Abstract
Heterogeneous ruthenium-based catalysts were applied in the selective, aerobic oxidation of 5-hydroxymethylfurfural, a versatile biomass-derived chemical, to form 2,5-furandicarboxylic acid. The oxidation reactions were performed in water with dioxygen as the oxidant at different pressures without added base. Catalysts were prepared by depositing catalytically active Ru(OH)x species on a number of different supports, such as titanium-, aluminum-, cerium-, zirconium-, magnesium- and lanthanum oxides, magnetite, spinel, hydrotalcite and hydroxyapatite. All the catalysts were found to be active in the oxidation reactions, and the choice of support was demonstrated to be important for the catalytic performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the interest for production of fine and bulk chemicals from biomass-based resources has increased significantly [1]. An example of such a bio-based chemical is 2,5-furandicarboxylic acid (FDA) [2]. FDA can be prepared by selective oxidation of 5-hydroxymethylfurfural (HMF) which is available from hexose monosaccharides, e.g. glucose and fructose, by acid-catalyzed dehydration [3, 4]. This makes FDA a bio-renewable feedstock directly available from biomass. FDA has in particular been promoted as an important renewable building block for production of plastic, due to its similarity to the fossil feedstock terephthalic acid [1].
Selective oxidation of organic molecules has attracted increasing attention over the past decade, especially with molecular oxygen [5–9]. In aerobic oxidations, air or molecular oxygen are used as the oxidants instead of classical metal oxides, containing, e.g. chromium or manganese. Oxygen is considered a “green” oxidant because it produces water as the only by-product unlike the aforementioned metal oxides, which generate stoichiometric amounts of metal waste. From an economic point of view, aerobic oxidation is also very attractive due to the low cost of oxygen and its unlimited accessibility.
A number of different heterogeneous catalyst systems have previously been reported for the selective oxidation of HMF to FDA. One of the first successful studies was made by Vinke et al. [10] using supported Pd or Pt catalysts under alkaline conditions. Here, ruthenium supported on carbon was also shown to be an active catalyst for the reaction, though not giving quantitative yield and possessing low catalyst stability. A Pt/C catalyst promoted with bismuth was further applied for HMF oxidation by Kröger et al. [11]. Recently, Corma and coworkers further showed that also gold nanoparticles supported on different metal oxides can catalyze the selective oxidation of HMF to FDA in good yields, although only in presence of base [12].
Several reactions are known in the literature to be catalyzed by ruthenium-based catalysts [13–16], including oxidation reactions [17]. However, the number of reports on heterogeneous ruthenium-based oxidation catalysts is limited and (beside Vinke et al. [10]) primarily reported by the groups of Kaneda [18, 19] and Mizuno [20, 21] for oxidations of alcohols to oxo compounds in organic solvents.
Very recently we have shown that it is possible to oxidize HMF aerobically to FDA using a heterogeneous, ruthenium oxide-hydroxide catalyst [22]. In this study, Ru(OH)x was supported on three different magnesium-containing supports: MgO (magnesium oxide), MgAl2O4 (spinel) and Mg6Al2(CO3)(OH)16·4H2O (hydrotalcite), and a clear support dependence on the catalyst activity and FDA yield was found.
Here we present the usage of other heterogeneous Ru(OH)x-based catalysts in the selective aerobic oxidation of HMF to FDA. Several catalysts were prepared with different supports, characterized by electron paramagnetic resonance (EPR), x-ray powder diffraction (XRPD), nitrogen sorption (BET area), transmission electron microscopy (TEM) and energy dispersive spectroscopy (EDS) and their catalytic activity compared. Notably, the oxidations were conducted in water and without the addition of base (Scheme 1). The effect of oxygen pressure and reaction time on the yield of FDA was examined.
2 Experimental
2.1 Materials
HMF (>99%), 2-furoic acid (98%), levulinic acid (LA) (98%), formic acid (FA) (98%), ruthenium(III) chloride (purum), hydrotalcite (HT), magnetite (>98%), hydroxyapatite (HAp) (>97%), aluminium oxide (>99.9%), zirconium oxide (99%), lanthanum(III) nitrate hexahydrate (99.99%) and sodium hydroxide (>98%) were acquired from Sigma-Aldrich. Ruthenium(III) nitrate hexahydrate (99.9%) and magnesium nitrate hexahydrate (p.a.) were obtained from Merck. Cerium oxide (99.5%) and lanthanum(III) oxide (99.9%) were purchased from Alfa Aesar. Magnesium oxide (p.a.) was purchased from Riedel-de Haën AG. 2,5-Diformylfuran (DFF) (98%) was obtained from ABCR GmbH & Co. 2,5-Furandicarboxylic acid (FDA) (>99%) and 5-hydroxymethyl-2-furan-carboxylic acid (HMFCA) (> 99%) were purchased from Toronto Research Chemicals Inc. and dioxygen (99,5%) from Air Liquide Denmark. All chemicals were used as received.
2.2 Catalyst Preparation
Magnesium-lanthanum oxide was prepared by co-precipitation and supported Ru(OH)x catalysts by deposition–precipitation procedures described elsewhere [20, 21, 23].
21.7 g (0.05 mol) La(NO3)3·6H2O and 38.4 g (0.15 mol) Mg(NO3)2·6H2O were dissolved in 250 mL water. Then 1 M solution of KOH was added in small portions to maintain pH around 12 over a time period of 8 h. Hereafter, the formed precipitate was filtered, washed with water and calcined at 650 °C for 6 h.
4.88 g of support (i.e. TiO2, Al2O3, Fe3O4, CeO2, ZrO2, MgO, MgAl2O4, HT, La2O3 or HAp) was added to 143 mL of 8.3 mM aqueous RuCl3 solution (1.19 mmol Ru). After stirring for 15 min, 28 mL of 1 M NaOH solution was added and the mixtures were stirred for 18 h. Then the catalysts were filtered off, washed thoroughly with water (colourless filtrates suggested absence of ruthenium ions) and dried at 140 °C for 40 h. A similar preparation procedure was applied for MgO·La2O3 supported catalyst, except that no base was added to the mixture. Approximately 4.9 g of each catalyst was obtained containing 2.4 wt% Ru.
2.3 Oxidation Reactions
HMF oxidation reactions were carried out in stirred Parr autoclaves equipped with internal thermocontrol (T316 steel, Teflon™ beaker insert, 100 mL). In each reaction the autoclave was charged with 63 mg of HMF (0.5 mmol) and 10 mL of water. This initial HMF concentration (0.05 M) was chosen to ensure complete dissolution by extrapolation of the experimental data on FDA solubility in water to 140 °C. Subsequently, the supported Ru(OH)x catalyst was added (0.025–0.105 g, 0.006–0.025 mmol Ru) and the autoclave was flushed and then pressurized with dioxygen (1–40 bar, ca. 1.6–64 mmol) and maintained at 140 °C for a given period of time under stirring (700 rpm).
After the reaction, the autoclave was rapidly cooled on ice bath to room temperature (i.e. 20 °C) and a sample taken out for HPLC analysis (Agilent Technologies 1200 series, Aminex HPX-87H column from Bio-Rad, 300 mm × 7.8 mm × 9 μm, flow 0.6 mL/min, solvent 5 mM H2SO4, temperature 60 °C) after filtering off the catalyst and measuring of the pH value. FDA concentration was measured in a similar way, after addition of 1 mL of 1 M NaOH solution to the post-reaction mixture. Reference samples were used to quantify the products. In recycling studies the catalyst was filtered off from the post-reaction mixture, washed with 0.1 M NaOH and water, and dried at 140 °C for 12 h before reuse.
2.4 Catalyst Characterization
XRPD patterns were recorded using a Huber G670 powder diffractometer (Cu-Kα radiation, λ = 1.54056 Å) in the 2θ interval 5–100°.
EPR spectra (X band) were measured with a Bruker EMX-EPR spectrometer at room temperature with a rectangular 4102 ST cavity operating in the TE102 mode. The microwave source was a Bruker ER 041 XG Microwave bridge with frequencies around 9.22 GHz.
TEM images were recorded on a FEI Tecnai Transmission Electron Microscope at 200 kV with samples deposited on a carbon support. EDS analysis was performed with an Oxford INCA system.
Surface areas were determined by nitrogen sorption measurements at liquid nitrogen temperature on a Micromeritics ASAP 2020. The samples were outgassed in vacuum at 150 °C for 4 h prior to the measurements. The total surface areas were calculated according to the BET method.
Inductively coupled plasma spectrometry (ICP) analysis was performed on diluted post-reaction mixture and quantified with ICP standard solutions (Fluka) on a Perkin Elmer ELAN 6000 with cross-flow nebulizer and argon plasma.
3 Results and Discussion
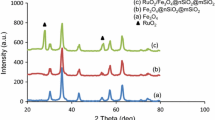
XRPD analysis of the prepared Ru(OH)x catalysts with TiO2, CeO2 or MgO·La2O3 support (2.4 wt% Ru) did not reveal crystalline ruthenium oxide phases. However, at higher metal loading, corresponding to 40 wt% Ru, ruthenium dioxide was clearly found (diffractograms of Ru(OH)x/TiO2 materials are shown in Fig. 1). This observation could indicate that amorphous ruthenium oxide might also be present in the 2.4 wt% Ru catalysts.
In the recorded EPR spectra of the catalysts (not shown) trace amounts of Ru(III) could only be identified in the hydrotalcite-supported 2.4 wt% catalyst. However, in the Ru(OH)x/TiO2 material with 40 wt% Ru, ruthenium(III) was also identified, thus suggesting that both oxidation states were present in the ruthenium oxide, i.e. RuO2·Ru2O3, with RuO2 as the major component and Ru(III) oxide in trace amount [24].
TEM images of the prepared Ru(OH)x/TiO2 and Ru(OH)x/CeO2 catalysts are presented in Fig. 2. Ruthenium species were not observed on the surface of titania, possibly due to their small size and the microscope resolution.
EDS analysis was performed on both whole catalyst samples and on random areas on the catalysts (see Fig. 2). Atomic ratios of Ru:Ti and Ru:Ce were determined to be 1.8:98.2 and 3.7:96.3, respectively, on both the whole catalyst and random area measurements. Thus, the weight percentage of Ru on titania and ceria was found to be 2.32 and 2.26 wt%, respectively, which is in good accordance with the expected content calculated from the preparation procedure.
In previous work, we explored the oxidation reaction of HMF to FDA in water solutions with added base using titania-supported gold nanoparticle catalyst [4]. Here, we initially investigated Ru(OH)x/TiO2 as catalyst in the HMF oxidation reaction in aqueous media without added base.
Firstly, experiments were carried out at 1 bar dioxygen pressure at 140 °C. After 2 h of reaction most of the HMF remained unconverted under these reaction conditions with less than 1% of FDA being formed. However, already at this reaction time decomposition to formic acid (FA) occurred resulting in a yield of 13.8% which increased to 55.4% after 20 h of reaction. FDA yield amounted after this reaction time to only 2.3%.
Further, we investigated the reaction at increased oxygen pressures. The products formed in oxidation reactions at 2.5 and 20 bars of dioxygen as a function of reaction time are presented in Fig. 3a and b, respectively.
The observed intermediate products were identified as HMFCA and DFF, as also previously found [4, 22].
At both examined oxygen pressures a significant formation of formic acid occurred, which was not the case in the analogous Au/TiO2-catalyzed aqueous oxidation [4]. Here, presence of sodium hydroxide facilitated FDA formation and prevented the formation of the acid-catalyzed degradation products [1, 4, 25]. Nevertheless, at 20 bars of dioxygen the formation of FDA occurred significantly faster than at 2.5 bar with the Ru(OH)x/TiO2 catalyst, whereas the reaction rate for degradation did not seem to increase. Thus, by performing oxidation of HMF in water solutions with Ru(OH)x/TiO2 catalyst at elevated pressure, it proved possible to obtain high selectivity towards 2,5-furandicarboxylic acid and high substrate conversion, while avoiding the formation of degradation by-products, such as FA and LA.
Different metal oxide supports, spinel (MgAl2O4), hydrotalcite [HT; Mg6Al2(CO3)(OH)16·4(H2O)] and hydroxyapatite [HAp; Ca10(PO4)6(OH)2] were screened in order to find a system with supported Ru(OH)x species that could provide high selectivity towards desirable oxidation products.
Characteristics of the screened supports and corresponding catalysts are compiled in Table 1. The results obtained in HMF oxidation with the catalysts are shown in Fig. 4.
As seen in Fig. 4, catalysts with basic magnesium-containing support generally showed high efficiency in HMF to FDA oxidation, whereas the usage of other oxides (e.g. ZrO2 and Al2O3) induced the formation of formic acid. Fe3O4 and hydroxyapatite supported Ru(OH)x catalyst revealed good selectivities towards FDA formation, however, in both cases formation of solid humins was observed constituting approximately 30% of the mass balance.
The supported catalysts with basic carrier materials, i.e. MgO, MgO·La2O3 and HT gave excellent selectivities and substrate conversions resulting in FDA yields above 95%. However, ICP analysis of the post-reaction solutions showed presence of magnesium ions, indicating that the support dissolved to a certain extent during reaction [23, 26]. This was confirmed by the relative high pH values measured in post-reaction mixtures with these supports, which was obtained from basic hydroxides formed upon dissolution of the support accompanied by formation of salts of the acid products.
In order to elucidate the effect of the magnesium-containing supports, a control experiment was conducted in which Ru(OH)x/TiO2 catalyst was used together with two mole equivalents of MgCl2. The reaction was carried out at reaction conditions identical to the support screening experiments (0.05 M HMF, 140 °C, 6 h). Although HMF was fully converted in the control experiment, only 3 and 2% of FDA and HMFCA were formed, respectively, while the rest constituted formic acid. This strongly suggested that the support played an important role with respect to the catalyst performance, rather than simply providing magnesium ions.
A similar pattern was also observed in a blank experiment when no catalyst was introduced into the reaction mixture. Here, formic acid was formed in 92% yield, while yield of FDA and other oxidation products was less than 1%.
Apart from magnesium-containing supports, good oxidation performance was also observed for ceria-supported catalyst, as seen in Fig. 4. Although selectivity towards FDA was only moderate in the time frame of 6 h, no degradation products were here observed. Hence, Ru(OH)x/CeO2 was further tested in the catalyzed HMF oxidation at different pressures (Fig. 5).
The obtained data clearly suggested that it was possible to avoid formation of undesirable degradation products by use of elevated pressures, whereas ambient pressure (i.e. 1 bar of O2) led to formation of 3% formic acid after 1 h of reaction. Therefore, with a desire to perform the reaction at lowest possible pressure, we investigated the product formation over time at 2.5 bars pressure (Fig. 6).
As observed from Fig. 6, the FDA yield constituted 38% under applied conditions after 6 h, which is higher than the FDA yield observed when Ru(OH)x/TiO2 was used as the catalyst under the same reaction conditions (see Fig. 3a). The yield of FDA increased to 60% after 18 h of reaction. However, HMFCA contributed 10% to the mass balance while no formic acid or levulinic acid were detected in the post-reaction mixture, possibly due to degradation of the formed FA and LA at extended reaction times.
Importantly, upon re-use the Ru(OH)x/CeO2 catalyst revealed no loss of activity, providing 38 and 36% yield of FDA after 6 h of reaction in second and third runs, respectively. This clearly demonstrated the applicability of the ceria catalyst system in line with the study performed by Corma and coworkers [12], in which gold nanoparticles deposited on ceria showed superior performance in aerobic oxidations compared to Au/TiO2 in the absence of base.
4 Conclusions
A number of Ru(OH)x/support catalysts were prepared and identified as highly efficient catalysts for aerobic oxidation of HMF to FDA under base-free and low to moderate oxygen pressures. Especially, ceria-supported catalysts showed higher activities and selectivities compared to those based on TiO2 as a support.
Further development of the catalytic systems, screening of different substrates and additional catalyst characterization are in progress.
References
Bozell JJ, Petersen GR (2010) Green Chem 12:539
Boisen A, Christensen TB, Fu W, Gorbanev YY, Hansen TS, Jensen JS, Klitgaard SK, Pedersen S, Riisager A, Ståhlberg T, Woodley JM (2009) Chem Eng Res Des 87:1318
Moreau C, Belgacem MN, Gandini A (2004) Top Catal 27:11
Gorbanev YY, Klitgaard SK, Woodley JM, Christensen CH, Riisager A (2009) ChemSusChem 2:672
Mallat T, Baiker A (2004) Chem Rev 104:3037
ten Brink G-J, Arends IWCE, Sheldon RA (2000) Science 287:1636
Christensen CH, Jørgensen B, Rass-Hansen J, Egeblad K, Madsen R, Klitgaard SK, Hansen SM, Hansen MR, Andersen HC, Riisager A (2006) Angew Chem Int Ed 45:4648
Marsden C, Taarning E, Hansen D, Johansen L, Klitgaard SK, Egeblad K, Christensen CH (2008) Green Chem 10:168
Kegnæs S, Mielby J, Mentzel UV, Christensen CH, Riisager A (2010) Green Chem 12:1437
Vinke P, van der Poel W, van Bekkum H (1991) Stud Surf Sci Catal 59:385
Kröger M, Prüße U, Vorlop K-D (2000) Top Catal 13:237
Casanova O, Iborra S, Corma A (2009) ChemSusChem 2:1138
Hansen TW, Wagner JB, Hansen PL, Dahl S, Topsøe H, Jacobsen CJH (2001) Science 294:1508
Klerke, Klitgaard SK, Fehrmann R (2009) Catal Lett 541:541
Schwab P, Grubbs RH, Ziller JW (1996) J Am Chem Soc 118:100
Rovik K, Klitgaard SK, Dahl S, Christensen CH, Chorkendorff I (2009) Appl Catal A 358:269
Pagliaro M, Campestrini S, Ciriminna R (2005) Chem Soc Rev 34:837
Ji H-B, Ebitani K, Mizugaki T, Kaneda K (2002) Catal Commun 3:511
Mori K, Kanai S, Hara T, Mizugaki T, Ebitani K, Jitsukawa K, Kaneda K (2007) Chem Mater 19:1249
Nikaidou F, Ushiyama H, Yamaguchi K, Yamashita K, Mizuno N (2010) J Phys Chem C 114:10873
Mizuno N, Yamaguchi K (2008) Catal Today 132:18
Gorbanev YY, Kegnæs S, Riisager A (2011) Catal Lett (accepted)
Veldurthy B, Clacens JM, Figueras F (2005) Adv Synth Catal 347:767
Kiwi J, Prins R (1986) Chem Phys Lett 126:579
Ståhlberg T, Grau Sørensen M, Riisager A (2010) Green Chem 12:321
Lakshmi Kantam M, Pal U, Choudary BM, Bhargava S (2008) Adv Synth Catal 350:1225
Acknowledgments
We thank Bodil Holten and Ass. Prof. Susanne L. Mossin (Centre for Catalysis and Sustainable Chemistry, Department of Chemistry, Technical University of Denmark) for BET and EPR measurements, Jacob S. Jensen (Department of Chemical and Biochemical Engineering, Technical University of Denmark) for FDA solubility data and Andras Kovacs (Center for Electron Nanoscopy, Technical University of Denmark) for TEM and EDS measurements. The work was supported by The Danish National Advanced Technology Foundation and Novozymes A/S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorbanev, Y.Y., Kegnæs, S. & Riisager, A. Effect of Support in Heterogeneous Ruthenium Catalysts Used for the Selective Aerobic Oxidation of HMF in Water. Top Catal 54, 1318 (2011). https://doi.org/10.1007/s11244-011-9754-2
Published:
DOI: https://doi.org/10.1007/s11244-011-9754-2