Abstract
The decomposition of toluene on γ-alumina, MnO2-alumina and Ag2O-alumina catalysts in a plasma-catalytic reactor is tested. A comparison between catalytic, catalyst-after-plasma and catalyst-in-plasma systems is made in 150–400 °C temperature range. An Arrhenius plot is made in order to deduce the mechanism of plasma activation. It was found that there is no difference between the measured activation energy for catalytic and catalyst-after-plasma systems. On the other hand it was found that plasma could activate catalyst placed inside of the discharge. Plasma treatment decreases the activation energy for the silver-alumina catalyst but does not increase the number of active centers on the surface of Ag2O-alumina. In case of MnO2-alumina, the activation mechanism is different: plasma does not change the activation energy and but does increase its efficiency due to formation of additional active centers. The mechanism of catalyst activation in plasma, which includes the structural change of manganese ions, is suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-thermal, atmospheric pressure plasma processing is now accepted as a proven environmental clean-up technology for the removal of pollutants (e.g. NOx, particulates, solvents, and chlorofluorocarbons, etc.) from waste gas streams. This technology is currently being investigated for the removal of NOx [1, 2], odours [3], and volatile organic compounds (VOCs) [4, 5] from many industrial fields.
However, it was found that the plasma treatment leads to the formation of various unwanted byproducts [5–7]. Hence, in order to improve and increase the efficiency of VOC decomposition, an innovative technology has been proposed where a plasma reactor was coupled with a catalytic reactor [8–13]. Such a combination helps overcome some of the disadvantages of both catalytic and plasma treatments. The combination of non-thermal plasma with catalyst can be achieved in either single stage or two-stage processes. In the two-stage system, where the catalyst is located after the plasma reactor, the main role of plasma is partial conversion of pollutants and the production of intermediate species such as ozone to facilitate catalytic reactions over downstream catalysts. In the case of the single-stage configuration, the catalyst is placed in the plasma reactor, and is activated by high energy particles (electrons, excited molecules and photons) produced by the non-thermal plasma.
In this paper, plasma-catalytic decomposition of toluene on alumina, MnO2-alumina and Ag2O-alumina catalysts is reported. A comparison between catalytic, catalyst-after-plasma and catalyst-in-plasma systems is made over a large temperature range. An Arrhenius plot is made in order to deduce the mechanism of plasma activation. It is shown that plasma plays an important role in enhancing catalytic activity.
Experiment
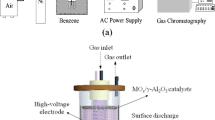
The experimental setup was based upon that used previously [14] modified for the introduction of a catalyst and supplied with heating [15–17]. The experiments were carried out using a non-thermal, atmospheric pressure plasma, consisting of a BaTiO3 dielectric packed-bed reactor: a quartz tube of 24 mm internal diameter with two electrodes apart through which the gas passes. The space between the electrodes was packed: (a) with 3.5 mm barium titanate beads in the case of the catalyst-after-plasma and plasma only system, or (b) the gaps between BaTiO3 were filled with (0.85–1.7) mm granules of catalyst in case of the catalyst-in-plasma system. A volume ratio 5:12 of catalyst to BaTiO3 were used here. A heater around the plasma reactor was used to control the temperature of the catalyst-in-plasma system. On the other hand, only the catalyst was heated for the catalyst-after-plasma system as shown in Fig. 1, the plasma reactor being unheated. The same volume (7 ml) of a catalyst was applied in both catalyst-in-plasma and catalyst-after-plasma systems.
An alternating current (AC) voltage between 16.5 k and 17.5 k Vpk-pk at a frequency between 10.25 kHz and 13.25 kHz is applied between the electrodes. Using a digital storage oscilloscope (Tektronix TDS 3012), current and voltage waveforms can be recorded for the discharge by using a calibrated high-voltage probe and measuring the current across a 1 kΩ resistor in the return earth path from the reactor. This produces an average power of 1 W.
The dry “air” carrier gas used was made by blending nitrogen (800 cm3/min) and oxygen (200 cm3/min) (BOC Gases) to give a total gas flow of 1 L min−1. For toluene (VWR International. Ltd.), nitrogen gas was passed through the gas bubbler, the gas flow was varied to obtain an initial concentration of 500 ppm.
The toluene decomposition was investigated on the surface of γ-Al2O3, Ag2O(7%)/Al2O3 and MnO2(7%)/Al2O3 catalysts. The conventional wetness impregnation method was applied for the catalyst preparation. Silver and manganese were loaded on the alumina pellets using silver (VWR International. Ltd.) and manganese (Alfa Aesar) nitrate precursors. Nitrate solutions were added to a pellet type alumina with the diameter of granules being 3–4 mm and total surface area being 180 m2/g. Then the catalysts were dried at 120 °C and calcinated at 400 °C. The prepared catalyst pellets were crushed and 0.85–1.7 mm fractions were utilized for investigation.
The identity of the end products of the plasma processing and the degree of destruction were monitored on line by infrared spectroscopy using the integrated gas analysis FTIR spectrometer (Gasmet DX-4010, Telmet, 9.8 m pathlength, resolution ∼7.7 cm-1).
Results
It is necessary to recognise that the majority of studies of VOC decomposition using plasma has been done for the “cold” plasma condition, i.e. without additional external heating. In the present investigation some experiments (catalyst-in-plasma) were carried out at high temperature in the plasma reactor. So it is important to determine the influence of temperature on the toluene decomposition rate as well as its thermal degradation on the surface of barium titanate. It is clear from Fig. 2 that the efficiency of plasma treatment (without catalyst) goes up with temperature from 40% to 75% in a temperature interval 140–400 °C. The concentration of ozone formed in discharge decreases with increasing temperature as shown here. On the other hand, Fig. 2 shows that a poor catalytic decomposition of toluene on the BaTiO3 surface is observed. A similar temperature dependence was obtained for the plasma decomposition of acetone, benzene, ethylene [17]. It was demonstrated that non-thermal plasma process removes VOCs more effectively as the process temperature increases. It is possible to explain these results with the increased kinetic reaction rates of O radicals at elevated temperature.
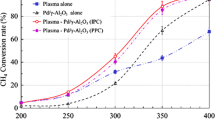
A comparison between catalytic, catalyst-after-plasma and catalyst-in-plasma systems is presented in Figs. 3, 4, 5 for the γ-Al2O3, Ag2O/Al2O3 and MnO2/Al2O3 catalysts correspondingly. An evaluation of the different catalysts shows that both silver and manganese catalysts show similar trends in toluene decomposition but alumina shows a low efficiency even at high temperature as might be expected.
The main problem of VOC removal in a plasma system is by-product formation. In a plasma reactor, ions, excited molecules and free radicals are formed initiating sequential chemical reactions. The radicals interact with VOC molecules, but there is often not enough energy for complete oxidation so it leads to the formation of by-products such as CO, O3, aerosol particles and polymers [7, 18].
Similar data were obtained in the present investigation. The analysis of carbon balance shows that toluene decomposition is not a complete oxidation to CO2 and the quantity of “lost carbon” depends on temperature as it shown on the Figs. 6, 7, 8. The “lost carbon” was calculated as a difference between the inlet carbon in the form of toluene and the outlet carbon in the form of CO2. It is clear that the catalysts move the process towards total oxidation at high temperature. The by-products formed during the plasma treatment are oxidized to CO2, so the main disadvantage of plasma treatment can be successfully solved by catalyst application. A high level of the “lost carbon” in case of catalyst alone system could be explained by incomplete oxidation of toluene to CO2 at low catalytic temperature. The main by-product formed in the discharge was CO, so the “lost carbon” was in form of CO and a small quantity of unidentified compounds. Fig. 9 shows the temperature dependence of CO concentration formed in plasma, catalyst-after-plasma and catalyst-after-plasma reactors for different catalysts. Unfortunately, the plasma generates high concentrations of carbon monoxide and it seems to be that even catalyst application cannot completely remove CO. Perhaps, higher temperature and additional or alternative catalysts are needed for total CO oxidation.
Carbon monoxide formation in plasma catalytic reactor as a function of temperature: (★) plasma only, alumina: (■) catalyst only, (□) catalyst-after-plasma, (⬓) catalyst-in-plasma Ag2O/Al2O3: (▴) catalyst only, (Δ) catalyst-after-plasma, (Δ) catalyst-in-plasma MnO2/Al2O3(●) catalyst only, (○) catalyst-after-plasma, (◒) catalyst-in-plasma
Discussion
It is clear from Figs. 3–5 that the catalyst-in-plasma method shows the best efficiency for the all investigated catalysts. This fact could be explained by the dual effect of plasma treatment at high temperature. Firstly, as was said before the efficiency of plasma decomposition increases with temperature so catalyst-in-plasma system should be definitely more effective in comparison with catalyst-after-plasma (only the catalyst is heated here). In addition, plasma provides extra activation of the catalyst as can be seen from calculated activation energies for the catalytic, catalyst-after-plasma and catalyst-in-plasma systems. Fig. 10, 11 show the Arrhenius plot of the toluene decomposition for the silver-alumina and manganese-alumina systems, where Co and C are initial and final concentrations of toluene. The determined activation energy and A parameter of the Arrhenius equation are presented in Table 1. It is clear that the physical meaning of the A parameter could be defined as a characteristic number of active centres on the catalyst surface. Unfortunately it was not possible to get Arrhenius plots for pure alumina due to a low decomposition rate on its surface.
First of all, it is necessary to acknowledge from the comparison of temperature dependence that no catalyst activation by plasma in catalyst-after-plasma reactor is seen for both silver and manganese oxides. It seems to be that plasma simply decreases the toluene concentration without any catalyst promotion. These data differ from the investigation of VOC decomposition in catalyst-after plasma system published previously [19], where in order to determine the mechanism of the plasma treatment some amount of ozone was added to the gas flow. VOCs were found to react with ozone at lower temperature than with oxygen, and also with lower activation energy. The authors suggested that the mechanism of the observed catalyst improvement by plasma included reaction of ozone with catalyst surface. Ozone decomposed on the active centers and generated surface oxygen radicals. All of this led to an increase in the efficiency of the catalytic centers. The improvement was higher at low catalyst temperature. It was suggested that the lifetime of ozone molecules at high temperatures was too short for any increase in catalyst efficiency [19].
The presence or absence of ozone could explain our results too. The ambient operating temperature of the BaTiO3 dielectric packed-bed reactor in the present work was nearly 140 °C and it produced low concentration of ozone from the air carrier gas, less then 5 ppm. It would seem that this quantity of ozone is not enough for significant activation of the catalyst and the influence of other excited molecules like O-atoms or radicals will be quite small because of their short lifetime. This could explain why we could not detect a big difference between the measured activation energies for catalytic and catalyst-after-plasma systems.
On the other hand, plasma could activate catalyst placed inside of the discharge. It is clear that plasma treatment decreases the activation energy for silver-alumina due to generated surface oxygen radicals as is suggested in [19]. It seems to be that plasma does not increase the number of active centers on the surface of Ag2O. In the case of manganese dioxide, the activation mechanism is quite different: plasma does not change the activation energy and the catalyst increases the destruction efficiency because of formation of additional active centers within plasma treatment.
The activation of catalyst placed inside of a BaTiO3 packed-bed plasma reactor was also observed in [11, 12]. It was reported that plasma could initiate the benzene decomposition on photocatalyst (TiO2, Ag/TiO2 and Pt/TiO2) at as low temperature as 100 °C. The authors did not suggest an activation mechanism but it was admitted that the mechanism of plasma activation differed from mechanism of photo activation.
In our case, the formation of additional active centers could be explained by changing the oxidation state of manganese ions. The investigation of MnO2/SiO2 catalyst showed that the manganese metal center was in a higher oxidation state than in the normal dioxide i.e. closer to a +5 oxidation state as opposed to the expected +4 value [20]. In addition, it was reported that that manganese oxides supported on alumina allow the structural changes during ozone decomposition reaction and Mn is oxidized to the higher oxidation state [21] and the mechanism of ozone decomposition on MnOx/Al2O3 catalyst proceeds by electron transfer from the Mn site to ozone and Mn is reduced back in the desorption of the oxygen species [22]:
The plasma reactor utilized in the present work generated low ozone concentration due to ozone decomposition into O-atom and molecular oxygen at high temperature (Fig. 2). Nevertheless we suggest that interaction between O-atoms and Mn-sites can lead the increase of oxidation states of the Mn ions too.
It has been found that efficient ethanol oxidation on metal-oxide catalysts can be associated with the availability of empty electronic energy states in the metal oxide-support complex: a higher oxidation state implies a larger number empty d-states and greater reaction ability (electron accepting nature) of the active center [23].
In our opinion the mechanism of catalyst activation in plasma might result in change of the oxidation state of the Mn ions, which were un-reactive in normal condition, under the influence of high voltage. The interaction of active molecules (ozone, atoms, and radicals) with the catalyst surface can promote electron transfer from Mn sites too. Further investigations are necessary to find out the contribution of these effects as well as to find out the difference between different catalysts. Activity of silver-oxide catalyst in hydrocarbon decomposition depends on oxidation state of silver ions too [24], but at the present stage it is difficult to explain the fact that plasma does not change the number of active centers on silver oxide surface.
Conclusion
The effectiveness of plasma-catalytic reactor for decomposing of toluene on alumina, MnO-alumina and Ag2O-alumina catalysts is demonstrated in the present studies. A comparison between catalytic, catalyst-after-plasma and catalyst-in-plasma systems is made for a large temperature range. An Arrhenius plot is made in order to deduce the mechanism of plasma activation. It was found that there is no a big difference between activation energy for catalytic and catalyst-after-plasma systems. On the other hand, plasma could activate catalyst placed inside of discharge. Plasma treatment decreases the activation energy for the silver-alumina system but does not increase the number of active centers on the surface of Ag2O. In case of manganese dioxide, the activation mechanism is quite different: plasma does not change the activation energy and catalyst increases its efficiency due to formation additional active centers.
References
Dinelli G, Rea M (1990) J Electrostat 25:23
Kwak JH, Peden CHF (2006) J Szanyi, Cat Lett 109:1
Zhang R, Yamamoto T, Bundy DS (1996) IEEE Trans. Ind. Applicat 32:113
Yamamoto T, Ramanathan K, Lawless PA, Ensor DS, Newsome JR (1992) IEEE Trans Ind Appl 28:528
Oda T, (2003) J Electrostat 57:293
Oda T, Takahashi T, Yamaji K (2002) IEEE Trans Ind Appl 38:873
Demidiouk V, Moon SI, Chae JO (2003) Catal Commun 4:51
Kim HH, Takashima K, Katsura S, Mizuno A (2001) J Phys D Appl Phys 34:604
Francke KP, Miessner H, Rudolph R (2000) Plasma Chem Plasma Process 20:393
Ogata A, Itoa D, Mizunoa K, Kushiyamaa S, Gala A, Yamamoto T (2002) Appl Catalysis A General 236:9
Bubnov AG, Burova EYu, Grinevich VI,Rybkin VV, Kim JK, Choi HS, (2006) Plasma Chem Plasma Process 26:19
Kim HH, Lee YH, Ogata A, Futamura S (2003) Catal Commun 4:347
Kim HH, Oh SM, Ogata A, Futamura S, (2005) Appl Catalysis B Environmental 56:213
Pringle KJ, Whitehead JC, Wilman JJ, Wu J (2004) Plasma Chem and Plasma Process 24:421
Harling AM, Wallis AE, Whitehead JC, Plasma Proc and Polymers (submitted)
Harling AM, Whitehead JC, Futamura S, Kim H-H, J Phys Chem C (submitted)
Hsiao MC, Penetrante BM, Merrit BT, Vogtlin GE, Wallman PH (1997) J Adv Oxid Technol 2:306
Guo YF, Ye D-Q, Chen KF, He JC, Chen WL (2006) J Mol Catal A Chemical 245:93
Demidiouk V, Chae JO (2005) IEEE Trans Plasm Science 33:157
Brown NMD, Mcmonagle JB, Greaves GN (1984) J Chem. Soc Faraday Trans 1 80:589
Einaga H, Harada M, Futamura S, (2005) Chem Phys Lett 408:377
Radhakrishnan R, Oyama ST, Chen JG, Asakura K, (2001) J Phys Chem B 105:4245
Zhang W, Desikan A, Oyama ST, (1995) J Phys Chem 99:14468
Kundakovic L, Flytzani-Stephanopoulos M (1999) Appl Catal A General 183:35
Acknowledgements
The present work was carried out with the support of a Marie Curie International Incoming Fellowship from the European Community (EC Contract Number: MIF1-CT-2005-007801).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demidyuk, V., Whitehead, J.C. Influence of Temperature on Gas-Phase Toluene Decomposition in Plasma-Catalytic System . Plasma Chem Plasma Process 27, 85–94 (2007). https://doi.org/10.1007/s11090-006-9045-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-006-9045-z