Abstract
Silver(I) N-heterocyclic carbene complexes were synthesized in good yields by the reactions of 1,3-dialkylperhydrobenzimidazolium salts with silver(I) oxide in dichloromethane. The silver complexes were used as carbene-transfer agents to synthesize palladium(II) N-heterocyclic carbene complexes. All of the complexes were characterized by physicochemical and spectroscopic methods. The new palladium complexes were tested as catalysts in the direct C5 arylation of 2-n-butylfuran, 2-n-butylthiophene and 2-n-propylthiazole with aryl bromides at 130 °C in N,N-dimethylacetamide. The arylation reactions proceeded selectively at the C5 position of the heteroaromatic compounds, and the corresponding coupling products were obtained in moderate to good yields by using 0.5 mol% of the palladium complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first metal complexes bearing N-heterocyclic carbene (NHC) ligands were independently reported by Wanzlick [1] and Öfele [2] in 1968. After these initial reports, most chemists except Lappert et al. [3] directed little attention toward NHC ligands for the next two decades. The isolation of the first stable NHC species in 1991 by Arduengo [4] and utilization of NHC complexes in catalysis led to a renewed interest in NHC chemistry. N-heterocyclic carbenes exhibit good σ-donor and weak π-acceptor properties, and they are very strong nucleophiles. Due to their strong σ-electron-donating properties, NHC ligands form stronger bonds with transition metals than classical ligands such as phosphines, often giving stable transition metal complexes that are generally resistant to decomposition [5, 6]. Additionally, the NHC ligands can be easily modified by changing the substituents on the nitrogen atoms or the carbene ring, which provides various ligands for organometallic chemistry [7–9]. Metal complexes of N-heterocyclic carbenes have received much interest as catalysts and display higher stabilities and reactivities than the corresponding phosphine complexes in various catalytic processes [10–15]. In particular, palladium NHC complexes have been successfully developed as highly active precatalysts for C–C and C–N coupling, CO-ethylene copolymerization and direct arylation reactions [16–19].
In recent years, the synthesis of arylated heterocycles has received much attention due to their biological and physical properties [20]. Such compounds can be prepared by conventional methods such as Stille, Suzuki, Kumada or Negishi couplings [21–24]. However, these methods require the preliminary synthesis of organometallic nucleophiles and produce stoichiometric amounts of side products. In addition, the high reactivities of these reagents as both nucleophiles and bases sometimes give rise to limitations in the synthesis. In 1990, Ohta et al. [25] reported the direct arylation of heteroaromatics (thiophenes, furans and thiazoles) with aryl halides in moderate to good yields, using [Pd(PPh3)4] as the catalyst. Since then, the palladium-catalyzed direct arylation of heteroaryl derivatives with aryl halides has become a valuable method for the synthesis of arylated heterocycles [26–30]. So far, a few examples of (NHC)Pd(II)-catalyzed arylation of heterocycles using aryl halides have been described [31–35]. To the best of our knowledge, however, Pd-carbene complexes bearing a perhydrobenzimidazol-2-ylidene ligand for direct arylation of heteroaromatic compounds have not been reported to date. The number, nature, and position of the substituents on the nitrogen atoms or NHC ring have considerable influence on the rate of catalysis and stability of the NHC complexes against heat, moisture and air. Therefore, thousands of free and metal-coordinated N-heterocyclic carbenes have been reported, although NHCs with cyclohexyl carbene backbones are still relatively rare. We now report the synthesis and characterization of new silver(I) and palladium(II) complexes with perhydrobenzimidazol-2-ylidene as a ligand, and the use of the palladium complexes as catalysts for the direct C5-arylation of heteroaromatics with various aryl halides.
Experimental
All reactions for the preparation of the Ag(I) and Pd(II) NHC complexes were carried out under argon in flame-dried glassware using standard Schlenk techniques. The solvents were purified by distillation over the drying agents indicated and were transferred under Ar: THF, Et2O (Na/K alloy), CH2Cl2 (P4H10), hexane, toluene (Na). All reagents were purchased from Sigma-Aldrich, Merck or Fluka. 1H and 13C NMR spectra were recorded in CDCI3 using a Bruker AC300P FT spectrometer operating at 300.13 MHz (1H) or 75.47 MHz (13C). Chemical shifts (δ) are given in ppm relative to TMS, coupling constants (J) in hertz. FTIR spectra were recorded as KBr pellets between 400 and 4000 cm−1 on a Mattson 1000 spectrophotometer (wavenumbers, cm−1). Chromatograms were recorded by GC-FID on an Agilent 6890 N gas chromatograph equipped with an HP-5 column of 30 m length, 0.32 mm diameter and 0.25 μm film thickness. Melting points were measured in open capillary tubes with an Electrothermal-9200 melting point apparatus and are uncorrected. Elemental analyses were performed at Inönü University research center.
Synthesis of perhydrobenzimidazolium salts 1
A mixture of the required N,N′-dialkyl-1,2-diaminocyclohexane (6.2 mmol), NH4Cl (6.2 mmol) and triethyl orthoformate (10 mL) was heated for 12 h at 110°C. Upon cooling to room temperature, colorless crystals were obtained. These were filtered off, washed with diethyl ether (3 × 15 mL) and dried under vacuum. The crude product was recrystallized from EtOH/Et2O.
Synthesis of silver(I) complexes 2
A solution of the appropriate perhydrobenzimidazolium chloride (0.94 mmol), Ag2O (0.47 mmol) and activated 4 Å molecular sieves in dichloromethane (20 mL) was stirred for 24 h at room temperature in the dark under argon. The reaction mixture was filtered through Celite, and the solvent was removed under reduced pressure. The crude product was recrystallized from dichloromethane/hexane (1:2) at room temperature. The resulting white solid was isolated by filtration and dried in vacuum.
Chloro-1,3-bis(4-isopropylbenzyl)perhydrobenzimidazol-2-ylidenesilver(I) 2a. Yield: 0.415 g, 83 %; en: 158 °C. IR: ν (NCN) = 1663 cm−1. Anal. Calc. for C27H37AgClN2: C, 60.84; H, 6.94; N, 5.25. Found: C, 60.89; H, 6.97; N, 5.29 %. 1H NMR (CDCl3) δ: 1.12–1.23 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.66–1.83 and 2.03–2.11 (m, 4H, NCHCH2CH2CH2CH2CHN), 2.83–2.94 (m, 2H, NCHCH2CH2CH2CH2CHN), 1.26 (d, 12H, J = 7.03 Hz, CH2C6H4CH(CH3)2-p), 3.12–3.22 (m, 2H, CH2C6H4CH(CH3)2-p), 4.44, 4.61, 4.91 and 5.07 (d, 4H, J = 15.0 Hz, CH2Ar), 7.08 and 7.36 (d, 8H, J = 8.1 Hz, Ar–H). 13C NMR (CDCl3) δ: 23.86 (NCHCH2CH2CH2CH2CHN), 28.06 (NCHCH2CH2CH2CH2CHN), 50.28 (NCHCH2CH2CH2CH2CHN), 23.95 (CH2C6H4CH(CH3)2-p), 33.97 (CH2C6H4CH(CH3)2-p), 66.42 (CH2Ar), 126.41, 128.02, 132.35 and 147.58 (Ar–C).
Chloro-1,3-bis(4-tert-butylbenzyl)perhydrobenzimidazol-2-ylidenesilver(I) 2b. Yield: 0.44 g, 91 %, en: 202–204 °C, IR: ν (NCN) = 1645 cm−1. Anal. Calc. for C29H41AgClN2: C, 62.08; H, 7.31; N, 4.99. Found: C, 62.01; H, 7.37; N, 4.92 %. 1H NMR (CDCl3) δ 1.13–1.32 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.75–1.78 and 2.04–2.07 (m, 4H, NCHCH2CH2CH2CH2CHN), 2.95–2.98 (m, 2H, NCHCH2CH2CH2CH2CHN), 1.33 (s, 18H, CH2C6H4C(CH3)3-p), 4.60 and 4.91 (d, 4H, J = 15.0 Hz CH2Ar), 7.24 and 7.38 (d, 8H, J = 8.2 Hz, Ar–H). 13C NMR (CDCl3) δ: 23.84 (NCHCH2CH2CH2CH2CHN), 28.04 (NCHCH2CH2CH2CH2CHN), 53.08 (NCHCH2CH2CH2CH2CHN), 31.32 (CH2C6H4C(CH3)3-p), 34.60 (CH2C6H4C(CH3)3-p), 66.42 (CH2Ar), 125.83, 127.35, 132.00 and 151.23 (Ar–C).
Chloro-1,3-bis(2,4,6-trimethoxybenzyl)perhydrobenzimidazol-2-ylidenesilver(I) 2c. Yield: 0.687 g, 77 %, en: 180–182 °C, IR: ν (NCN) = 1610 cm−1. Anal. Calc. for C27H37AgClN2O6: C, 51.55; H, 5.88; N, 4.45. Found: C, 51.50; H, 5.83; N, 4.49 %. 1H NMR (CDCl3) δ: 1.34–1.49 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.64–1.71 and 1.75–1.85 (m, 4H, NCHCH2CH2CH2CH2CHN), 3.57–3.69 (m, 2H, NCHCH2CH2CH2CH2CHN), 3.80 and 3.82 (s, 18H, CH2C6H2(OCH3)3-2,4,6), 4.35, 4.46, 4.75 and 4.90 (d, 4H, J = 14.5 Hz, CH2Ar), 6.08 and 6.12 (s, 4H, Ar–H). 13C NMR (CDCl3) δ: 22.10 (NCHCH2CH2CH2CH2CHN), 24.69 (NCHCH2CH2CH2CH2CHN), 40.96 (NCHCH2CH2CH2CH2CHN), 55.47 and 55.97 (CH2C6H2(OCH3)3-2,4,6), 59.28 (CH2Ar), 90.27, 101.68, 105.12, 159.63, 159.77 and 161.21 (Ar–C).
Synthesis of palladium(II) complexes 3
A solution of the required silver(I) NHC complex (0.74 mmol) and PdCl2(PhCN)2 (0.37 mmol) in dichloromethane (20 mL) was stirred for 24 h at room temperature in the dark. The resulting mixture was filtered through Celite, and the solvent was removed under reduced pressure. The crude product was recrystallized from dichloromethane/diethyl ether (1:2) at room temperature. The white crystals were filtered off, washed with diethyl ether (3 × 10 mL) and dried under vacuum.
Bis[1,3-di(4-isopropylbenzyl)perhydrobenzimidazol-2-ylidene]dichloropalladium(II) 3a. Yield: 0.28 g, 81 %, en: 268–270 °C. IR: ν (NCN) = 1511 cm−1. Anal. Calc. for C54H74N4PdCl2: C, 67.85; H, 7.74; N, 5.86. Found: C, 67.93; H, 7.71; N, 5.81 %. 1H NMR (CDCl3) δ: 0.99–1.02 (m, 8H, NCHCH2CH2CH2CH2CHN), 1.59–1.61 and 1.84–1.88 (m, 8H, NCHCH2CH2CH2CH2CHN), 2.83–2.85 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.22 (d, 24H, J = 6.92 Hz, CH2C6H4CH(CH3)2-p), 2.87–2.92 (m, 4H, CH2C6H4CH(CH3)2-p), 4.94, 5.03, 5.45 and 5.57 (d, 8H, J = 15.3 Hz, CH2Ar), 7.01 and 7.52 (d, 16H, J = 8.1 Hz, Ar–H). 13C NMR (CDCl3) δ: 23.99 (NCHCH2CH2CH2CH2CHN), 28.12 (NCHCH2CH2CH2CH2CHN), 51.92 (NCHCH2CH2CH2CH2CHN), 23.97 (CH2C6H4CH(CH3)2-p), 33.73 (CH2C6H4CH(CH3)2-p), 66.27 (CH2Ar), 126.35, 128.54, 133.60 and 147.73 (Ar–C), 203.72 (Pd–C).
Bis[1,3-di(4-tert-butylbenzyl)perhydrobenzimidazol-2-ylidene]dichloropalladium(II) 3b. Yield: 0.33 g, 89 %, en: 297–298 °C. IR: ν (NCN) = 1514 cm−1. Anal. Calc. for C58H82N4PdCl2: C, 68.84; H, 8.11; N, 5.53. Found: C, 68.90; H, 8.18; N, 5.59 %. 1H NMR (CDCl3) δ: 0.99–1.15 (m, 8H, NCHCH2CH2CH2CH2CHN), 1.59–1.61 and 1.84–1.87 (m, 8H, NCHCH2CH2CH2CH2CHN), 2.87–2.88 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.27 (s, 36H, CH2C6H4C(CH3)3-p); 4.95, 4.99, 5.49 and 5.54 (d, 8H, J = 15.3 Hz, CH2Ar); 7.26, 7.27, 7.50 and 7.54 (d, 16H, J = 8.4 Hz, Ar–H). 13C NMR (CDCl3) δ: 23.99 (NCHCH2CH2CH2CH2CHN), 28.12 (NCHCH2CH2CH2CH2CHN), 51.92 (NCHCH2CH2CH2CH2CHN), 31.39 (CH2C6H4C(CH3)3-p); 34.44 (CH2C6H4C(CH3)3-p); 66.41 (CH2Ar); 125.20, 128.16, 133.28 and 149.95 (Ar–C), 203.79 (Pd–C).
Bis[1,3-di(2,4,6-trimethoxybenzyl)perhydrobenzimidazol-2-ylidene]dichloropalladium(II) 3c. Yield: 0.30 g, 70 %, en: 247–249 °C. IR: ν (NCN) = 1498 cm−1. Anal. Calc. for C54H74N4O12PdCl2: C, 56.49; H, 6.45; N, 4.88. Found: C, 56.40; H, 6.49; N, 4.81 %. 1H NMR (CDCl3) δ: 0.84–1.16 (m, 8H, NCHCH2CH2CH2CH2CHN), 1.34–1.63 and 1.71–1.84 (m, 8H, NCHCH2CH2CH2CH2CHN), 3.31–3.42 (m, 4H, NCHCH2CH2CH2CH2CHN), 3.80 and 3.83 (s, 36H, CH2C6H2(OCH3)3-2,4,6), 4.49, 4.84, 5.42 and 5.60 (d, 8H, J = 14.1 Hz, CH2Ar), 6.04 and 6.11 (s, 8H, Ar–H). 13C NMR (CDCl3) δ: 22.44 (NCHCH2CH2CH2CH2CHN), 24.24 (NCHCH2CH2CH2CH2CHN), 38.46 (NCHCH2CH2CH2CH2CHN), 55.43 and 55.96 (CH2C6H2(OCH3)3-2,4,6), 59.69 (CH2Ar), 89.90, 101.90, 106.92, 159.68, 160.39 and 162.21 (Ar–C), 200.53 (Pd–C).
General procedure for direct C5 arylations
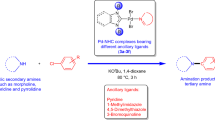
The required heteroaryl derivative (2 mmol), aryl halide (1 mmol), Pd complex 3a–c (0.005 mmol), KOAc (1 mmol) and DMAc (2 mL) were placed in a Schlenk tube equipped with a magnetic stirring bar. The Schlenk tube was purged several times with argon and then placed in a preheated oil bath at 130 °C, and the reaction mixture was stirred for 1 h. The mixture was analyzed by gas chromatography to determine the conversion of the aryl bromide and the yield of product. The solvent was removed by heating the reaction vessel under vacuum, and the residue was charged directly onto a silica gel column. The products were eluted using diethyl ether/pentane (1:3).
Results and discussion
Synthesis of the complexes
In general, silver(I) NHC complexes can be easily prepared by deprotonation of two equivalents of azolium salt with the mild base Ag2O [36]. This method is very useful because the free carbene ligand, which is often hard to handle, does not need to be isolated. Our previously reported [37, 38] symmetrical 1,3-dialkylperhydrobenzimidazolium salts were synthesized in high yields from the N,N′-dialkylcyclohexan-1,2-diamines, triethyl orthoformate and ammonium chloride. The silver(I) NHC complexes 2a–c were prepared by reaction of the 1,3-dialkylperhydrobenzimidazolium salts as NHC ligand precursors with Ag2O in dichloromethane at room temperature in the dark (Scheme 1). The silver NHC complexes 2a–c were obtained in high yields as white solids, soluble in halogenated solvents. The formation of complexes 2a–c was confirmed by the loss of the perhydrobenzimidazolium C2 proton resonance, observed at 10.74 ppm for 1a and 1b, 8.62 ppm for 1c in the 1H NMR spectra. In the 13C NMR spectra of 1a, 1b and 1c, the C2 carbon is observed at 161.90, 162.02 and 162.28 ppm, respectively; for complexes 2a–c, the equivalent resonances for the carbene carbon were not detected, which has also been mentioned in the literature and given as an indicator of the fluxional behavior of such NHC complexes [39]. The silver NHC complexes exhibit characteristic υ(C=N) bands, at 1663, 1645 and 1610 cm−1, for 2a, 2b and 2c, respectively.
Various synthetic methods for the synthesis of Pd(II) NHC complexes have been described in the literature. One of these is the generation of a silver(I) NHC complex, followed by transfer of the carbene ligand to palladium. This reaction has been successfully applied for a variety of metals, including ruthenium, rhodium, iridium, gold and nickel [40]. The palladium(II) NHC complexes 3a–c were prepared in good yields by treatment of the corresponding silver complexes with PdCl2(PhCN)2 in dichloromethane at room temperature (Scheme 1). These complexes are stable both in solution and in the solid state against air, light and moisture and are soluble in chlorinated solvents. Suitable single crystals of these complexes for X-ray diffraction studies could not be obtained. The new complexes were therefore characterized by 1H NMR, 13C NMR, IR spectroscopy and elemental analysis, which support the proposed structures. They show a characteristic υ(NCN) band at 1511, 1514 and 1498 cm−1 for 3a, 3b and 3c, respectively. NMR analyses of the complexes showed that the N-heterocyclic carbene ligands had successfully transferred from silver to palladium. The 13C NMR spectra of the Pd complexes exhibited a singlet assigned to the C2 carbon at 203.72, 203.79 and 200.53 ppm for 3a, 3b and 3c, respectively. This is consistent with reported values for other [PdCl2(NHC)2] complexes.
Catalytic studies
Use of benzimidazol-2-ylidene or imidazolin-2-ylidene ligands in the palladium-catalyzed direct arylation of thiazoles, thiophenes and furans was originally reported by Doucet et al. [31]. They found that the treatment of aryl bromides with thiazoles, thiophenes or furans in the presence of 1 mol% of a Pd-NHC complex in N,N-dimethylacetamide at 150 °C for 20 h under argon gave the corresponding arylation products in moderate to good yields. In this study, we performed catalytic experiments under mild conditions including short reaction time, low catalyst loading and relatively low temperature. Based on previous results, in this study, we chose N,N-dimethylacetamide (DMAc) as the solvent and potassium acetate as the base. The catalytic reactions were performed at 130 °C for 1 h in the presence of complexes 3a–c. Under these reaction conditions, 2-n-butylfuran, 2-n-butylthiophene and 2-n-propylthiazole were allowed to react with four aryl bromides bearing electron-donating or electron-withdrawing groups at the para position to furnish the arylated products in moderate to good yields (Tables 1, 2, 3). In the absence of palladium complex, the reactions of 4-bromoacetophenone with 2-n-butylfuran, 2-n-butylthiophene or 2-n-propylthiazole resulted in only 1 % yields under these reaction conditions. When aryl chlorides were used as substrate, the reactivities dropped significantly and longer reaction times were required to afford the arylated products. For example, 4-chloroacetophenone was coupled with 2-n-butylfuran in the presence of 3c to give 5-(4-acetylphenyl)-2-n-butylfuran in 52 % yield at 130 °C after 20 h. The arylation reactions were regioselective, such that in all cases, only the C5-arylated products were formed.



Initially, we investigated the reactions of 2-n-propylthiazole with 4-bromoacetophenone, 4-methoxybromobenzene, 4-methylbromobenzene and bromobenzene for the direct C5 arylation reactions using complexes 3a–c as catalysts. The arylation products were obtained in good yields for all three catalysts (Table 1, entries 1–12). Aryl bromides with an electron-donating group such as methoxy or methyl on the aromatic ring reacted with 2-n-propylthiazole to give the coupled products in excellent yields (Table 1, entries 4–9). However, aryl bromides without an electron-donating group on the aromatic ring showed slightly lower reactivities, as demonstrated in the arylation reactions using 4-bromoacetophenone and bromobenzene (Table 1, entries 1–3 and 10–12). For all aryl bromides, the best conversion (of 93–99 %) was achieved with complex 3c.
Next, we examined the reactivity of 2-n-butylthiophene using the same coupling partners under similar reaction conditions (Table 2). As with 2-n-propylthiazole, aryl bromides with an electron-donating group coupled with 2-n-butylthiophene to give the corresponding arylation products in good yields (Table 2, entries 4–9). Thus, the 5-arylated thiophenes were obtained in high yields. 4-Bromoacetophenone was successfully coupled with 2-n-butylthiophene using complexes 3a–c as catalysts to give 5-(4-acetylphenyl)-2-n-butylthiophene in 66–72 % yields. High conversions for 2-n-butylthiophene were obtained using the electron-rich 4-bromoanisole and 4-methylbromobenzene in the presence of all three complexes (Table 2, entries 5–8). The catalytic activities of complexes 3a–c in these reactions were similar to those in the direct C5 arylation of 2-n-propylthiazole.
Finally, for the direct arylation reactions, in place of 2-n-propylthiazole or 2-n-butylthiophene, 2-n-butylfuran was used. The reactions were performed with 4-substituted aryl bromides at 130 °C for 1 h in DMAc to obtain 5-aryl-2-n-butylfurans with the results summarized in Table 3. Four aryl bromides were used successfully. Reactivities of these aryl bromides using complexes 3a–c were also examined for the direct arylation of 2-n-butylfuran. All of the aryl bromides gave moderate to high yields of C5 arylation products in the presence of 0.5 mol% of catalyst. High conversions for 2-n-butylfuran were obtained using electron-deficient 4-bromoacetophenone (Table 3, entries 1–4) as compared to direct arylation of 2-n-propylthiazole or 2-n-butylthiophene. Among the three complexes, complex 3c bearing NHC ligands with methoxy substituents exhibited better catalytic activity than the others.
Conclusion
In summary, silver(I) and palladium(II) NHC complexes were successfully synthesized and characterized by physicochemical and spectroscopic methods. The catalytic activities of the palladium complexes were investigated in direct C5 arylation of thiazole, thiophene and furan derivatives in the presence of potassium acetate. All three complexes demonstrated excellent catalytic activities in these reactions. Furthermore, the catalyst loading and reaction temperature were both lower, and the reaction time shorter, compared to previous reports. It should be noted that these arylation reactions are very selective, such that in all cases, only the C5-arylated products were formed; the 3- or 4-arylated products were not detected by GC analysis of the reaction mixtures.
References
Wanzlick H-W, Schönherr H-J (1968) Angew Chem Int Ed Engl 7:141
Öfele K (1968) J Organomet Chem 12:42
Cardin DJ, Çetinkaya B, Lappert MF, Manojlovic-Muir L, Muir KW (1971) J Chem Soc Chem Commun 400
Arduengo AJ III, Harlow RL, Kline M (1991) J Am Chem Soc 113:361
Jafarpour L, Nolan SP (2000) Adv Organomet Chem 46:181
Peris E, Crabtree RH (2004) Coord Chem Rev 248:2239
Herrmann WA (1997) Angew Chem Int Ed Engl 36:2162
Benhamou L, Chardon E, Lavigne G, Bellemin-Laponnaz S, Cesar V (2011) Chem Rev 111:2705
Levin E, Ivry E, Diesendruct CE, Lemcoff NG (2015) Chem Rev 115:4607
Scholl M, Trnka TM, Morgan JP, Grubbs RH (1999) Tetrahedron Lett 40:2247
Şahin Z, Gürbüz N, Özdemir İ, Şahin O, Büyükgüngör O, Achard M, Bruneau C (2015) Organometallics 34:2296
Günay ME, Çoğaşlıoğlu GG (2016) Turk J Chem 40:296
DePasquale J, Kumar M, Zeller M, Papish ET (2013) Organometallics 32:966
Yiğit B, Yiğit M, Özdemir İ, Çetinkaya E (2012) Transit Met Chem 37:297
Busetto L, Cassani MC, Femoni C, Mancinelli M, Mazzanti A, Mazzoni R, Solinas G (2011) Organometallics 30:5258
Li F, Hu JJ, Koh LL, Hor TSA (2010) Dalton Trans 39:5231
Hartwig JF, Kawatsura M, Hauck SI, Shaughnessy KH, Alcazar-Roman LM (1999) J Org Chem 64:5575
Chen JCC, Lin IJB (2000) Organometallics 19:5113
Toure BB, Lane BS, Sames D (2006) Org Lett 8:1979
Li JJ, Gribble GW (2000) Palladium in heterocyclic chemistry. Pergamon, Amsterdam
Wolf C, Lerebours R (2003) J Org Chem 68:7077
Allegretti M, Arcadi A, Marinelli F, Nicolini L (2001) Synlett 5:609
Organ MG, Abdel-Hadi M, Avola S, Hadei N, Nasielski J, O’Brien CJ, Valente C (2006) Chem Eur J 13:150
L’Helgoual’ch J-M, Seggio A, Chevallier F, Yonehara M, Jeanneau E, Uchiyama M, Mongin F (2008) J Org Chem 73:177
Ohta A, Akita Y, Ohkuwa T, Chiba M, Fukunaga R, Miyafuji A, Nakata T, Tani N, Aoyagi Y (1990) Heterocycles 31:1951
Satoh T, Miura M (2007) Chem Lett 36:200
Xi P, Yang F, Qin S, Zhao D, Lan J, Gao G, Hu C, You J (2010) J Am Chem Soc 132:1822
Beydoun K, Doucet H (2011) J Organomet Chem 696:1749
Srinivasan R, Kumaran RS, Nagarajan NS (2015) Catal Commun 58:187
Kamimoto N, Schollmeyer D, Mitsudo K, Suga S, Waldvogel SR (2015) Chem Eur J 21:8257
Özdemir İ, Gök Y, Özeroğlu Ö, Kaloğlu M, Doucet H, Bruneau C (2010) Eur J Inorg Chem 12:1798
Ghosh D, Lee HM (2012) Org Lett 14:5534
Akkoç S, Gök Y, Akkurt M, Tahir MN (2014) Inorg Chim Acta 413:221
Bernhammer JC, Singh H, Huynh HV (2014) Organometallics 33:4295
Karaca EÖ, Gürbüz N, Özdemir İ, Doucet H, Şahin O, Büyükgüngör O, Çetinkaya B (2015) Organometallics 34:2487
Wang HMJ, Lin IJB (1998) Organometallics 17:972
Yigit M (2009) Molecules 14:2032
Murat Y, Bayam G, Yiğit B, Özdemir İ (2013) Heterocycles 87:897
Nielsen DJ, Cavell KJ, Skelton BW, White AH (2003) Inorg Chim Acta 352:143
Lin IJB, Vasam CS (2007) Coord Chem Rev 251:642
Acknowledgments
We thank the Adıyaman University Research Fund (FEFMAP/2015-0004) for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yiğit, B., Yiğit, M., Dağdeviren, Z. et al. Synthesis of silver(I) and palladium(II) N-heterocyclic carbene complexes and their use as catalysts for the direct C5 arylation of heteroaromatic compounds. Transit Met Chem 41, 751–757 (2016). https://doi.org/10.1007/s11243-016-0075-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0075-y