al. 2005). LBVaV, previously called Lettuce big-vein virus, was first described by Kuwata et al. (1983) and was thought to be a causal agent of big-vein disease for nearly two decades, although there was no evidence that LBVaV induced big-vein symptoms in lettuce. The second virus, MiLV, was then found by Roggero et al. (2000). Lot et al. (2002) and Sasaya et al. (2008) reported that lettuce plants infected with LBVaV did not develop big-vein symptoms in the absence of MiLV and that lettuce plants infected with MiLV developed big-vein symptoms regardless of the presence or absence of LBVaV. Therefore, MiLV but not LBVaV is now regarded as the causal agent of lettuce big-vein disease.
The best method to control big-vein disease is the development of genetic resistance (Ryder and Robinson 1995). Resistant cultivars have been developed by conventional breeding using several sources that provide moderate resistance (Ryder 1981; Ryder and Robinson 1991); however, cultivars released to date do not exhibit high level of resistance because breeding sources with high resistance to the disease have not been found in lettuce Lactuca sativa L. (Bos and Huijberts 1990; Ryder and Robinson 1995). Complete resistance to the disease is found only in wild lettuce L. virosa (Bos and Huijberts 1990). Introgression of the resistance from L. virosa into cultivated lettuce has been attempted, and partial resistance has been introgressed into L. sativa cultivars (Hayes and Ryder 2007). However, introgression of complete resistance into L. sativa has not been successful. Transgenic strategies are therefore attractive alternative methods against lettuce big-vein disease.
Generation of transgenic plants with virus resistance has been demonstrated as an effective strategy against virus infections through the expression of coat protein genes, viral replicase genes, or other viral sequences (Baulcombe 1996). This phenomenon is termed pathogen-derived resistance and includes protein-mediated resistance and RNA-mediated resistance. The mechanism of RNA-mediated resistance involves RNA silencing, in which sequence-specific RNA degradation occurs. In RNA silencing, double-stranded RNA is cut into small interfering RNAs (siRNAs) of 21–26 nt by the RNase III-like Dicer or Dicer-like enzymes (Hamilton et al. 2002). The antisense strand of siRNA is loaded into an RNA-induced silencing complex (RISC) and links the complex to the target RNA strand by base-pairing. The RISC cuts the target RNA strand, and the RNA is subsequently degraded.
RNA silencing can be induced by dsRNA, and virus-resistant transgenic plants can be produced efficiently by introducing inverted repeats of a viral gene that encode dsRNA (Smith et al. 2000). Since nucleotide sequences of MiLV have been reported (Kawazu et al. 2003; van der Wilk et al. 2002), we engineered inverted repeats of the MiLV CP gene. In this study, we report the development and characterization of lettuce expressing such MiLV construct and the selection of transgenic lines resistant to MiLV.
Material and methods
Lettuce transformation
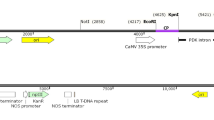
The β-glucuronidase (GUS) gene in the binary vector pBI121 was replaced by a cassette containing inverted repeats of the 5′ end (500 nt) of the MiLV CP gene (GenBank accession number AF532872) and the intron of a L. sativa resistance protein candidate (RGC2a) gene (GenBank accession number AF017752). The resultant binary vector pYK23 was transformed via Agrobacterium tumefaciens-mediated transformation into lettuce (L. sativa L. cv ‘Kaiser’) by the leaf disc method (Curtis et al. 1995). Explants were regenerated on medium containing 100 mg/L kanamycin. PCR was carried out to detect the transgene in transgenic plants using the following primers: 5′-GTAAA TATAA ATTTT TAATG CTAAT AC-3′ (lettuce intron region) and 5′-TTTTC CCAGT CACGA CGTTG-3′ (downstream of the inverted repeats).
Virus inoculation and detection
Inoculation and detection of MiLV and LBVaV was conducted as described by Kawazu et al. (2006). Roots of susceptible cultivar ‘Cisco’ plants infected with O. brassicae carrying MiLV and LBVaV were homogenized with water, and the homogenized roots were poured onto the base of each lettuce plant. Inoculated plants were kept in a plant growth chamber at 20°C during the day (14-h photoperiod) and 15°C at night (10 h), or transferred to infested soil on a bed in a glasshouse. Protein extracts were obtained from leaf or root tissue of lettuce plants. DAS-ELISA for detection of MiLV and Western blot analysis for detection of LBVaV were performed as described by Kawazu et al. (2006).
Southern blot analysis
Genomic DNA was isolated from lettuce leaves using a DNeasy Plant Mini Kit (QIAGEN Inc., The Netherlands). Approximately 2.5 μg of genomic DNA were digested with EcoRV or SacI, and the resulting fragments were electrophoresed in a 1.2% agarose gel and transferred to a Hybond-N+ membrane (GE Healthcare Bio-Sciences Corp., USA) by capillary blotting (Sambrook and Russell 2001). RNA probes from the full coding sequence of the MiLV CP gene (1.3 kb) were prepared using DIG RNA Labeling Kit (SP6/T7) (Roche, Switzerland). Hybridization was done in NorthernMax Prehybridization/Hybridization Buffer (Ambion Inc., USA) at 40°C overnight. The Hybond-N+ membrane was washed with 2× SSC and 0.2% SDS at room temperature for 5 min (twice), then washed with 2× SSC and 0.2% SDS at 40°C for 15 min (twice). The immunological detection of the DIG-labeled probe was performed using DIG Wash and Block Buffer Set (Roche), Anti digoxigenin-AP conjugate (Roche), and CDP-star Substrate (Applied Biosystems, USA).
Northern blot analysis
Total RNA was extracted from leaf tissue of lettuce plants using ISOGEN (Nippon Gene Co. Ltd., Japan). Extracted RNA solution was mixed with an equal amount of PEG/NaCl solution (10% polyethylene glycol 6000, 1 M NaCl), kept on ice for 30 min, then centrifuged at 15,000g for 10 min. Low-molecular weight RNA in the supernatant was precipitated with EtOH. Approximately 10 μg of low-molecular weight RNA were electrophoresed in 15% acrylamide gel containing 7 M urea and transferred to a Hybond-N+ membrane (GE Healthcare Bio-Sciences Corp.) using a semi-dry blotter V20-SDB (SCIE-PLUS, UK). Hybridization and detection were performed as described above (Southern blot analysis), but hybridization was done at 35°C overnight, and the Hybond-N+ membrane was washed with 2× SSC and 0.2% SDS at room temperature for 5 min (twice), and then washed with 2× SSC and 0.2% SDS at 35°C for 15 min (twice).
Results
Selection of transgenic lines for MiLV resistance
Inverted-repeats of the fragment of the MiLV CP gene with a lettuce-derived intron as a spacer were transformed into lettuce. Regenerated lettuce plants (T0 generation) were analyzed for the transgene by PCR, transgene-positive plants were self-pollinated, and seeds (T1 generation) were produced from 40 independent lines. At least three seeds were obtained from each T0 plant. To screen for resistant lines, three to 16 plants of each T1 line were inoculated with MiLV using O. brassicae and kept in a growth chamber. DAS-ELISA for MiLV detection in leaves was performed 37 days post-inoculation (dpi), and two lines (lines 408 and 495) were selected as resistant lines. Percentages of MiLV-negative plants of lines 408 and 495 were 40% (six of 15 plants) and 71% (10 of 14 plants), respectively. Percentages of MiLV-negative plants of the other 38 lines were less than 40%. Five MiLV-negative plants of line 408 or 495 were self-pollinated, and seeds (T2 generation) were produced from two plants of line 408 and from three plants of line 495. A resistance test in a growth chamber was then performed for these five lines (lines 408-1, 408-2, 495-1, 495-2, and 495-3; Table 1). MiLV was detected in nine of 15 transgene-positive plants of line 408-1 and in 14 of 16 transgene-positive plants of line 408-2. This result shows that the resistance of lines 408-1 and 408-2 to MiLV was weak. Almost all transgene-negative plants of lines 495-1, 495-2, and 495-3 were MiLV-positive (three of three, four of five, and three of three, respectively). However, all transgene-positive plants of lines 495-1, 495-2, and 495-3 were MiLV-negative. These results indicate that plants of lines 495-1, 495-2, and 495-3 are resistant to MiLV if they have the transgene. Western blot analysis for LBVaV detection was also performed, and all plants tested were LBVaV-positive, indicating that all transgenic lines were susceptible to LBVaV (Table 1).
Resistance tests of transgenic line 495-1 and its progeny
The transgenic line 495-1 was used for further analysis. A resistance test in a growth chamber was performed again, and not only MiLV and LBVaV but also symptoms of lettuce big-vein were checked (Table 2). Line 495-1 plants with the transgene, line 495-1 plants without the transgene, and non-transformed ‘Kaiser’ plants were inoculated with O. brassicae carrying MiLV and LBVaV. Both MiLV and LBVaV were detected in leaves of all ‘Kaiser’ plants and all line 495-1 plants without the transgene at 50 dpi. All the plants were also symptomatic. LBVaV was detected in leaves of all transgene-positive plants at 50 dpi, but all the plants were MiLV-negative in leaves and without symptoms. Line 495-1 plants were self-pollinated, and transgenic plants of T3 generation (line 495-1-1) were inoculated with MiLV. All T3 plants were without symptoms at 43 dpi, while all ‘Kaiser’ plants were symptomatic (Fig. 1). These results indicate that line 495-1 and its progeny are resistant to big-vein symptoms expression.
We searched for the presence of MiLV not only in leaves but also in roots of lettuce plants after MiLV inoculation (Table 2). MiLV was detected in roots of all ‘Kaiser’ plants and line 495-1 plants without the transgene, but only some transgenic plants (two of five) were MiLV-positive in roots at 43 and 50 dpi. All transgenic plants were MiLV-positive in roots at 70 and 116 dpi, but MiLV-negative in leaves. We performed a similar experiment using T4 plants in a glasshouse (Table 3). T4 plants and ‘Kaiser’ plants were grown on infested soil in a glasshouse. MiLV and LBVaV were detected in leaves and roots of all ‘Kaiser’ plants. LBVaV was also detected in leaves and roots of all T4 plants. On the other hand, all T4 plants were MiLV-positive in roots but MiLV-negative in leaves and without symptoms. These results suggest that resistance to MiLV is less effective in roots than in leaves.
The copy number of the transgene in line 495-1 or 495-1-1
To determine the copy number of the transgene in the transgenic lettuce, Southern blot analysis was performed on genomic DNA from three transgenic plants of line 495-1-1 (T3 generation) (Fig. 2). Genomic DNA digested with EcoRV or SacI was hybridized with the MiLV CP probe, and only one band was detected. Southern blot analysis was also performed using three transgenic plants of line 495-1 (T2 generation), and only one band was detected as well (data not shown). These results show that only one copy of the transgene was present in the transgenic lettuce.
Southern blot analysis of transgenic lettuce line 495-1-1 (T3 generation) for determining the copy number of the transgene. Genomic DNA of line 495-1-1 was digested with EcoRV or SacI and hybridized with the MiLV CP probe. Lanes 1–3: genomic DNA digested with EcoRV; lanes 4–6: genomic DNA digested with SacI. The size of the DNA ladder marker is indicated on the left. The same result was obtained for line 495-1 (T2 generation)
Detection of small interfering RNAs (siRNAs) in transgenic plants
siRNAs are indicative of RNA silencing, so transgenic lettuce plants without MiLV inoculation were analyzed for the presence of siRNAs. Low-molecular weight RNA was extracted from leaves of line 495-1 plants with the transgene and ‘Kaiser’ plants, and hybridized with the MiLV CP probe. Two bands of expected size for siRNAs (between 18 and 26 nt) were detected only in transgenic plants (Fig. 3). This suggests that RNA silencing is active against MiLV CP gene-derived RNA in line 495-1.
Northern blot analysis of small interfering RNAs (siRNAs) isolated from line 495-1 plants (T2 generation) without MiLV inoculation. The upper panel shows detection of siRNAs in low-molecular weight RNAs extracted from leaves of transgenic or nontransgenic plants. Lanes 1–4: ‘Kaiser’ plants; lanes 5–8: line 495-1 plants. The size of the RNA ladder marker is indicated on the left. The lower panel shows tRNA and 5S rRNA stained with ethidium bromide
We also analyzed for the presence of siRNAs in transgenic lines susceptible to MiLV (lines 451-1 and 452-1) and line 408-1 (Fig. 4). Plants were analyzed for the transgene by PCR, and leaves of transgene-positive plants were used for siRNA detection. Figure 4 shows the results on representative plants for each transgenic line. siRNAs were detected in all plants of line 495-1 (11 transgene-positive plants were analyzed). On the other hand, no siRNA was detected in line 451-1 (eight transgene-positive plants were analyzed) or line 452-1 (five transgene-positive plants were analyzed). This suggests that RNA silencing is not active against MiLV CP gene-derived RNA in line 451-1 or 452-1. Line 408-1 which showed weak resistance to MiLV (Table 1) was also analyzed (lanes 3 and 4 in Fig. 4). No siRNA was detected in seven of nine transgene-positive plants of line 408-1, but siRNAs were detected in two plants. This result suggests that RNA silencing is active against MiLV CP gene-derived RNA in some plants of line 408-1.
Detection of small interfering RNAs (siRNAs) in leaves of resistant or susceptible transgenic lines (T2 generation) without MiLV inoculation. The upper panel shows detection of siRNAs in low-molecular weight RNAs extracted from leaves of transgenic plants and the lower panel shows tRNA and 5S rRNA stained with ethidium bromide in each lane. Only transgene-positive plants were used for the analysis. Lines 451-1 and 452-1 are susceptible to MiLV
We compared the amounts of siRNAs in leaves and roots of line 495-1-1-12 (T4 generation) (Fig. 5). Approximately 10 μg of low-molecular weight RNA were used for Northern blot analysis, and fewer amounts of siRNAs were detected in RNA extracted from roots than in RNA extracted from leaves.
Detection of small interfering RNAs (siRNAs) in leaves and roots of line 495-1-1-12 (T4 generation) without MiLV inoculation. The upper panel shows detection of siRNAs in low-molecular weight RNAs extracted from leaves (L) or roots (R) of transgenic plants. The lower panel shows low-molecular weight RNAs stained with ethidium bromide. The top of the lower panel shows the top of the acrylamide gel and the bottom line corresponds to the size of 30-nucleotide RNA marker. Three plants were used for the analysis
Discussion
In this study, 40 T1 lines carrying inverted repeats of a fragment of the MiLV CP gene were produced, and two lines (lines 408 and 495) were selected as resistant to MiLV. Two T2 lines from line 408 and three T2 lines from line 495 were tested for resistance to LBVaV and MiLV (Table 1). LBVaV was detected in all tested plants, showing that these T2 lines are susceptible to LBVaV. MiLV was detected in many plants of lines 408-1 and 408-2, indicating that resistance of these lines to MiLV is weak. Lines 495-1 to 495-3 showed higher resistance to MiLV because no MiLV was detected when they had the transgene (Table 1).
A resistance test of line 495-1 was repeated in a growth chamber, and all plants with the transgene were without symptoms and MiLV-negative, but they were LBVaV-positive in leaves at 43, 50, and 70 dpi (Table 2). We also performed a resistance test of line 495-1-1-12 (T4 generation) in a glasshouse, and all T4 plants tested were without symptoms and MiLV-negative but LBVaV-positive in leaves (Table 3). These results suggest that the suppression of symptom expression was caused by suppression of MiLV infections in leaves. This is consistent with previous reports that MiLV but not LBVaV induced lettuce big-vein disease (Lot et al. 2002; Sasaya et al. 2008).
In the RNA interference process, siRNA is produced. Two distinct siRNAs, short (21–22 nt) and long (24–26 nt) siRNAs, accumulate in plants, and these siRNAs have different roles (Hamilton et al. 2002). The long siRNA correlates with systemic silencing and methylation of homologous DNA, while the short siRNA correlates with mRNA degradation. In this paper, two siRNAs specific for the MiLV CP gene were detected in line 495-1 (Figs. 3, 4). We also analyzed for the presence of siRNAs in transgenic lines susceptible to MiLV (lines 451-1 and 452-1), and no siRNA was detected in these lines although they had the transgene (Fig. 4). These results suggest that production of siRNAs derived from the transgene is needed for MiLV resistance. siRNAs were detected in some plants of line 408-1, which is consistent with the result that some plants of line 408-1 were MiLV-negative in the resistance test (Table 1).
RNA silencing-mediated resistance to Beet necrotic yellow vein virus (BNYVV) is less effective in roots than in leaves (Andika et al. 2005). Transgenic Nicotiana benthamiana plants that were immune to foliar rub-inoculation with BNYVV could be infected by viruliferous zoospores of the vector fungus Polymyxa betae. Virus titer was reduced in symptom-free leaves of the plants showing the recovery phenotype, but it was high in roots of the same plants. In our study, lettuce plants were inoculated with MiLV using the vector fungus O. brassicae, and the presence of MiLV was examined in leaves and roots (Table 2). MiLV was detected in roots of some transgenic plants, but the percentage of the transgenic plants that were MiLV-positive in roots was lower than that of non-transgenic plants that showed MiLV-positive in roots at 43 or 50 dpi, suggesting that roots of the transgenic lettuce have some resistance to MiLV. However, all transgenic plants were MiLV-positive in roots, but MiLV-negative in leaves at 70 and 116 dpi. Two transgenic plants were MiLV-positive in roots at 43 dpi, but these plants were still MiLV-negative in leaves at 116 dpi. A similar experiment was performed using T4 plants in a glasshouse (Table 3). MiLV was detected in leaves and roots of all ‘Kaiser’ plants. On the other hand, all T4 plants were MiLV-positive in roots but MiLV-negative in leaves. These results suggest that resistance to MiLV is less effective in roots than in leaves. Next we compared the amounts of siRNAs in leaves and roots of T4 plants (Fig. 5). Fewer amounts of siRNAs were detected in RNA extracted from roots than in RNA extracted from leaves, but it was difficult to compare the accumulation levels of siRNAs precisely because different patterns of low-molecular weight RNA were seen on an acrylamide gel: the amounts of tRNA and rRNA were fewer and the amounts of RNA which was smaller than tRNA and rRNA were more in roots than in leaves (the lower panel in Fig. 5). It is possible that weak resistance of roots to MiLV is due to fewer amounts of siRNAs in roots, but another possibility is that MiLV suppresses RNA silencing in a root-specific manner. It has been reported that RNA4-encoded p31 of BNYVV is not able to suppress RNA silencing in leaves, but that p31 enhances the ability of BNYVV to suppress silencing in roots (Rahim et al. 2007).
Production of transgenic plants with virus-derived nucleotides is an effective method for virus resistance. Transgenic lettuce with resistance to big-vein disease was produced by introducing an antisense construct of the LBVaV CP gene (Kawazu et al. 2006). The transgenic lettuce showed resistance not only to LBVaV but also to MiLV and big-vein symptoms expression, but the mechanism of resistance to MiLV is unknown. In this paper, a new transgenic lettuce with resistance to big-vein disease was produced using the MiLV CP gene. This is the first report of virus-resistant plants produced using a nucleotide sequence derived from the genus Ophiovirus. The MiLV-resistant lettuce produced in our study can be used as a resistant cultivar or as a breeding source.
References
Andika IB, Kondo H, Tamada T (2005) Evidence that RNA silencing-mediated resistance to Beet necrotic yellow vein virus is less effective in roots than in leaves. Mol Plant Microbe Interact 18:194–204. doi:10.1094/MPMI-18-0194
Baulcombe DC (1996) Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell 8:1833–1844
Bos L, Huijberts N (1990) Screening for resistance to big-vein disease of lettuce (Lactuca sativa). Crop Prot 9:446–452. doi:10.1016/0261-2194(90)90135-T
Curtis IS, Davey MR, Power JB (1995) Leaf disk transformation. In: Gartland KMA, Davey MR (eds) Methods in molecular biology, vol 44. Humana Press Inc., New Jersey, pp 59–70
Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) (2005) Virus taxonomy:eighth report of the international committee on the taxonomy of viruses. Elsevier Academic Press, San Diego, CA
Hamilton A, Voinnet O, Chappell L, Baulcombe D (2002) Two classes of short interfering RNA in RNA silencing. EMBO J 21:4671–4679. doi:10.1093/emboj/cdf464
Hayes RJ, Ryder EJ (2007) Introgression of partial resistance to big vein disease from Lactuca virosa into cultivated lettuce. HortScience 42:35–39
Kawazu Y, Sasaya T, Morikawa T, Sugiyama K, Natsuaki T (2003) Nucleotide sequence of the coat protein gene of Mirafiori lettuce virus. J Gen Plant Pathol 69:55–60. doi:10.1007/s10327-002-0019-6
Kawazu Y, Fujiyama R, Sugiyama K, Sasaya T (2006) A transgenic lettuce line with resistance to both Lettuce big-vein associated virus and Mirafiori lettuce virus. J Am Soc Hortic Sci 131:760–763
Kuwata S, Kubo S, Yamashita S, Doi Y (1983) Rod-shaped particles, a probable entity of Lettuce big vein virus. Ann Phytopathol Soc Jpn 49:246–251
Lot H, Campbell RN, Souche S, Milne RG, Roggero P (2002) Transmission by Olpidium brassicae of Mirafiori lettuce virus and Lettuce big-vein virus, and their roles in lettuce big-vein etiology. Phytopathology 92:288–293. doi:10.1094/PHYTO.2002.92.3.288
Rahim MD, Andika IB, Han C, Kondo H, Tamada T (2007) RNA4-encoded p31 of beet necrotic yellow vein virus is involved in efficient vector transmission, symptom severity and silencing suppression in roots. J Gen Virol 88:1611–1619. doi:10.1099/vir.0.82720-0
Roggero P, Ciuffo M, Vaira AM, Accotto GP, Masenga V, Milne RG (2000) An Ophiovirus isolated from lettuce with big-vein symptoms. Arch Virol 145:2629–2642. doi:10.1007/s007050070012
Ryder EJ (1981) ‘Thompson’ lettuce. HortScience 16:687–688
Ryder EJ, Robinson BJ (1991) ‘Pacific’ lettuce. HortScience 26:437–438
Ryder EJ, Robinson BJ (1995) Big-vein resistance in lettuce: identifying, selecting, and testing resistant cultivars and breeding lines. J Am Soc Hortic Sci 120:741–746
Sambrook J, Russell DW (2001) Molecular cloning. A laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sasaya T, Fujii H, Ishikawa K, Koganezawa H (2008) Further evidence of Mirafiori lettuce big-vein virus but not of Lettuce big-vein associated virus with big-vein disease in lettuce. Phytopathology 98:464–468. doi:10.1094/PHYTO-98-4-0464
Smith NA, Singh SP, Wang M-B, Stoutjesdijk PA, Green AG, Waterhouse PM (2000) Total silencing by intron-spliced hairpin RNAs. Nature 407:319–320. doi:10.1038/35036500
van der Wilk F, Dullemans AM, Verbeek M, van den Heuvel JF (2002) Nucleotide sequence and genomic organization of an ophiovirus associated with lettuce big-vein disease. J Gen Virol 83:2869–2877
Acknowledgement
This work was supported by National Agriculture and Food Research Organization (NARO), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawazu, Y., Fujiyama, R. & Noguchi, Y. Transgenic resistance to Mirafiori lettuce virus in lettuce carrying inverted repeats of the viral coat protein gene. Transgenic Res 18, 113–120 (2009). https://doi.org/10.1007/s11248-008-9200-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-008-9200-9