Abstract
The formation of N2O has been studied by means of isothermal lean-rich experiments at 150, 180 and 250 °C over Pt–Ba/Al2O3 and Pt/Al2O3 catalysts with H2 and/or C3H6 as reductants. This allows to provide further insights on the mechanistic aspects of N2O formation and on the influence of the storage component. Both gas phase analysis and surface species studies by operando FT-IR spectroscopy were performed. N2O evolution is observed at both lean-to-rich (primary N2O) and rich-to-lean (secondary N2O) transitions. The production of both primary and secondary N2O decreases by increasing the temperature. The presence of Ba markedly decreases secondary N2O formation. FT-IR analysis shows the presence of adsorbed ammonia at the end of the rich phase only for Pt/Al2O3 catalyst. These results suggest that: (i) primary N2O is formed when undissociated NO in the gas phase and partially reduced metal sites are present; (ii) secondary N2O originates from reaction between adsorbed NH3 and residual NOx at the beginning of the lean phase. Moreover, N2O reduction was studied performing temperature programming temperature experiments with H2, NH3 and C3H6 as reducing agents. The reduction is completely selective to nitrogen and occurs at temperature higher than 250 °C in the case of Pt–Ba/Al2O3 catalyst, while lower temperatures are detected for Pt/Al2O3 catalyst. The reactivity order of the reductants is the same for the two catalysts, being hydrogen the more efficient and propylene the less one. Having H2 a high reactivity in the reduction of N2O, it could react with N2O when the regeneration front is developing. Moreover, also ammonia present downstream to the H2 front could react with N2O, even if the reaction with stored NOx seems more efficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
After Euro 6 emission standards coming into force for light duty vehicle from 2014, the car manufactures of diesel-engine and light-duty direct-injection (DI) gasoline-engine in Europe are facing severe challenges in terms of NOx emissions reduction [1, 2].
The NOx storage and reduction (NSR) catalysts, also referred as lean NOx traps (LNTs), represent a viable solution for the NOx removal from the exhaust gas of lean gasoline and diesel powered engines. The catalysts contain three fundamental components: a high surface area support (like γ-Al2O3), NOx storage materials (typically Ba, K) and precious metals (such as Pt, Rh, Pd). Promoters and additives like Ce, Zr may be also included to increase thermal stability and add OSC (Oxygen Storage Capacity) functionality. The removal of nitrogen oxides is accomplished under transient operation; during long lean periods (60–90 s) NOx are adsorbed over the storage component in form of nitrites/nitrates [3]; then, upon rich spikes (1–3 s) of the engine, the surface is regenerated. The reductants needed for the catalyst regeneration may also be originated from direct fuel injection in the exhausts, or from an upstream fuel reformer to give a reducing gas containing mainly CO and H2. Catalyst regeneration mainly leads to N2, however other byproducts like N2O, NH3 or unconverted NO may also be detected.
Nitrous oxide is highly undesired byproduct due to its high global warming potential, ~ 300 times higher than CO2 [4]. N2O emissions during operation of LNT catalyst are typically observed during both the lean-to-rich and rich-to-lean phase switching (primary and secondary N2O, respectively) when using H2, CO or C3H6 as reductant [5,6,7,8]. In the case of primary N2O formation, it was suggested that the extent of N2O formation depends on the reducibility of the noble metal (PGM) sites [9]. Depending on the efficiency of the reductant in the oxygen removal from the PGM sites, different amounts of N2O will be detected, i.e. the lower is the reduction capacity, the higher is the N2O production. Different factors affect the N2O formation when the catalytic system is switched from rich to lean (secondary emissions): in this case the residual reduced species like NHx (x = 1–3), isocyanate or hydrocarbons could react with gaseous NO/O2 to give N2O.
N2O emission control is an important issue nowadays for any emission technology, and several ways have been proposed to reduce them. The catalytic decomposition over a catalyst placed downstream the LNT brick might be a viable solution to decompose/reduce N2O after its formation (deN2O reaction). A variety of catalytic systems has been proposed and tested for this purpose [10], including metal oxides [11,12,13] and ion-exchange zeolites [14, 15]. Furthermore, some authors report high activity for catalytic systems based on cobalt, copper, iron, and especially noble metals [16, 17].
In this work, the focus is on the pathways involved in the formation of N2O during the cyclic operations of a typical LNT catalysts. For these purposes, lean/rich cycles under isothermal conditions have been carried out over two homemade model catalysts with and without the storage component, i.e. Pt-Ba/Al2O3 (1/20/100 w/w) and Pt/Al2O3 (1/100 w/w). To get more insights on the reaction pathways occurring in the evolution of nitrous oxide during lean/rich cycles, the decomposition/reduction of N2O has also been investigated over the same catalytic systems. Accordingly, temperature-programmed reduction (TPR) experiments have been performed to investigate the N2O decomposition and reduction with different reductants.
In these experiments hydrogen has been employed as reducing agent, being this species the most effective (and idealized) NOx reductant thanks to its simple chemistry and effectiveness [18,19,20,21,22]. For comparison purposes, C3H6 has also been used as more representative reducing species present in the exhausts.
Flow-reactor experiments and operando FT-IR spectroscopy are used as complementary tools to combine the catalyst surface species with gas-phase data.
2 Materials and Methods
Homemade Pt/γ-Al2O3 (1/100 w/w) and Pt–Ba/γ-Al2O3 (1/20/100 w/w) catalysts have been prepared by standard incipient wetness impregnation of commercial γ-alumina support. The obtained catalysts have a specific surface area of 182 and 133 m2/g (BET method) and pore volume 0.94 and 0.68 cm3/g (cumulative adsorption BJH method), respectively.
All experiments have been performed in a quartz flow-microreactor of 7 mm I.D. In each run 60 mg of catalyst (70–100 μm) and total flow of 100 Ncc/min have been used. A complete quantitative detection of the reaction products has been obtained through a combined use of MS (Thermostar 200, Pfeiffer Vacuum), UV–Vis NOx and NH3 analyzer (LIMAS 11HW, ABB), MultiGas FT-IR (mod. 2030, MKS) and a micro-gas chromatograph (3000A, Agilent), as detailed in previous works [23, 24].
The formation of N2O has been studied by means of lean-rich cycles in the range 150–250 °C. These experiments consist in alternating rectangular step feeds of NO (1000 ppm) + 3% v/v O2 in flowing He (lean phase), with rich phases (H2 2000 ppm or C3H6 450 ppm + 2.5% v/v H2O in He), until reproducible cycles are obtained. Catalyst conditioning involves the modification of the active storage Ba sites from the initial carbonate form (as loaded in the reactor) to the actual form under reaction conditions, i.e. carbonate, hydroxide, oxide [22, 25]. The relative amounts of the various Ba species depend on the gas phase composition, i.e. nature of the reducing agent, presence/absence of CO2 and water. The reactivity of the different Ba species in the NOx storage-reduction has been analyzed elsewhere [26].
The evolution of the surface species at 150 °C has been also analyzed by FT-IR spectroscopy performed under operando conditions with an IR reactor cell (ISRI Infrared Reactor, Granger, IN, USA) by using the catalysts in form of self-supported wafers (13 mg, diameter = 13 mm). The IR spectra were collected with a FT-IR Vertex 70 (Bruker, Billerica, MA, USA) spectrometer with 4 cm−1 spectral resolution and accumulation of 64 scans using a mercury–cadmium–telluride (MCT) detector. The NOx storage (NO (1000 ppm) + O2 (3% v/v) in He) and reduction (H2 (2000 ppm) in He) were performed under a flow of 50 Ncc/min. As in the case of the catalytic tests, the catalysts have been pre-conditioned with several adsorption/reduction cycles. The spectra are reported as difference spectra in the figures. The background spectrum is always that obtained after the conditioning treatment.
In the choice of the operating conditions, the length of the lean and the rich phases is not representative of real operating conditions but it has been exaggerated to better enlighten the processes occurring at the surface. Besides, the reductant concentration has been kept low to avoid excessive temperature effects during the switches.
Finally, the reactivity of N2O (500 ppm in Ar) was investigated by temperature-programmed reduction (TPR) experiments in the temperature range 40–500 °C (heating rate 10 °C/min) by using H2 (2000 ppm in He) and C3H6 (1000 ppm in He) as reductants. Since ammonia is also formed during lean-rich experiments, the reactivity of this species (1000 ppm in He) has also been investigated. Experiments have been performed in the presence of CO2 (1000 ppm) and H2O (2.5% v/v).
3 Results and Discussion
3.1 N2O Formation
The results of isothermal lean-rich experiments carried out over Pt–Ba/Al2O3 catalyst with H2 as reductant (gas-phase and surface analysis) carried out at low temperature (150 °C) are shown in Figs. 1 and 2, respectively. Note that the complete experiments comprehend many lean-rich cycles up to reaching the steady-state conditions, while in the Figure the most representative one is reported for each catalyst.
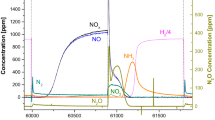
Upon NO addition (lean phase, Fig. 1), the NO concentration increases monotonically with time showing a non-negligible dead time, while NO2 is observed in negligible amounts due to the low temperature (the NO to NO2 oxidation is not relevant). No other species have been observed, if one excludes a minor N2O peak at the rich-to-lean transition.
Upon the subsequent lean-rich switch, the NOx concentration decreases with a tail and instantaneous production of N2 is observed and of NH3 later on. Significant amounts of N2O are also detected while the NO concentration is decreasing.
The surface FT-IR analysis of the lean phase is reported in Fig. 2a and shows the formation of mainly ionic nitrites on Ba sites (bands at 1350 cm−1, νsym.(NO2) and at 1215 cm−1, νasym.(NO2)). Upon increasing the exposure time, a small band at 1540 cm−1 is also observed, which is related to bidentate nitrates [3]. On the other hand, FT-IR spectra recorded during the reduction (Fig. 2b) show the gradual consumption of nitrites, upon H2 exposure, without the formation of other surface species. Only minor amounts of nitrites are still present at the end of the rich phase.
On Pt/Al2O3, upon the admission of NO during the lean phase a very small dead-time in the NO breakthrough is observed at the reactor outlet (Fig. 3); small amount of NO2 are observed due to the higher oxidation activity of the Ba-free catalyst. A significant N2O peak has also been observed at the rich-to-lean transition.
Upon the lean-rich switch, a tail is observed in the NOx concentration; simultaneously minor amounts of N2 and NH3 are detected at the reactor outlet. Non-negligible amounts of N2O are also detected, although in smaller amounts than those observed in the case of the Pt–Ba/Al2O3 catalyst.
Surface analysis obtained during the lean phase (Fig. 4a) shows the main formation of nitrites on Al2O3 sites (both ionic, νasym.(NO2) and νsym.(NO2) modes at 1230 and 1314 cm−1, respectively, and linear, ν(N–O) and ν(N=O) modes at 1079 and 1548 cm−1, respectively). Minor amounts of bidentate nitrates (ν(N=O) modes at 1610 and 1470 cm−1) are also observed. Upon H2 admission (Fig. 4b), the progressive consumption of nitrites is observed. Minor amounts of nitrites are still present at the end of the rich phase. However, as opposed to Pt–Ba/Al2O3, over the Pt/Al2O3 catalyst the reduction of nitrites is also accompanied by the appearance of new bands, i.e. at 1256 and 1620 cm−1. According to the literature, these bands can be related to coordinated ammonia species on surface Lewis sites (symmetric deformation and asymmetric deformation, respectively), while the lower intensities bands at 1397, 1486 and 1688 cm−1 can be associated to surface ammonium ions, whose formation enlightens the presence of Brønsted acid sites of alumina [27,28,29]. Moreover, this assignment is in line with results from a dedicated experiment where the ammonia interaction (i.e. 150 ppm in He) with Pt/Al2O3 at 150 °C was tested (see dotted line in Fig. 4b).
Lean-rich cycles at 150 °C have also been carried out over Pt–Ba/Al2O3 catalyst using propylene as reductant in the presence of water. A representative cycle in these conditions is reported in Fig. 5. The reactivity of the catalyst when propylene is used as reducing agent is very poor: the dead time for NO breakthrough is very small, near 10 s, much shorter than in the case of H2 as reductant. Minor amounts of NO2 are also observed at the reactor outlet, together with CO2 at the beginning of the lean phase due to the decomposition of Ba-carbonates upon formation of the adsorbed NOx species [25, 30]. The presence of surface carbonates is expected when propylene is used as reductant: the reduction process produces CO2 that is adsorbed as carbonates. At the beginning of the lean phase, N2O evolution is also observed.
When switching rich, NOx concentration decreases with a small tail, and negligible amount of N2 are observed together with a small peak of N2O. Propylene rapidly reaches the inlet steady state concentration, due to the limited amounts of NOx stored on the surface. This is likely related to both the presence of surface carbonates (that decrease the NOx storage) and to the low activity of propylene as reductant, which is not able to fully reduce surface NOx at such low temperature.
Lean-rich cycles have been performed also at higher temperature, e.g. 180 and 250 °C over Pt–Ba/Al2O3 and Pt/Al2O3 catalysts. It is important to underline that the temperature has an important effect on both the NOx storage capacity and the efficiency of the reducing agent. These two aspects influence the total amounts of the reduction products (being related to the NOx stored amounts) and the selectivity of the process (i.e. the relative amounts of the same reduction products). As expected, by increasing the temperature the NOx storage capacity increases as well [26], and the reducing agent becomes more efficient in the reduction [31]. Temperature also affects N2O formation, as summarized in Fig. 6 showing the outlet N2O concentration profiles and the amounts of N2O emitted at different temperatures in the case of Pt–Ba/Al2O3 when using H2 (Fig. 6a, b) or propylene (Fig. 6e, f) as reductants, and in the case of the Pt/Al2O3 (Fig. 6c, d) catalyst (H2 reductant).
In the case of Pt–Ba/Al2O3, when H2 is used as reductant the primary N2O formation is higher than the secondary at all the investigated temperatures (see Fig. 6a, b); temperature strongly decreases both the primary and secondary the N2O formation. In the case of primary N2O emissions (lean-to-rich transition), it has been suggested that N2O is formed at the regeneration front, upon admission of the reductant (H2) that leads to the release of gaseous NO upon decomposition of the stored NOx. Evolved NO is then reduced over the platinum sites; at the beginning of the reduction phase, Pt sites are only partially reduced (they were in the oxidized state during the previous lean phase) and accordingly NO is reduced primarily to N2O according to the following scheme:
As the reduction process goes on, Pt–O sites are more reduced by the reductant thus restoring Pt metal sites (reactions (4)–(5)):
Over fully reduced metal sites, NO is readily decomposed to N- and O-adspecies; while O-adspecies are scavenged by H2 leading to water (reaction (5)), N-adspecies lead to N2 and NH3 formation (reactions (6) and (7)):
It is therefore clear that the oxidation degree of the Pt metal sites and the relative surface concentrations of the N-, H-, and O-adsorbed entities are the main factors governing the reduction products distribution, and as consequence the selectivity of the reduction process [32].
Notably, the product selectivity measured at the reactor outlet (see Fig. 7a in the case of H2) is affected by the development of a reduction front along the catalytic bed, due to the integral nature of the trap. In fact, according with the sequential mechanism already proposed in the literature [33, 34], the so-formed ammonia may further react with NOx species stored downstream the front leading to the formation of N2. This has been very well documented by reaction studies were ammonia has been used as reductant [18, 21, 34]. In this respect, the chance of N2O to further react with some surface species is also of interest, and will be discussed later on.
At variance, over the investigated Pt–Ba/Al2O3 sample, secondary N2O formation is very small, and is visible only at low temperature. It has been suggested that N2O formation occurs in this case due to the reaction between NO/O2 fed upon the lean phase and reductive species (like NCO, CO or NH3) in an adsorbed state, as reported also by Bártová et al. [5, 9]. In view of the significant NH3 production observed in this case at low temperatures, it is very likely that part of the formed ammonia remains adsorbed on the surface (in small amounts due to the basicity of the surface) and this is responsible for the small N2O peak observed upon the rich-to-lean transition.
Temperature strongly decreases both primary and secondary N2O formation (see Fig. 6a) and pushes the selectivity to N2 (Fig. 7a). In the case of the primary N2O formation, this is related to the fast reduction of the Pt sites that accordingly efficiently decompose NO to N- and O-adspecies, thus favoring N2, NH3 and H2O formation through reactions (1), (2), (4), (5) and (7). Along similar lines, secondary N2O formation decreases with temperature due to the decrease of the adsorbed ammonia.
In the case of Pt/Al2O3 (Fig. 6c, d), primary N2O formation is much smaller due to the lower amounts of stored NOx and hence due to the decrease of gas-phase NO concentration released upon the rich phase. At variance, the secondary N2O peak, originated at the rich-to-lean transition, is higher than the primary one and it is much higher that observed in the case of the Pt–Ba/Al2O3 sample. As pointed out by FTIR measurements, in the case of Pt/Al2O3 catalyst at the end of the rich phase some ammonia is still present at the catalyst surface (Fig. 4b). This ammonia can react with gaseous NO/O2 fed at the beginning of the lean phase, giving N2O, in line with the well-known selectivity to nitrous oxide of the NH3+NO reaction in the presence of O2 over PGM-based catalysts [35]. The higher amounts of adsorbed ammonia with respect to Pt–Ba/Al2O3 are in line with the higher acidity of the Pt/Al2O3 sample. Again, also in this case temperature increase decreases secondary N2O formation due to the decrease of the amounts of ammonia stored on the catalyst surface.
Finally, in the case of Pt–Ba/Al2O3 (Fig. 6e, f) when propylene is used as reductant, a more complex picture is observed since primary N2O formation increases with temperature in the investigated T-range, whereas the opposite is seen for secondary N2O formation. The increase with temperature of the primary N2O formation is related to the increase in the amounts of stored NOx and to the poor reactivity of propylene as reductant. In fact, in this case even at 250 °C the reduction of the Pt sites proceeds rather slowly and accordingly significant amounts of N2O could be formed. At variance, concerning secondary N2O formation, as apparent from Fig. 8 isocyanates species and CO adsorbed on Pt sites are well detected by FT-IR. Indeed, as it appears in Fig. 8 for the rich phase at 250 °C, bands characteristic of CO adsorbed on Pt (band at 2067 cm−1 which shifts towards 2050 cm−1) and of isocyanates NCO− on Ba sites (band at 2170 cm−1) rapidly grow up showing a maximum after 2 min and an intensity loss for higher contact time [31], a typical behavior of intermediate species. CO is formed during the reduction of stored NOx with C3H6 according to a reforming reaction; the so-formed CO then reacts with adsorbed NOx leading to adsorbed isocyanates species, that remain onto the catalytic surface at the end of the rich phase. At the rich-to-lean switch NCO species could react with NO/O2 leading to secondary N2O formation.
3.2 N2O Reactivity
In order to check whether N2O is a terminal species or it may be involved in subsequent reactions once formed, the reactivity on N2O alone (N2O decomposition) or using different reductants (N2O reduction) has been investigated over Pt–Ba/Al2O3 and Pt/Al2O3 catalysts. The results of the decomposition studies, carried out upon heating the samples from room temperature up to 500 °C are shown in Fig. 9a, b in the case of Pt–Ba/Al2O3 and Pt/Al2O3, respectively. The onset temperature for N2O decomposition in both cases is near 400 °C; at 500 °C (maximum ramp temperature) the N2O conversion is near 30%. N2O decomposition leads to the production of N2 and O2. As shown by the figures, the reactivity of N2O in the decomposition reaction is very poor, and therefore does not occur in the investigated temperature range of lean-rich cycles.
The reactivity of N2O towards different reducing agent (H2, NH3, C3H6) has also been studied over both Pt–Ba/Al2O3 and Pt/Al2O3, and results are compared in Fig. 10 in terms of N2O concentration. H2 and C3H6 have been considered being the actual reducing agents investigated in this work; the reactivity of ammonia has also been investigated being NH3 formed upon reaction of the stored NOx with H2.
In the case of H2, over the ternary system the reduction of N2O starts near 90 °C (Fig. 10a) leading to the formation of N2 only (not shown). A slightly lower temperature is observed in the case of the binary system (i.e. near 80 °C, Fig. 10b). However, whereas N2O consumption is complete below 250 °C in the case of the Pt–Ba/Al2O3 sample, over the Ba-free sample complete consumption of N2O is attained slightly below 300 °C. This indicates that the presence of Ba slightly increase the catalyst reactivity.
There is now a general consensus on the fact that the N2O decomposition/reduction over noble metal catalysts involves the formation of O-adspecies (reactions (8), (9)) [10 and ref herein reported]; in the absence of reducing agents, the rate determining step is the recombination of the oxygen adsorbed species with release of molecular oxygen (reaction (11)):
As shown in Fig. 9, this process likely occurs only at high temperatures, being the decomposition of N2O observed only above 300 °C.
At variance, in the presence of a reductant (H2), adsorbed oxygen species are scavenged by H2 and the reaction proceeds at much low temperatures (reaction 12):
Since the rate determining step for the N2O reduction is the removal of surface oxygen, the efficiency of N2O reduction/decomposition depends on the capacity of the reductants and/or the catalyst to facilitate these reactions.
This reaction scheme is supported by several authors. In particular, Cant et al. [36] have demonstrate that the reduction of 15N14NO by hydrogen over a Rh/SiO2 catalyst yields 15N14N as the exclusive nitrogen-containing product. This result rules out a suggestion in the literature that the reduction proceeds through cleavage of the N–N bond. Moreover, McCabe et al. [37] report that the reaction orders and apparent activation energy are consistent with a mechanism involving reaction between adsorbed reductant (in that case CO) and adsorbed N2O dissociation products.
Figure 10 previously discussed in the case of H2 as reducing agent points out that N2O can be reduced at low temperatures by H2. This may suggest that there is a chance, at the regeneration front, for the forming (native) N2O to be at least partially reduced by H2. However downstream the regeneration front, i.e. in the absence of gaseous H2, N2O does not react with the catalyst surface.
Figure 10a, b also compares the N2O concentration profiles measured during the reduction with the different reducing agents as function of temperature over Pt–Ba/Al2O3 and Pt/Al2O3, respectively.
Comparing the onset temperature for N2O consumption the following order of reactivity is apparent: H2 ~ NH3 > C3H6. The same reactivity order is observed in the case of Pt–Ba/Al2O3 and Pt/Al2O3, although on the Ba-free sample ammonia reacts faster than hydrogen even if its onset temperature is higher.
As already discussed in the case of hydrogen, the main role of the reducing agent is to remove adsorbed oxygen species from the surface allowing the N2O reduction. O2 species are scavenged by ammonia and propylene according to the following overall reactions:
Since propylene exhibits a lower capacity than hydrogen and ammonia to promote these reactions, the onset temperature of N2O consumption is observed at higher temperatures.
Interestingly, the temperature range where ammonia is able to convert N2O is the same where N2O evolution is seen during the experiments. Since ammonia, like N2O, is formed at the regeneration front and travels along the catalyst bed, there is a chance for this species to decrease N2O concentration. However, since ammonia also reacts with NOx stored downstream the H2 regeneration front at such temperatures, very likely its efficiency in the N2O reduction is limited, although a role of ammonia in the decrease of N2O emissions cannot be ruled out.
4 Concluding Remarks
In this paper, mechanistic aspects involved in the formation and reduction of N2O over a model Pt–Ba/Al2O3 LNT catalyst have been investigated by means of simultaneous gas phase and surface analysis using FT-IR spectroscopy. N2O evolution is observed both at the lean-to-rich (primary N2O) and rich-to-lean (secondary N2O) switches. In particular, when hydrogen is used as reductant: (i) primary N2O formation is significant, secondary N2O is negligible; (ii) without the supported Ba phase (Pt–Al2O3 sample), also secondary N2O is significant and higher with respect to the primary one; (iii) without the Ba phase, surface intermediate species, i.e. ammonia and ammonium ions, not present in the case of Pt–Ba/Al2O3, are detected; (iv) in all cases, the production of both primary and secondary N2O decreases on increasing temperature. Moreover, when a poor reductant like propylene is used, also in the case of Pt–Ba/Al2O3 the secondary N2O production is higher than the primary one and surface intermediate species, i.e. isocyanates and CO adsorbed on Pt sites, are well detected by FT-IR.
All these results suggest that primary N2O is formed when undissociated NO in the gas phase and partially reduced metal sites are present, while secondary N2O originates from reaction between surface intermediate species and residual NOx at the beginning of the lean phase.
The reactivity of N2O towards different reducing agent (H2, NH3, C3H6) has been studied under programming temperature (TPR). Comparing the onset temperature of N2O consumption, it appears that, as expected, stronger is the reductant lower is the onset temperature; their reactivity order is the same for Pt–Ba/Al2O3 and Pt/Al2O3 catalyst, that is: H2 ~ NH3 > CO > C3H6. The reduction is completely selective to nitrogen and occurs at temperature higher than 250 °C in the case of Pt–Ba/Al2O3 catalyst, while lower temperatures are detected for Pt/Al2O3 catalyst. The rate determining step for deN2O is the removal of surface oxygen; as a consequence, the efficiency of N2O reduction/decomposition depends on the capacity of the reductants and/or the catalyst to facilitate these reactions. Considering that H2 has a high reactivity in the reduction of N2O, it may reacts with N2O that is forming at the regeneration front. Moreover, also ammonia present downstream to the H2 front has the chance to reduce N2O, although this species is more likely involved in the reaction with NOx stored downstream the H2 front.
References
Bielaczyc P, Woodburn J, Szczotka A (2014) Appl Energy 117:134–141
Chen Y, Borken-Kleefeld J (2014) Atmos Environ 88:157–164
Lietti L, Daturi M, Blasin-Aubé V, Ghiotti G, Prinetto F, Forzatti P (2012) ChemCatChem 4(1):55–58
Lashof DA, Ahuja DR (1990) Nature 344(6266):529
Bártová Š, Kočí P, Mráček D, Marek M, Pihl JA, Choi J-S, Toops TJ, Partridge WP (2014) Catal Today 231:145–154
Choi J-S, Partridge WP, Pihl JA, Kim M-Y, Kočí P, Daw CS (2012) Catal Today 184(1):20–26
Clayton RD, Harold MP, Balakotaiah V (2009) AIChE J 55(3):687–700
Dasari P, Muncrief R, Harold MP (2013) Top Catal 56(18):1922–1936
Kočí P, Bártová Š, Mráček D, Marek M, Choi J-S, Kim M-Y, Pihl JA, Partridge WP (2013) Top Catal 56(1):118–124
Jabłońska M, Palkovits R (2016) Catal Sci Technol 6:7671–7687
Ohnishi C, Asano K, Iwamoto S, Chikama K, Inoue M (2007) Catal Today 120:145–150
Xue L, He H, Liu C, Zhang C, Zhang B (2009) Environ Sci Technol 43:890–895
Grzybek G, Stelmachowski P, Gudyka S, Indyka P, Sojka Z, Guillén-Hurtado N, Rico-Pérez V, Bueno-López A, Kotarba A (2016) Appl Catal B 180:622–629
Zou W, Xie P, Hua W, Wang Y, Kong D, Yue Y, Ma Z, Yang W, Gao Z (2014) J Mol Catal A 394:83–88
Zhang X, Shen Q, He C, Ma C, Cheng J, Liu Z, Hao Z (2012) Catal Sci Technol 2:1249–1258
Piumetti M, Hussain M, Fino D, Russo N (2015) Appl Catal B 165:158–168
Kim SS, Lee SJ, Hong SC (2011) Chem Eng J 169:173–179
Cumaranatunge L, Mulla SS, Yezerets A, Currier NW, Delgass WN, Ribeiro FH (2007) J Catal 246(1):29–34
Tamm S, Andonova S, Olsson L (2014) Catal Lett 144(7):1101–1112
Nova I, Lietti L, Castoldi L, Tronconi E, Forzatti P (2006) J Catal 239(1):244–254
Partridge WP, Choi J-S (2009) Appl Catal B 91(1):144–151
Forzatti P, Lietti L, Castoldi L (2015) Catal Lett 145(2):483–504
Castoldi L, Righini L, Matarrese R, Lietti L, Forzatti P (2015) J Catal 328:270–279
Castoldi L, Matarrese R, Morandi S, Righini L, Lietti L (2018) Appl Catal B 224:249–263
Lietti L, Forzatti P, Nova I, Tronconi E (2001) J Catal 204:175–191
Morandi S, Prinetto F, Ghiotti G, Castoldi L, Lietti L, Forzatti P, Daturi M, Blasin-Aubé V (2014) Catal Today 231:116–124
Centi G, Perathoner S, Biglino D, Giamello E (1995) J Catal 152:75–92
Ramis G, Larrubia MA (2004) J Mol Catal A 215:161–167
Sobczyk DP, Hesen JJG, van Grondelle J, Schuring D, de Jong AM, van Santen RA (2004) Catal Lett 94:37–43
Righini L, Kubiak L, Morandi S, Castoldi L, Lietti L, Forzatti P (2014) ACS Catal 4:3261–3272
Castoldi L, Matarrese R, Kubiak L, Daturi M, Artioli N, Pompa S, Lietti L (2018) Catal Today. https://doi.org/10.1016/j.cattod.2018.01.026 (in press)
Lietti L, Artioli N, Righini L, Castoldi L, Forzatti P (2012) Ind Eng Chem Res 51:7597–7605
Bhatia D, Clayton RD, Harold MP, Balakotaiah V (2009) Catal Today 147S:S250
Lietti L, Nova I, Forzatti P (2008) J Catal 257:270
Scheuer A, Hauptmann W, Drochner A, Gieshoff J, Vogel H, Votsmeier M (2012) Appl Catal B 111:445–455
Cant NW, Chambers DC, Liu IO (2011) J Catal 278:162–166
McCabe RW, Wong C (1990) J Catal 121:422–431
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castoldi, L., Matarrese, R., Liu, C. et al. Dynamics and Selectivity of N2O Formation/Reduction During Regeneration Phase of Pt-Based Catalysts. Top Catal 61, 1672–1683 (2018). https://doi.org/10.1007/s11244-018-1022-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-1022-2