Abstract
4-Vinylbenzyl-substituted Ag(I) N-heterocyclic carbene (NHC) complexes and Ru(II) NHC complexes have been synthesized. The Ag(I) complexes were synthesized from the imidazolium salts and Ag2O in dichloromethane at room temperature. The Ru(II) complexes were prepared from Ag(I) NHC complexes by transmetallation. The six 4-Vinylbenzyl-substituted Ag(I) NHC complexes and six 4-Vinylbenzyl-substituted Ru(II) NHC complexes have been characterized by spectroscopic techniques and elemental analyses. The Ru(II) NHC complexes show catalytic activity for the transfer hydrogenation of ketones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the coordination chemistry of N-heterocyclic carbene (NHC) ligands has drawn much attention [1–3]. In general, NHCs that form stable bonds with different metals and particularly with transition metals are considered to be an alternative for tertiary phosphines. Transition metal–NHC complexes show interesting electronic properties such as high σ-basicity and low π-acidity. Furthermore, by tuning the steric and electronic properties of the NHC, the activity and selectivity of metal–NHC complexes can be easily varied [4–6]. Such complexes can show good catalytic activity [7, 8]. In recent years, metal–NHC complexes have been reported comprising both simple N-alkyl or aryl substituents and also N-functionalized NHCs integrating various functional groups such as pyridyl, phosphinyl and pyrazolyl. The emergence of functionalized NHCs as ligands [9–12] in transition metal-catalyzed reactions, such as the hydrogenation of olefins [13–17] and the transfer hydrogenation of ketones [18–24], has provided an effective strategy in the efficient transformation of small molecules [25, 26].
Ag(I) NHC complexes have drawn continuous attention [27]. Suitable transfer reagents for many other metal–NHC complexes can be obtained from Ag(I) NHC complexes, which are the most popular choice for NHC transfer reagents for the synthesis of Ru(II) NHC complexes [28, 29].
Ru(II) NHC complexes can contain functional ligands such as hydride or alkylidene. Important aspects of Ru(II) NHC complexes are their broad range of oxidation states and coordination geometries of ruthenium. Ru(II) NHC complexes with large structural motifs have found applications in catalysis [30–32].
Hydrogen transfer reactions of C=O and C=NR groups have used metal complexes as catalysts. Ru(II) NHC complexes [33–36] have revealed especially good activity in transfer hydrogenation reactions. In recent years, transition metal-catalyzed hydrogen transfer reactions using isopropanol as a source of hydrogen have been described in several useful applications [37, 38].
In the course of the our studies involving the use of functionalized NHC ligands, we here report the synthesis and structures of a number of Ag(I) NHC and Ru(II) NHC complexes. The Ru(II) NHC complexes have been investigated as catalysts for the transfer hydrogenation of a range of ketones.
Experimental
All syntheses of Ag(I) complexes 2 and Ru(II) complexes 3 were carried out under an inert atmosphere in flame-dried glassware using standard Schlenk techniques. Solvents were purified by distillation over the drying agents indicated and were transferred under Ar: Et2O (Na/K alloy), CH2Cl2 (P4O10), hexane and toluene (Na).
All other reagents were obtained commercially from Aldrich and used without further purification. Melting points were measured in glass capillaries under air with an Electrothermal-9200 melting point apparatus. FT IR spectra were recorded as KBr pellets in the range 400–4,000 cm−1 on an AT, UNICAM 1000 spectrometer. Proton (1H) and Carbon (13C) NMR spectra were recorded using a Varian AS 400 Merkur spectrometer operating at 400 MHz (1H) or 100 MHz (13C) in CDCl3 and DMSO-d6 with tetramethylsilane as an internal reference. Products were investigated with an Agilent 6890 N GC system by GC–FID with a HP-5 column of 30 m length, 0.32 mm diameter and 0.25 μm film thickness. Column chromatography was performed using silica gel 60 (70–230 mesh). Elemental analyses were performed by the Turkish Research Council (Ankara, TURKEY) Microlab.
Synthesis of complex 2a
To a solution of 1-(4-vinylbenzyl)-3-benzylimidazol chloride (0.500 g, 1.6 mmol) in dichloromethane (30 mL), silver(I) oxide (0.185 g, 0.8 mmol) and activated 4 molecular sieves were added. The reaction mixture was stirred for 24 h, at room temperature in the dark. The reaction mixture was then filtered through Celite, and the solvents were evaporated under vacuum to afford the product as a white solid. The crude product was recrystallized from dichloromethane/diethyl ether (1:3) at room temperature. Yield: 0.50 g (75 %). m.p.: 146–148 °C; ν(CN): 1,669 cm−1. Anal. Calc. for C19H21AgClN2: C: 54.2; H: 5.0; N, 6.7 %. Found: C: 54.2; H: 5.0; N: 6.6 %. 1H NMR (300 MHz, DMSO), δ 3.52 (s, 4H, NCH 2 CH 2 N); 4.72 (s, 4H, NCH 2 C6H5 and NCH 2C6H4CH = CH2); 5.31 and 5.88 (d, 1H, J: 10.8 and 17.7 Hz, NCH2C6H4CH = CH 2); 6.74 (dd, 1H, J:28.5 NCH2C6H4CH = CH2); 7.24–7.50 (m, 9H, Ar–H).13C NMR (300 MHz, DMSO), δ 49.1 (NCH2 CH2N); 54.3 and 54.4 (NCH 2 C6H5 and NCH 2 C6H4CH = CH2); 125.9, 126.1, 126.7, 127.6, 128.1, 128.6, 129.1, 136.3, 136.6, 136.8, 137.1 and 141.2 (NCH2C6H4 CH = CH2 and Ar–C); 203.5 (Ag–C carb.).
Synthesis of complex 2b
The synthesis of 2b was carried out in the same way as that described for 2a, but 1-(4-vinylbenzyl)-3-(2-methylbenzyl)imidazol chloride (0.523 g, 1.6 mmol) was used instead of 1-(4-vinylbenzyl)-3-benzylimidazol chloride. Yield: 0.56 g (80 %). m.p.: 97–100 °C; ν(CN): 1,664 cm−1. Anal. Calc. for C20H23AgClN2: C: 55.3; H: 5.3; N: 6.4 %. Found: C: 55.2; H: 5.3; N: 6.4 %. 1H NMR (300 MHz, DMSO), δ 2.32 (s, 3H, C6H5CH 3 ); 3.52 (s, 4H, NCH 2 CH 2N); 4.72 and 4.73 (s, 4H, NCH 2C6H4 and NCH 2C6H4CH = CH2); 5.28 and 5.85 (d, 1H, J: 10.9 Hz and 17.7 NCH2C6H4CH = CH 2); 6.74 (dd, 1H, J:28.6 NCH2C6H4CH = CH2); 7.23–7.51 (m, 8H, Ar–H).13C NMR (300 MHz, DMSO), δ 19.6 (C6H5 CH3); 48.9–49.3 (NCH2 CH2N); 52.5 (NCH 2 C6H5); 54.2 (NCH 2 C6H4CH = CH2); 115.1,126.7, 126.8, 127.0, 128.3, 128.4, 131.0, 131.2, 134.5, 136.3,136.5, 136.6, 136.8 and 137.2, (C6H4 CH = CH2 and Ar–C); 203.7 (Ag–C carb.).
Synthesis of complex 2c
The synthesis of 2c was carried out in the same way as that described for 2a, but 1-(4-vinylbenzyl)-3-(3-methylbenzyl)imidazol chloride (0.523 g, 1.6 mmol) was used instead of 1-(4-vinylbenzyl)-3-benzylimidazol chloride. Yield: 0.54 g (78 %). m.p.: 109–111 °C; ν(CN):1,666 cm−1. Anal. Calc. for C20H23AgClN2: C: 55.3; H: 5.3; N: 6.4. %. Found: C: 55.2; H: 5.3; N: 6.4. %. 1H NMR (300 MHz, DMSO), δ 2.32 (s, 3H, C6H5CH 3 ); 3.52 (s, 4H, NCH 2 CH 2 N); 4.72 and 4.73 (s, 4H, NCH 2C6H4CH = CH2 and NCH 2 C6H4); 5.23 and 5.85 (d, 1H, J: 10.9 Hz and 17.7 Hz NCH2C6H4CH = CH 2); 6.74 (dd, 1H, J: 28.6 Hz NCH2C6H4CH = CH2); 7.23–7.51 (m, 8H, Ar–H). 13C NMR (300 MHz, DMSO), δ 19.6 (C6H5 CH3); 48.9–49.3 (NCH2 CH2N); 52.5 (NCH2C6H5); 54.2 (NCH 2 C6H4CH = CH2); 115.1, 126.7, 126.8, 127.0, 128.3, 128.4, 131.0, 131.2, 134.5, 136.3, 136.5, 136.6, 136.8 and 137.2. (NCH2C6H4 CH = CH2 and Ar–C); 203.7 (Ag–C carb.).
Synthesis of complex 2d
The synthesis of 2d was carried out in the same way as that described for 2a, but 1-(4-vinylbenzyl)-3-(4-methylbenzyl)imidazol chloride (0.523 g, 1.6 mmol) was used instead of 1-(4-vinylbenzyl)-3-benzylimidazol chloride. Yield: 0.54 g (79 %). m.p.: 143–145; ν(CN): 1,668 cm−1. Anal. Calc. for C20H23AgClN2: C: 55.3; H: 5.3; N: 6.4. %. Found: C: 55.2; H: 5.3; N: 6.4. %. 1H NMR (300 MHz, DMSO), δ 2.30 (s, 3H, C6H5CH 3 ); 3.50 (s, 4H, NCH 2 CH 2 N); 4.67 (s, 2H, NCH 2 C6H4); 4.70 (s, 2H, NCH 2C6H4CH = CH2); 5.28 and 5.84 (d, 1H, J: 10.2 Hz and 17.7 Hz NCH2C6H4CH = CH 2); 6.74 (dd, 1H, J: 28.5 Hz NCH2C6H4CH = CH2); 7.12–7.50 (m, 8H, Ar–H). 13C NMR (300 MHz, DMSO), δ 21.2 (C6H4 CH3); 49.1 (NCH2 CH2N); 54.1 and 54.2 (NCH 2 C6H4CH = CH2 and NCH2C6H5); 115.0, 127.0, 128.2, 128.5, 129.1, 129.8, 133.6, 136.3, 137.2 and 137.6. (NCH2C6H4 CH = CH2 and Ar–C); 203.3 (Ag–C carb.).
Synthesis of complex 2e
The synthesis of 2e was carried out in the same way as that described for 2a, but 1-(4-vinylbenzyl)-3-(2,4,6-trimethylbenzyl)imidazol chloride (0.568 g, 1.6 mmol) was used instead of 1-(4-vinylbenzyl)-3-benzylimidazol chloride. Yield: 0.61 g (82 %). m.p.: 225–227; ν(CN):1,669 cm−1. Anal. Calc. for C22H28AgClN2: C: 57.1; H: 5.9; N: 6.0. %. Found: C: 57.1; H: 5.8; N: 6.0. %. 1H NMR (300 MHz, DMSO), δ 2.22 and 2.31 (s, 9H, C6H5(CH 3 )3); 3.43 (s, 4H, NCH 2 CH 2 N); 4.65 (s, 2H, NCH 2 C6H5); 4.66 (s, 2H, NCH 2C6H4CH = CH2); 5.27 and 5.84 (d, 1H, J: 11.1 Hz and 17.7 Hz NCH2C6H4CH = CH 2); 6.73 (dd, 1H, J: 28.5 Hz NCH2C6H4CH = CH2); 6.90–7.49 (m, 6H, Ar–H). 13C NMR (300 MHz, DMSO), δ 20.5 and 21.1 [C6H5(CH3)3]; 48.6–48.7 (NCH2 CH2N); 54.3 (NCH 2 C6H4CH = CH2 and NCH2C6H5); 115.0, 127.0, 128.3, 129.7, 136.4, 136.6, 137.2, 137.7 and 137.9 (NCH2C6H4 CH = CH2 and Ar–C); 203.4 (Ag–C carb.).
Synthesis of complex 2f
The synthesis of 2f was carried out in the same way as that described for 2a, but 1-(4-vinylbenzyl)-3-(2,3,5,6-tetramethylbenzyl)imidazol chloride (0.590 g, 1.6 mmol) was used instead of 1-(4-vinylbenzyl)-3-benzylimidazol chloride. Yield: 0.60 g (79 %). m.p.: 225–227; ν(CN):1,669 cm−1. Anal. Calc. for C23H30AgClN2: C: 57.9; H: 6.1; N: 5.9: Found: C: 57.9; H: 6.1; N: 5.8. %. 1H NMR (300 MHz, DMSO), δ 2.19 and 2.20 [s, 12H, C6H(CH 3 )4]; 3.38 (s, 4H, NCH 2 CH 2 N); 4.66 and 4.67 (s, 4H, NCH 2C6H4CH = CH2 and NCH 2 C6H5); 5.27 and 5.84 (d, 1H, J: 10.8 and 17.7 Hz NCH2C6H4CH = CH 2); 6.72 (dd, 1H, J: 28.2 Hz NCH2C6H4CH = CH2); 6.97–7.52 (m, 5H, Ar–H). 13C NMR (300 MHz, DMSO), δ 16.3 and 20.7 (C6H(CH3)4); 48.9–49.6 (NCH2 CH2N); 54.6 (NCH 2 C6H4CH = CH2 and NCH2C6H5); 115.0, 127.0, 128.3, 131.8, 132.1, 133.8, 134.2, 136.3, 136.4, 136.6 and 137.2 (NCH2C6H4 CH = CH2 and Ar–C); 203.2 (Ag–C carb.).
Synthesis of complex 3a
To a solution of chloro[1-(4-vinylbenzyl)-3-benzylimidazol-2-ylidene]silver(I) (0.117 g, 0.28 mmol) in dichloromethane (30 mL), di-μ-chloro-bis[chloro(η6-1-isopropyl-4-methylbenzene)ruthenium(II)] (0.086 g, 0.14 mmol) was added. The reaction mixture was stirred for 24 h at room temperature in the dark and then filtered through Celite, and the solvents were evaporated under vacuum to afford the product as a red-brown solid. The crude product was recrystallized from dichloromethane/diethyl ether (1:3) at room temperature. Yield: 0.11 g (70 %). m.p.: 206–208 °C; ν(CN): 1,496 cm−1. Anal. Calc. for RuC30H38Cl2N2: C: 60.2; H: 6.4; N: 4.7. %. Found: C: 60.2; H: 6.4; N: 4.7. %. 1H NMR (300 MHz, CDCl3); δ 1.29 [d, 6H, J: 5.8 Hz, p-CH3C6H4CH(CH 3 )2]; 2.18 [s, 3H, p-CH 3C6H4CH(CH 3 )2]; 2.87 [h, 1H, J: 5.8 Hz, p-CH3C6H4CH(CH 3 )2]; 3.41 and 3.56 (m, 4H, NCH 2 CH 2 N); 4.93 (m, 2H, NCH 2 C6H5 and NCH 2 C6H4CH = CH2); 5.17 and 5.44 (d, 4H, J: 5.7 Hz, p-CH3C6 H 4CH(CH 3 )2); 5.34 (m, 2H, NCH 2 C6H4CH = CH2); 5.30 and 5.81 (m, 2H, C6H4CH = CH 2 ); 6.74 (m, 1H, C6H4CH = CH2); 7.28–7.48 (m, 9H, Ar–H); 13C NMR (300 MHz, CDCl3); δ 18.8 [p-CH3C6H4CH(CH 3 )2]; 22.6 [p-CH3C6H4CH(CH 3 )2]; 30.7 [p-CH3C6H4 CH(CH 3 )2]; 48.9 (NCH2 CH2N); 55.8 and 55.9 (NCH 2 C6H5 and NCH 2 C6H4CH = CH2); 83.6, 85.8, 97.7, 97.8, 108.3 and 108.4 [p-CH3 C 6H4CH(CH 3 )2]; 113.9, 125.5, 125.7, 126.5, 127.3, 127.7, 128.1, 128.9, 136.4, 136.7, 137.1, 137.4 and 138.0. (Ar–C); 208.3 (Ru–C carb.).
Synthesis of complex 3b
The synthesis of 3b was carried out in the same way as that described for 3a, but chloro[1-(4-vinylbenzyl)-3-(2-methylbenzyl)imidazol-2-ylidene]silver(I) (0.121 g, 0.28 mmol) was used instead of chloro[1-(4-vinylbenzyl)-3-benzylimidazol-2-ylidene]silver(I). Yield: 0.12 g (74 %). m.p.: 208–210 °C; ν(CN): 1,489 cm−1. Anal. Calc. for RuC31H40Cl2N2: C: 60.9; H: 6.6; N: 4.6. %. Found: C: 60.8; H: 6.6; N: 4.6. %. 1H NMR (300 MHz, CDCl3); δ 1.25 [d, 6H, J: 6.3 Hz, p-CH3C6H4CH(CH 3)2]; 2.19 [s, 3H, p-CH 3C6H4CH(CH3)2]; 2.34 (s, 3H, CH2C6H4CH 3-2); 2.77 [h, 1H, J: 6.3 Hz, p-CH3C6H4CH(CH3)2]; 3.53 and 3.63 (m, 4H, NCH 2 CH 2 N); 4.86 and 5.51 (s, 4H, NCH 2C6H4CH3-2 and NCH 2C6H4CH = CH2); 5.17 and 5.45 [d, 4H, J: 6.3 Hz, p-CH3C6 H 4CH(CH 3 )2]; 5.24 and 5.87 (m, 2H, NCH2C6H4CH = CH 2); 6.72 (m, 1H, NCH2C6H4CH = CH2); 7.13–7.70 (m, 8H, Ar–H). 13C NMR (300 MHz, CDCl3); δ 18.4 [p-CH3C6H4CH(CH3)2]; 22.3 (NCH2C6H4 CH3-2); 22.6 [p-CH3C6H4CH(CH3)2]; 30.8 [p-CH3C6H4 CH(CH3)2]; 48.9 and 49.0 (NCH2 CH2N); 52.7 and 55.7 (NCH2C6H4 CH3-2 and NCH2C6H4CH = CH2); 83.6, 84.0, 84.5, 85.8, 98.0 and 107.5 [p-CH3 C 6H4CH(CH3)2]; 114.0, 124.8, 126.3, 127.1, 128.1, 130.8, 135.8, 135.9, 136.4, 136.6, 136.7 and 137.0. (Ar–C); 209.2 (Ru–C carb.).
Synthesis of complex 3c
The synthesis of 3c was carried out in the same way as that described for 3a, but chloro[1-(4-vinylbenzyl)-3-(3-methylbenzyl)imidazol-2-ylidene]silver(I) (0.121 g, 0.28 mmol) was used instead of chloro[1-(4-vinylbenzyl)-3-benzylimidazol-2-ylidene]silver(I). Yield: 0.13 g (80 %). m.p.: 209–211 °C; ν(CN): 1,496 cm−1. Anal. Calc. for RuC31H40Cl2N2: C: 60.8; H: 6.6; N: 4.6. %. Found: C: 60.8; H: 6.6; N: 4.7. %. 1H NMR (300 MHz, CDCl3); δ 1.26 [d, 6H, J: 6.0 Hz, p-CH3C6H4CH(CH 3)2]; 2.18 [s, 3H, p-CH 3C6H4CH(CH3)2]; 2.34 (s, 3H, NCH2C6H4CH 3-3); 2.88 [h, 1H, J: 6.0 Hz, p-CH3C6H4CH(CH3)2]; 3.45 and 3.523 (m, 4H, NCH 2 CH 2 N); 4.92 (s, 2H, NCH 2C6H4CH3-3); 5.26 (m, 2H, NCH2C6H4CH = CH 2); 5.17 and 5.36 (d, 4H, J: 6.3 Hz, p-CH3C6 H 4CH(CH3)2); 5,36 (m, 2H, NCH 2C6H4CH = CH2); 6.73 (m, 1H, NCH2C6H4CH = CH2); 7.04–7.48 (m, 8H, Ar–H). 13C NMR (300 MHz, CDCl3); δ 18.8 [p-CH3C6H4CH(CH3)2]; 21.5 (NCH2C6H4 CH3-3); 22.6 [p-CH3C6H4CH(CH3)2]; 30.7 [p-CH3C6H4 CH(CH3)2]; 48.9 (NCH2 CH2N); 55.7 and 55.9 (NCH2C6H4 CH3-3 and NCH2C6H4CH = CH2); 83.5, 85.8, 97.7 and 108.4 [p-CH3 C 6H4CH(CH3)2]; 114.3, 124.6, 125.5, 125.7, 126.5, 127.3, 128.9, 136.4, 136.6, 136.7, 137.3, 137.5, 138.0 and 138.5. (Ar–C); 208.8 (Ru–C carb.).
Synthesis of complex 3d
The synthesis of 3d was carried out in the same way as that described for 3a, but chloro[1-(4-vinylbenzyl)-3-(4-methylbenzyl)imidazol-2-ylidene]silver(I) (0.121 g, 0.28 mmol) was used instead of chloro[1-(4-vinylbenzyl)-3-benzylimidazol-2-ylidene]silver(I). Yield: 0.14 g (84 %). m.p.: 157–159 °C; ν(CN): 1,489 cm−1. Anal. Calc. for RuC31H40Cl2N2: C: 60.8; H: 6.6; N: 4.6. %. Found: C: 60.8; H: 6.6; N: 4.6. %. 1H NMR (300 MHz, CDCl3); δ 1.29 [d, 6H, J: 6.0 Hz, p-CH3C6H4CH(CH 3)2]; 2.18 (s, 3H, p-CH 3C6H4CH(CH3)2); 2.36 (s, 3H, NCH2C6H4CH 3-4); 2.87 (h, 1H, J: 6.0 Hz, p-CH3C6H4CH(CH3)2); 3.40 and 3.53 (m, 4H, NCH 2 CH 2 N); 4.90 (m, 2H, NCH 2C6H4CH3-4); 5.26 and 5.76 (m, 2H, NCH2C6H4CH = CH 2); 5.17 and 5.44 (d, 4H, J: 6.0 Hz, p-CH3C6 H 4CH(CH3)2); 5,32 (m, 2H, NCH 2C6H4CH = CH2); 6.72(m, 1H, NCH2C6H4CH = CH2); 7.16–7.40 (m, 8H, Ar–H). 13C NMR (300 MHz, CDCl3); δ 18.8 [p-CH3C6H4CH(CH3)2]; 21.1 (NCH2C6H4 CH3-4); 22.6 [p-CH3C6H4CH(CH3)2]; 30.7 [p-CH3C6H4 CH(CH3)2]; 48.8 and 49.0 (NCH2 CH2N); 55.6 and 55.7 (NCH2C6H4 CH3-4 and NCH2C6H4CH = CH2); 83.6, 85.7, 97.8 and 108.3 [p-CH3 C 6H4CH(CH3)2]; 114.0, 126.5, 127.8, 128.1, 129.4, 134.0, 136.4, 136.7, 137.0 and 137.3. (Ar–C); 208.3 (Ru–C carb.).
Synthesis of complex 3e
The synthesis of 3e was carried out in the same way as that described for 3a, but chloro[1-(4-vinylbenzyl)-3-(2,4,6-trimethylbenzyl)imidazol-2-ylidene]silver(I) (0.129 g, 0.28 mmol) was used instead of chloro[1-(4-vinylbenzyl)-3-benzylimidazol-2-ylidene]silver(I). Yield: 0.15 g (86 %). m.p.: 207–209 °C; ν(CN): 1,488 cm−1. Anal. Calc. for RuC33H44Cl2N2: C: 61.9; H: 6.9; N: 4.4. %. Found: C: 61.8; H: 6.9; N: 4.4. %. 1H NMR (300 MHz, CDCl3); δ 1.34 [d, 6H, J: 6.0 Hz, p-CH3C6H4CH(CH 3)2]; 2.23 [s, 3H, p-CH 3C6H4CH(CH3)2]; 2.28 and 2.40 [s, 9H, NCH2C6H2(CH 3)3-2,4,6]; 2.92 [h, 1H, J: 6.0 Hz, p-CH3C6H4CH(CH3)2]; 3.18 and 3.30 (m, 4H, NCH 2 CH 2 N); 4.65 [s, 2H, NCH 2C6H2(CH3)3-2,4,6]; 4.84 (m, 2H, NCH 2C6H4CH = CH2); 5.22 and 5.53 [d, 4H, J: 6.0 Hz, p-CH3C6 H 4CH(CH3)2]; 5.75 (m, 2H, NCH2C6H4CH = CH 2); 6.71 (m, 1H, NCH2C6H4CH = CH2); 6.88–7.41 (m, 6H, Ar–H). 13C NMR (300 MHz, CDCl3); δ 18.8 [p-CH3C6H4CH(CH3)2]; 20.8 [p-CH3C6H4CH(CH3)2]; 20.9 and 29.7 [NCH2C6H2(CH3)3-2,4,6]; 31.0 (p-CH3C6H4 CH(CH3)2); 48.4 and 48.2 (NCH2 CH2N); 49.2 and 55.4 (NCH2C6H2 CH3-2,4,6 and NCH2C6H4CH = CH2); 84.0, 85.6, 97.8 and 107.7 (p-CH3 C 6H4CH(CH3)2); 113.9, 126.5, 128.0, 129.4, 129.5, 136.4, 136.9 and 137.9. (Ar–C); 208.0 (Ru–C carb.).
Synthesis of complex 3f
The synthesis of 3f was carried out in the same way as that described for 3a, but chloro[1-(4-vinylbenzyl)-3-(2,4,6-trimethylbenzyl)imidazol-2-ylidene]silver(I) (0.133 g, 0.28 mmol) was used instead of chloro[1-(4-vinylbenzyl)-3-benzylimidazol-2-ylidene]silver(I). Yield: 0.15 g (84 %). m.p.: 219-221 °C; ν(CN): 1,472 cm−1. Anal. Calc. for RuC34H46Cl2N2: C: 62.4; H: 7.1; N: 4.3. %. Found: C: 62.4; H: 7.1; N: 4.3. %. 1H NMR (300 MHz, CDCl3); δ 1.32 [d, 6H, J: 6.3 Hz, p-CH3C6H4CH(CH 3)2]; 2.19 [s, 3H, p-CH 3C6H4CH(CH3)2]; 2.21 [s, 12H, NCH2C6H(CH 3)4-2,3,5,6]; 2.90 [h, 1H, J: 6.3 Hz, p-CH3C6H4CH(CH3)2]; 3.21 and 3.33 (m, 4H, NCH 2 CH2N); 4.70 and 4.84 (m, 4H, NCH 2C6HCH3-2,3,5,6 and NCH 2C6H4CH = CH2); 5.22 and 5.52 [d, 4H, J: 6.2 Hz, p-CH3C6 H 4CH(CH3)2]; 5.75 (m, 2H, NCH2C6H4CH = CH 2); 6.71 (m, 1H, NCH2C6H4CH = CH2); 6.88–7.41 (m, 6H, Ar–H). 13C NMR (300 MHz, CDCl3); δ 18.8 [p-CH3C6H4CH(CH3)2]; 20.8 [p-CH3C6H4CH(CH3)2]; 21.0 and 21.7 [NCH2C6H(CH3)4-2,3,5,6]; 31.2 [p-CH3C6H4 CH(CH3)2]; 47.9 and 48.3 (NCH2 CH2N); 49.4 and 54.8 (NCH2C6H2 CH3-2,3,5,6 and NCH2C6H4CH = CH2); 84.0, 85.6, 97.8 and 107.7 [p-CH3 C 6H4CH(CH3)2]; 113.9, 126.5, 128.0, 129.4, 129.5, 136.4, 136.9 and 137.9. (Ar–C); 208.2 (Ru–C carb.).
General method for the transfer hydrogenation of ketones
The catalytic hydrogen transfer reactions were carried out in a closed Schlenk flask under argon atmosphere. A mixture of the required ketone (1 mmol), catalyst Ru(II) NHC complexes 3a–f (0.01 mmol) and KOH (4 mmol) was heated to reflux in 10 mL of i-PrOH for 1 h. The solvent was then removed under vacuum, and the residue was extracted with ethyl acetate/hexane (1:5), filtered through a pad of silica gel with copious washings, concentrated and purified by flash chromatography on silica gel. The product distribution was determined by 1H NMR spectroscopy, GC and GC–MS.
Results and discussion
Synthesis of Ag(I) NHC complexes
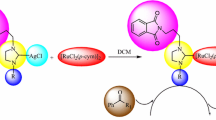
The synthetic route for the unsymmetrically substituted 4-vinylbenzyl Ag(I) NHC complexes 2a–f and their corresponding Ru(II) NHC complexes is illustrated in Scheme 1. The Ag(I) complexes 2a–f were prepared by stirring 1-(4-vinylbenzyl)-3-alkylimidazolidinium salts with 0.5 equivalents of silver(I) oxide in dichloromethane at room temperature for 24 h. The complexes were obtained as off white solids in 75–82 % yield. These Ag(I) complexes are soluble in halogenated solvents, but insoluble in nonpolar solvents. They were characterized by spectroscopic techniques (1H, 13C NMR and IR) and elemental analysis. 1H and 13C NMR spectra are consistent with the proposed formulae. In the 1H and 13C NMR spectra in d-DMSO, loss of signals for the imidazolium proton (NCHN) (9–11 ppm) and imidazolium carbon (NCHN) at (158–159 ppm) showed the formation of the expected complexes. In the 13C NMR spectra, resonances of the carbene carbon atoms are observed in the range δ 203.2–203.7 ppm, respectively, for 2a–f. These signals are shifted downfield compared with the carbene precursors, which further demonstrates the formation of the expected NHC complexes. The IR spectra for these complexes show a characteristic ν(C=N) band at 1,664–1,669 cm−1 for 2a–f. The NMR and FT IR spectra are similar to those reported for other Ag(I) NHC complexes [39].
Synthesis of Ru(II) NHC complexes
The 4-Vinylbenzyl-substituted Ru(II) NHC complexes 3a–f were prepared from the corresponding Ag(I) NHC complexes via transmetallation (Scheme 1). The air and moisture stable Ru(II) NHC complexes were soluble in solvents such as chloroform, toluene and dichloromethane, but insoluble in nonpolar solvents. They are red-brown solids, obtained in 70–86 % yield. These complexes were characterized by analytical and spectroscopic techniques. In their 1H NMR spectra, resonances for the isopropyl and methyl protons of the p-cymene group in the range 1.26–1.34 (methyl of isopropyl group), 2.77–2.92 (methyl of isopropyl group) and 2.18–2.23 ppm (p-methyl of p-cymene group), respectively, were consistent with formation of the Ru(II) NHC complexes. In their 13C NMR spectra, the resonances of the carbene carbon atoms are observed in the range δ 208.0–209.3 ppm. These signals are shifted downfield compared with the corresponding Ag(I) NHC complexes, where they were observed in the range of 203.2–203.7 ppm. The IR data for the Ru(II) NHC complexes show the characteristic ν(C=N) band at 1,472–1,496 cm−1.
Catalytic transfer hydrogenation of ketones
Ruthenium complexes have been used as active catalysts for transfer hydrogenation using 2-propanol as a hydrogen source [40]. In addition, 2-propanol is a popular solvent for such transfer hydrogenation reactions, since it is easy to handle and is relatively nontoxic, environmentally benign and inexpensive. Furthermore, the volatile acetone by-product can be easily removed.
We have investigated and compared the catalytic properties of our 4-vinyl substituted Ru(II) NHC complexes 3a–f in the transfer hydrogenation of various methyl aryl ketones. The reduction in acetophenone with 2-propanol to 1-phenylethanol was chosen as a model reaction. The reaction was carried out using the Ru(II) precatalyst (0.01 mmol), KOH (4 mmol) and substrate ketone (1.00 mmol) in 2-propanol at 80 °C. The conversion was monitored by GC and NMR. It is well known that such reactions are sensitive to the nature of the base. We surveyed K2CO3, Cs2CO3, NaOH, KOH, t-BuOK and NaOAc, and the highest rate was observed when KOH was used as base. In this way, a variety of ketones was transformed to the corresponding secondary alcohols, with the results summarized in Table 1.

All of these complexes 3a–f are seen to be very active in hydrogen transfer reactions; nevertheless, complex 3a turned out to be the most active. The reduction in acetophenone with 3a was complete within 1 h, with a yield reaching 92 %. In contrast, acetophenone was reduced within 1 h using the other complexes in conversions of 60–89 % (Table 1).
A variety of ketones could be converted to the corresponding secondary alcohols. The results are illustrated in Table 1. Under those conditions, p-methoxyacetophenone and p-fluoroacetophenone react cleanly and in good yields with 2-propanol (Table 1). The transformation of ketones with bulky substituents was less favorable. We tried this reaction with benzophenone within 1 h, but only low yields were obtained. Therefore, we have extended the duration of the experiments for benzophenone to 2 h. The benzophenone was reduced within 1 h using 3a and 3b with 21 and 23 % conversion, respectively. However, for reaction times of 2 h, the reduction in benzophenone with 3a and 3b gave 93 and 96 % conversions, respectively (Table 1).
Conclusions
In conclusion, via the Ag(I) NHC 2a–f transmetallation route, Ru(II) NHC complexes were readily accessible and proved to be effective catalyst precursors for the transfer hydrogenation of ketones. The combination of well-defined 4-Vinylbenzyl-substituted Ru(II) NHC complexes 3a–f gave stable and highly efficient catalysts for the transfer hydrogenation of ketones in isopropyl alcohol.
References
Herrmann WA (2002) Angew Chem Int Ed 41:1290
Poyatos M, Mata JA, Peris E (2009) Chem Rev 109:3677
Diez-Gonzalez S, Marion N, Nolan SP (2009) Chem Rev 109:3612
Huang J, Stevens ED, Nolan SP, Petersen JL (1999) J Am Chem Soc 121:2674
Huang J, Schanz H-J, Stevens ED, Nolan SP (1999) Organometallics 18:2370
Hillier AC, Sommer WJ, Yong BS, Petersen JL, Cavallo L, Nolan SP (2003) Organometallics 22:4322
Crabtree RH (2005) J Organomet Chem 690:5451
Diez-Gonzalez S, Nolan SP (2007) Coord Chem Rev 251:874
Liddle ST, Edworthy IS, Arnold PL (2007) Chem Soc Rev 36:1732
Lee HM, Lee CC, Cheng PY (2007) Curr Org Chem 11:1491
Normand AT, Cavell KJ (2008) Eur J Inorg Chem 2008:2781
Poyatos M, Mata JA, Peris E (2009) Chem Rev 109:3677
Cui XH, Burgess K (2005) Chem Rev 105:3272
Nanchen S, Pfaltz A (2006) Chem Eur J 12:4550
Chen D, Banphavichit V, Reibenspies J, Burgess K (2007) Organometallics 26:855
Horn S, Albrecht M (2011) Chem Commun 47:8802
Horn S, Gandolfi C, Albrecht M (2011) Eur J Inorg Chem 2011:2863
Corberán R, Sanaú M, Peris E (2007) Organometallics 26:3492
Zeng FL, Yu ZK (2008) Organometallics 27:6025
Dyson G, Frison JC, Whitwood AC, Douthwaite RE (2009) Dalton Trans 2009:7141–7151
Gnanamgari D, Sauer ELO, Schley ND, Butler C, Incarvito CD, Crabtree RH (2009) Organometallics 28:321
Cross WB, Daly CG, Boutadla Y, Singh K (2011) Dalton Trans 40:9722
Del Pozo C, Iglesias M, Sánchez F (2011) Organometallics 30:2180
Jimenez MV, Fernandez-Tornos J, Perez-Torrente JJ, Modrego FJ, Winterle S, Cunchillos C, Lahoz FJ, Oro LA (2011) Organometallics 30:5493
Hahn FE, Jahnke MC (2008) Angew Chem Int Ed 47:3122
Diez-Gonzalez S, Marion N, Nolan SP (2009) Chem Rev 109:3612
Garrison JC, Youngs W (2005) J Chem Rev 105:3978
Wang HMJ, Lin IJB (1998) Organometallics 17:972
Lin IJB, Vasam CS (2004) Commun Inorg Chem 25:75
Herrmann WA (2002) Angew Chem Int Ed 41:1290
Perry MC, Burgess K (2003) Tetrahedron Assymetry 14:951
Dragutan I, Dragutan V, Delaude L, Demonceau A (2005) ARKIVOC 10:206
Yigit B, Yigit M, Ozdemir I, Cetinkaya E (2012) Transit Met Chem 37:297
Wwn O, Lough AJ, Morris RH (2010) Chem Commun 46:8240
Ding N, Hor TSA (2010) Dalton Trans 39:10179
Dragutan V, Dragutan I, Delaude L, Demonceau A (2007) Coord Chem Rev 251:765
Nayori R (1994) Asymmetric catalysis in organic synthesis. Wiley, New York
I Ojima (2000) Catalytic asymmetric synthesis, 2nd edition, Wiley, New York
Gürbüz N, Özcan EÖ, Özdemir İ, Çetinkaya B, Şahin O, Büyükgüngör O (2012) Dalton Trans 41:2330
Noyori R, Hashiguchi S (1997) Acc Chem Res 30:97
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aktaş, A., Gök, Y. 4-Vinylbenzyl-substituted silver(I) N-heterocyclic carbene complexes and ruthenium(II) N-heterocyclic carbene complexes: synthesis and transfer hydrogenation of ketones. Transition Met Chem 39, 925–931 (2014). https://doi.org/10.1007/s11243-014-9877-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9877-y