Abstract
The combination of a sub-microsecond pulsed dielectric barrier discharge with Pd/γ-Al2O3 catalyst was investigated for the oxidation of low concentration of methane, propene, and toluene in air at atmospheric pressure. The systematic comparison of the plasma-catalysis with the thermal-catalysis and the plasma-alone have been made for temperature window of 20–400 °C and energy deposition in the range 23–148 J L−1. To emphasis the reactivity of plasma produced species on the activation of the catalyst, two reactor configurations were used [in-plasma catalysis (IPC), and post-plasma catalysis (PPC)]. Fourier transform infrared spectrometer was used to follow the VOCs conversion and to quantify the mineralized products. The plasma-catalyst combination leads to higher VOCs conversion and decreases the catalyst activation temperature in both IPC and PPC configurations. For hybrid system, the light-off temperature is always lower than those observed for catalytic oxidation. In addition, the formation of the by-products such as formaldehyde, formic acid, and ozone was reduced significantly. A significant synergetic effect of hybrid plasma-catalytic system is not really observed in the presence of CH4 and C3H6. However, for C7H8 oxidation the synergetic effect was demonstrated at low temperature (<150 °C) for the IPC configuration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Volatile organic compounds (VOCs) emitted from various industrial and domestic processes are important sources of air pollution and therefore, become a serious problem for the human health and the environment. The well-established technologies for the removal of VOCs such as thermal and catalytic oxidation [1] require generally high thermal temperatures and thus a large amount of energy. Thus, these processes become unsuitable and energetically more expensive for the case of moderate gas flows containing low concentrations of VOCs. As an alternative to the thermal and catalytic oxidation, extensive efforts have been made over the last two decades to study the oxidation of different types of hazardous pollutants by means of non-thermal plasmas (NTP) [2–5].
The NTP is well known to be an ionized gas containing mixture of ions, radicals and neutral molecules. The most significant advantage of NTP is that the temperature of the background gas remains close to room temperature. However, NTP technology also has disadvantages like the formation of undesirable by-products such as ozone (O3), aldehydes (CH2O, CH2O2), and nitrogen oxides (NOx). Recently, it was shown that a more effective use of NTP for air pollution control is possible by combining plasma with heterogeneous catalysts [6–10]. In addition, synergetic effects resulting from the interplay between plasma and catalyst are studied for various VOCs. The combination of NTP with a catalytic phase can be achieved either in a single stage (In plasma catalysis, IPC) or a two-stage configuration (Post-plasma catalysis, PPC). In the IPC, the catalysts are in contact with the plasma discharge and the active species are generated in the direct vicinity of the catalyst surface (e.g., excited-state atoms and molecules, reactive radicals, photons, and electrons). Moreover, in the IPC configuration the catalyst surface modification is generally observed [11]. On the contrary, in the PPC arrangement, the catalyst is exposed only to long-lived species that exit from the plasma zone, i.e. mainly O3 [12].
In this work, the oxidation of methane (CH4), propene (C3H6), and toluene (C7H8) in air at atmospheric pressure was investigated using a Dielectric Barrier Discharge (DBD) reactor combined or not with Pd(1 wt%)/γ-Al2O3 catalyst. Results are reported as a function of the temperature, the specific input energy (SIE), and the position of the catalyst with respect to the plasma (IPC and PPC). In addition, the synergetic effect between the plasma and the catalyst, for the specific VOCs, was studied.
2 Experimental Description
2.1 Plasma-Catalysis Reactor and Analytical System
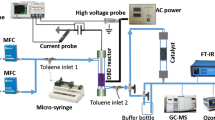
The experimental set-up was described in details elsewhere [13]. The plasma reactor is a dielectric-barrier discharge (DBD) reactor in a cylindrical configuration. A tungsten wire (0.9 mm-diameter) serves as high voltage electrode. It was fixed in the center of a quartz tube (15 mm inner diameter, 300 mm long) by two ceramic rings. The quartz tube was wrapped with a copper grid used as ground electrode. The discharge length was 100 mm and the corresponding discharge volume is 18 cm3. The plasma was driven by a pulsed high-voltage generator delivering an output voltage up to 20 kV into 500 ns pulses (FWHM—full width at half maximum) at a maximum frequency of about 1 kHz. The power delivered to the plasma was measured by means of a voltage (Tektronix P6015A, 1000:1, 5 ns rise time) and a current (Pearson 4100, 1 V/A, 10 ns rise time) probes connected to a digital oscilloscope (Tektronix DPO 3054). The pulse energy (Ep) was calculated by direct integration of the transient voltage and the current over one period. The specific input energy (SIE) was calculated using Eq. (1) where Ep, f, and Q denote the pulse energy, the pulse frequency, and the gas flow rate, respectively. For all experiments Ep is fixed at 13 mJ (applied voltage is 19 kV) and the frequency is varied between 10 and 200 Hz. Under these experimental conditions, the maximum obtained SIE value is 148 J L−1.
The VOCs conversion and the end-products analysis were carried out using a Fourier Transform Infrared spectrometer (FTIR-Nicolet 6700, Thermo-Scientific) equipped with liquid nitrogen cooled mercury-cadmium-tellurium (MCT) detector and 10 m gas path cell.
2.2 Catalyst Preparation and Characterization
The detailed results of catalysts preparation and characterization have been reported elsewhere [13]. The palladium supported catalysts were prepared by impregnating alumina beads (1 mm diameter, Sasol Germany GmbH), in metal precursor palladium tetramine nitrate solution (Pd(NH3)4(NO3)2; STREM Chemicals Inc.). The suspension was stirred for 3 h at 50 °C and at atmospheric pressure. The impregnated samples were dried at 120 °C for 24 h, and then calcined at 500 °C for 4 h at a heating rate of 3 °C min−1, under air flow, to obtain the final form of the supported catalysts.
The amount of Pd loading was determined by using the Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES, Horiba Jobin–Yvon ACTIVA-spectrometer). The specific surface area were determined by the multi-point Brunauer-Emmett-Tellet method (BET, Micromeritics ASAP 2010) using N2 as the sorbate at −196 °C. Before measurement, samples were outgassed at 300 °C at 5 × 10−3 Torr for 3 h. The prepared catalysts are named as Pd(X)/Al2O3, in which X denotes the theoretical content of Pd, and the obtained results are presented in Table 1.
XRD analyses were conducted using a Bruker D5005 powder diffractometer using Cu–Kα radiation. Samples were scanned (0.02°/step) in the 2θ range of 4–80°. For all catalysts, XRD spectra revealed the characteristic peaks of the γ-Al2O3 phase (JCPDS-ICDD 00-010-0425), Pd metal peaks and PdO peaks (JCPDS-ICDD 87-0638). As reported in Table 1, the Pd loading does not change the total surface area of the γ-Al2O3. The average crystallite sizes of the catalysts were calculated using the Scherrer equation. The obtained average PdO particle size is approximately 4.8 nm, and the average Pd metal particle size is about 6.3 nm. The chemical states of the Pd atoms on the support were investigated by an XPS spectrometer (AXIS Ultra DLD Kratos Analytical) using Al-Kα radiation. The XPS result showed Pd2+ peaks as a main oxidation state of Pd, corresponding to the PdO phase in agreement with the results reported by Otto et al. [14].
2.3 Experimental Procedure
For IPC configurations, about 2.2 g catalyst was placed in the middle of the plasma discharge zone. Similarly, for the PPC system, the same amount of catalyst was placed downstream the discharge zone. The corresponding catalyst volume in the reactor is 4.1 mL. In both cases, the catalysts were placed in between quartz wool.
The VOCs oxidation was performed with 1 L min−1 continuous gas flow which corresponds to a gas hourly space velocity (GHSV) of 21 500 h−1. The initial concentration of the VOCs was fixed at (1000 ± 5) ppm. The plasma-catalyst reactor was placed in a tubular furnace (ERALY) and the temperature was increased from 22 to 500 °C at a rate of 5 °C min−1. The temperature was measured by a K-type thermocouple placed on the reactor surface and the uncertainty in the measurements is ±3 °C. The conversion of VOCs has been carried out under steady state condition, i.e. after reaching the VOCs adsorption/desorption equilibrium and a stable temperature. The VOCs conversion was calculated using Eq. 2.
The uncertainty in the VOCs conversion was determined by repeating each experiment for four times. The determined uncertainty is less than ± 5 %. The CO and CO2 (COX) selectivity are calculated using Eq. 3:
where, Y denotes the number of carbon of the specific VOCs. It is worth to mention that, during the plasma process, apart from CO and CO2 many other oxygenated species were identified like CH2O, CH2O2, C6H6, and C7H6O. Hence, for all investigated VOCs, no attempts were made to calculate the total carbon mass balance.
3 Results and Discussion
3.1 Methane Oxidation
Pd supported catalysts have been extensively studied for the catalytic total oxidation of methane [1], nevertheless, only few studies have been devoted to plasma-catalytic systems [13–15]. It is widely reported that in order to achieve the complete oxidation of methane, temperatures higher than 400 °C should be reached. Therefore, it is proposed that by coupling the catalyst phases with a plasma the operating temperature could be reduced significantly [8, 13, 15]. Figure 1 shows the CH4 conversion in air as a function of temperature for plasma-alone, thermal-catalysis and plasma-catalysis hybrid systems (IPC and PPC).
For plasma-alone, i.e. without catalyst, above ∼250 °C, a quasi-linear dependence of the CH4 removal is observed. A maximum of about 67 % conversion is reached at 400 °C with CO and CO2 being the main products. Moreover, below 200 °C, unwanted products such as O3 and HNO3 are also observed which is in agreement with the literature [8].
In the presence of Pd/γ-Al2O3, i.e. in the absence of plasma, CH4 conversion starts around 250 °C, is about 70 % at 350 °C and reaches about 100 % at 400 °C. Finally, when the plasma is combined with the catalyst, in IPC configuration, the shape of the curve is similar to the catalytic system. However, in all the temperature range, about 20 % more CH4 conversion is always observed. The light off temperature T50 (i.e. temperature to achieve 50 % conversion), for plasma-catalysis is only 310 °C. This is 30 °C lower than the light-off temperature in the case of thermal-catalysis and however 70 °C lower than the plasma-alone process. This emphasizes the fact that using plasma-catalyst a high CH4 conversion in air could be achieved at low temperature.
In IPC configuration, the catalyst may affect the discharge properties and could influence the production of excited and short-lived reactive species [7, 16]. On the other hand, a fraction of unstable reactive species can be recombined to produce more stable species such as O3, NO, NO2, HNO2 and HNO3. Only these long-lived species could reach the catalyst placed down-stream the plasma (PPC). Therefore, during plasma operation, the catalyst surface could be modified differently depending on the position of the catalyst. These modifications, due to the combined effect of plasma and temperature, could result to enhance the dispersion of active sites on the catalyst surface [17], could also modify the oxidation state of the supported metal oxides [18], and could increase the specific surface area and/or modify the catalyst structure [19]. Consequently, the CH4 conversion is affected. When the plasma was switched-on, although the difference in CH4 conversion is low (< 6 %) we observed that the amounts of produced CO and CO2 were more important in IPC configuration than in the PPC system. However, after runs characterizations such as XRD and BET were carried out on Pd/γ-Al2O3. No significant changes were observed versus fresh catalyst leading to the conclusion that in our case, the catalytic surface could change in the presence of the plasma but the plasma does not affect the active sites themselves.
3.2 Propene Oxidation
3.2.1 Plasma, Thermal-Catalytic and Plasma-Catalytic Oxidation
Figure 2 compares the C3H6 conversion in air for plasma, thermal-catalysis and plasma-catalysis (IPC and PPC) systems. One can note that the C3H6 conversion, by thermal-catalysis, has a threshold temperature of about 130 °C, and then it steeply increases with increasing the temperature and 100 % conversion is reached at 250 °C. The plasma processing, in presence or in absence of the catalyst, exhibits much lower threshold temperature and the conversion starts already at room temperature. For all the investigated temperatures, the plasma-catalyst hybrid system, in both IPC and PPC, has shown higher C3H6 conversion. The most striking difference is that at 150 °C both IPC and PPC exhibit about 75 % conversion, whereas, the thermal-catalysis presents only 9 % conversion. Below 150 °C, IPC shows higher conversion efficiency than PPC. However, the plasma-alone has shown about 20 % more conversion than in IPC configuration. The lower conversion observed in the plasma-catalytic system can be explained by the presence of numerous by-products such as CO, CH2O, CH2O2, CH3NO3, O3, and NOx observed in all the range of the temperature. Some of these species can be adsorbed on the catalytic sites and inhibit the propene conversion.
3.2.2 Effect of Plasma Input Energy
Figure 3 shows the C3H6 conversion in air for IPC configuration as a function of temperature for different SIE. One can note that C3H6 conversion increases with increasing the plasma input energy. For example, with 55 J L−1, total conversion is achieved at 100 °C, whereas, in the absence of plasma (thermal-catalysis) about 250 °C is required. Indeed, above 87 J L−1, the SIE has shown no significant effect on the C3H6 conversion. Interestingly, T50 of C3H6 oxidation is reduced by more than 160 °C when the plasma discharge is ignited with an energy density of 55 J L−1. For CH4 under similar operating conditions, the difference in T50 between the plasma-alone and plasma-catalytic processes is only 30 °C (even at high values of SIE). Therefore, it can be assumed that the VOCs conversion in the plasma-catalytic system depends both on the input energy and on the nature of the VOCs and their interaction with the catalyst surface [20].
The influence of SIE on CO and CO2 selectivity at 150 °C for IPC configuration is presented in Fig. 4. The CO2 selectivity increases with increasing SIE while the CO formation is not significantly influenced by SIE. At 150 °C and the maximum SIE (148 J L−1), a complete conversion of C3H6 is achieved with about 76 % carbon mass balance (64 % CO2 + 12 % CO). The missing carbon mass balance can be attributed to the identified products such as CH2O and CH2O2.
As reported in Fig. 5, during C3H6 removal at 150 °C, the by-products concentration decrease with increasing SIE. Similar results were observed for methane [13]. The concentrations of CH2O and CH2O2, given in arbitrary units, are determined from the area of the spectral features in the wavenumber range 1850–1650 cm−1 for CH2O and 1250–1000 cm−1 for CH2O2. Therefore, it can be suggested that, at a given temperature, the plasma assist the complete oxidation of C3H6 to CO2 and H2O.
3.3 Toluene Oxidation
3.3.1 Plasma, Thermal-Catalytic, and Plasma-Catalytic Oxidation
The C7H8 conversion, for various configurations, as a function of temperature is reported in Fig. 6. In the case of IPC, below 100 °C, a significant C7H8 desorption has been observed which is not observed either for CH4 or C3H6 (see Figs. 1, 2). Moreover, under similar operating conditions, the C7H8 desorption is not observed for thermal-catalysis and PPC configuration, suggesting that it is mainly the plasma that modifies the C7H8 adsorption/desorption equilibrium on the catalyst surface and leads to a desorption into the gas phase. Similarly that we observed for C3H6 conversion, for thermal-catalysis, C7H8 conversion begins at 150 °C and strongly increases with increasing temperature and a complete conversion is reached at 250 °C. Indeed, for the plasma alone, the conversion begins at room temperature and a maximum of 60 % conversion is obtained at 250 °C. When the plasma is combined with the catalyst, the conversion begins at 100 °C and about 90 % is reached at 200 °C. Under similar operating conditions, only 20 % conversion is achieved by thermal-catalysis. Therefore, it can be assumed that, the plasma-catalyst coupling enhances the C7H8 oxidation due to synergetic effect [21]. Similarly to what we observed for C3H6, the IPC configuration led to a high efficiency for C7H8 oxidation.
3.3.2 Effect of the Plasma Input Energy
As reported in the prior section, compared to thermal-catalysis and PPC, IPC shows a higher conversion above 100 °C. This can be attributed to the catalyst activation by high-energy species such as OH, O and O3 produced by the plasma. In order to emphasis the catalyst activation by plasma, by keeping other parameters constant, the effect of SIE on C7H8 conversion is studied (Fig. 7). Below 100 °C, the C7H8 desorption increases with increasing SIE which supports the hypothesis proposed in the previous section that the plasma discharge modifies the C7H8 adsorption/desorption equilibrium and leads to desorption into the gas phase. The SIE not only increases the C7H8 desorption, but also increases the conversion from 100 °C to 200 °C in which 100 % conversion is found even for low SIE.
The influence of SIE on CO and CO2 selectivity at 150 °C is studied by fixing all other parameters and the results are reported in Fig. 8. For all the investigated SIE, the CO selectivity remains constant, whereas, until 87 J L−1, the CO2 selectivity steeply increases with increasing the SIE and reaches a maximum of about 45 %. Thereafter, it remains constant even with a maximum SIE of 148 J L−1. Therefore, it can be concluded that, above 87 J L−1, neither temperature nor SIE affect the C7H8 conversion and CO2 selectivity. It is worth to mention that along with CO and CO2, C6H6 and C7H6O are the main by-products identified downstream the reactor [22].
3.4 Plasma-Catalyst Synergetic Effect
The term synergetic effect is often used to highlight the enhanced additive effect obtained when two individual processes are combined under similar operation conditions. Under our experimental conditions, to emphasize the synergetic effect of plasma-catalyst hybrid system, the conversions of CH4 (300 °C and 148 J L−1), C3H6 (150 °C and 23 J L−1) and C7H8 (150 °C and 148 J L−1) in air are shown in Fig. 9. According to these data, it is clear that the synergetic effect between plasma and catalyst in IPC was observed only for C7H8 removal. When plasma and catalyst is coupled, about 20 % more conversion is obtained as compared to the sum of that individual conversion. These results are in agreement with the literature [8, 9]. The synergetic effects of plasma-catalytic systems have been already reported for VOCs such as C3H6, C3H8 and C7H8. However, as reported by Whitehead et al. [9] synergetic effect has not been observed for CH2Cl2. The authors suggested that the synergetic effect does not unanimously occur in plasma catalysis processes. It can be concluded that, synergetic effect depends on several factors such as the nature of catalysts, the type of plasma reactor, the operating conditions, and mainly the nature of the pollutants. Although, plasma-catalyst coupling does not show synergetic effect, it highlights the fact that plasma increases the conversion and total oxidation of CH4 and C3H6.
4 Conclusion
The combination of DBD plasma and Pd(1 wt%)/γ-Al2O3 catalyst was found to be an efficient solution to improve the VOCs conversion in air and to lower the light-off temperature of the catalyst. In plasma-catalyst hybrid system, CH4, C3H6, and C7H8 oxidation starts at much lower temperatures and exhibits higher CO2 selectivity compared to catalytic-alone and plasma-alone processes. The increase in SIE increases the VOCs conversion and CO2 selectivity. For CH4, about 20 % more conversion is obtained when the plasma is combined with the catalyst in IPC configuration. For C3H6, at 150 °C, a complete conversion and 50 % CO2 selectivity are obtained at 87 J L−1. Below 100 °C, it was shown that the plasma discharge modifies the C7H8 adsorption/desorption equilibrium and leads to desorption into the gas phase. In addition, at low temperatures, the C7H8 desorption increases with increasing the SIE and a complete conversion and about 40 % CO2 selectivity are obtained at 200 °C and 55 J L−1.
It was shown that, under similar operating conditions, the VOCs conversion, and the CO and CO2 selectivity depend on the nature of the VOCs and the affinity between the VOCs and the catalyst. The coupling of plasma and catalyst, at room temperature, decreases the formation of the by-products (CO, CH2O, CH2O2, HNO3). At high temperature, the thermal-catalytic effect is more dominant than the plasma-catalytic effect. In the IPC configuration, the synergetic effect between plasma and catalyst is observed only for the C7H8 oxidation process, whereas, in the PPC system, the synergistic effect was not observed for the three VOCs investigated.
References
Gélin P, Primet M (2002) App Catal B Environ 39:1–37
Kim HH (2004) Plasma Process Polym 1:91–110
Vandenbroucke AM, Morent R, De Geyter N, Leys C (2011) Hazard Mat 195:30–54
Khacef A, Cormier JM, Pouvesle JM (2002) J Phys D Appl Phys 35:1491–1498
Orlandini I, Riedel U (2004) Catal Today 89:83–88
Van Durme J, Dewulf J, Leys C, Van Langenhove H (2008) Appl Catal B Environ 78:324–333
Than Quoc AH, Pham Huu T, Le Van T, Cormier JM, Khacef A (2011) Catal Today 176:474–477
Baylet A, Marécot P, Duprez D, Jeandel X, Lombaert K, Tatibouët JM (2012) Appl Catal B Environ 113–114:31–36
Whitehead JC (2010) Pure Appl Chem 82:1329–1336
Djéga-Mariadassou G, Baudin F, Khacef A, Da Costa P (2012) In: Parvulescu et al (Ed.) Plasma chemistry and catalysis in gases and liquids, Wiley-VCH Verlag GmbH, pp 89–125
Guo YF, Ye DQ, Chen KF, He JC, Chen WL (2006) J Mol Catal A Chem 245:93–100
Sivachandiran L, Thevenet F, Rousseau A (2014) Chem Eng J 246:184–195
PhamHuu T, Gil S, DaCosta P, Giroir-Fendler A, Khacef A (2015) Catal Today 257:86–92
Otto K, Haack LP, DeVries JE (1992) Appl Catal B Environ 1:1–12
Demidyuk V, Whitehead JC (2007) Plasma Chem Plasma Process 27:85–94
Da Costa P, Marques R, Da Costa S (2008) Appl Catal B Environ 84:214–222
Hua W, Jin L, He X, Liu J, Hu H (2010) Catal Commun 11:968–972
Foix M, Guyon C, Tatoulian M, Da Costa P (2010) Catal Commun 12:20–24
Liu CJ, Yu K, Zhang Y, Zhu X, He F, Eliasson B (2004) Appl Catal B Environ 47:95–100
Burch R, Crittle DJ, Hayes MJ (1999) Catal Today 47:229–237
Sun Y, Zhou L, Zhang L, Sui H (2012) J Environ Sci 24:891–896
Fan X, Zhu TL, Wang MY, Li XM (2009) Chemosphere 75:1301–1306
Acknowledgments
The authors gratefully acknowledge S. Gil and A. Giroir-Fendler, IRCELYON, for assistance in catalysts analysis and the fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pham Huu, T., Sivachandiran, L., Da Costa, P. et al. Methane, Propene and Toluene Oxidation by Plasma-Pd/γ-Al2O3 Hybrid Reactor: Investigation of a Synergetic Effect. Top Catal 60, 326–332 (2017). https://doi.org/10.1007/s11244-016-0619-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-016-0619-6