Abstract
This study represents an optimized protocol for callus establishment and cell suspension culture of Matricaria chamomilla, and the impact of the static magnetic field (SMF) on flavonoid metabolism and antioxidant activity were examined for the first time. The effect of growth regulators was investigated to enhance biomass growth and apigenin production. Murashige and Skoog medium supplemented with 2,4-D (1.5 mg l−1) and Kinetin (0.5 mg l−1) showed the highest callus induction rate (100%), fresh weight, apigenin (0.82%) and apigenin-7-glucoside (1.57%) contents. Cell suspension culture was established, and the optimum subculture time was found to 13–15 days. SMF induced cell leaching and oxidative stress in all treated cells by an increase in H2O2 content and more stimulation of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) enzymes activities. Total phenolic, flavonoid and DPPH activity increased in cells treated to SMF, and the maximum content of apigenin (1.3%) and apigenin-7-glucoside (2.1%) were identified in cell treated to 4 mT. These results provided an effective method for the regulation of flavonoid biosynthesis in M. chamomilla cell suspension culture, and the use of SMF as a tool for the induction of apigenin production.
Key message
Cell suspension cultures of Matricaria chamomilla contain valuable medicinal flavonoids. Static magnetic field promoted apigenin production and antioxidative enzyme activities in M. chamomilla cell suspension.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chamomile (Matricaria chamomilla L.) is a medicinal plant of Asteraceae that exists all over the world. This plant is native to Iran and cultures in Europe, America, and Asia. Apigenin is a flavonoid compound that has detected in M. chamomilla seedling (Sayadiet al. 2014). Apigenin has extensive biological activities including anti-inflammatory, antioxidant, anti-cancer, neuroprotective, anti-microbial and anti-allergic impacts (Sebai et al. 2014; Zemestani et al. 2016; Patel et al. 2007; Ranpariya et al. 2011; Silvaet al. 2012). Apigenin exists in different plant species with low content such as Capsicum annum, Allium sativum, Averrhoa bilimbi, Pisum sativum, Psidium guajava and other plants (Miean and Mohamed 2001), while its content is higher in M. chamomilla than the mentioned plants. On the other hand, different populations of M. chamomilla from different countries showed various apigenin contents (Srivastava and Gupta 2007). Iranian M. chamomilla showed 0.74–1.11% apigenin content (Haghi et al. 2014).
Biotechnological approaches, especially plant cell and tissue culture as a supplement to traditional agriculture has potential for the high production of biological metabolites (Giri and Narasu 2000; Rao and Ravishankar 2002). Plants and cultivated cells produce secondary metabolites in qualitatively like pathways (Hellwig et al. 2004), and there are successful samples of tissue cultures to increase content of the secondary compounds in some medicinal plants. Besids, these contents were more as compared to wild plants (Estrada-Zuniga et al. 2009). In vitro cultures characterize a suitable tool for generating phenylpropanoids as a replacement for using wild plants (Estrada-Zuniga et al. 2009).
Numerous strategies are applied to improve the quality and quantity of secondary compounds generation in medicinal plant cell culture comprising a selection of suitable cell lines, immobilization of cells, alteration in compounds of the culture medium, various physical and chemical stresses, etc. (Tahsiliet al. 2014). Magnetic field (MF) is a physical stress has been recently proposed for plant secondary metabolite production and they have fewer dangerous for the environment (Aladjadjiyan 2010). MF effects on cells are related to the magnetic susceptibility, MF intensity, and gradient. Cells are containing various cellular components and combinations that could reply to the MF including mitochondria, cell membrane, protein and DNA (Pauling 1979). MF could modify electromagnetic characterizes of biomolecules, membrane permeability, and enzyme activity in biochemical pathways. Also, it has been stated MF is altered the concentration and activity of reactive oxygen species (ROS), membrane ionic currents, and cell growth and propagation (Çeliket al. 2009; Galland and Pazur2005; Payez et al. 2013; Sahebjamei et al. 2007). A few studies indicated that MF could induce various secondary metabolites in plants (Rezaei et al. 2010; Taghizadeh et al. 2019; Mansourkhaki et al. 2019), and its mechanism needs more investigation. Therefore, the purposes of this work were to investigate the impacts of the static magnetic field (SMF) on secondary metabolites and antioxidative responses in M. chamomilla cell suspension culture for the first time. Results from this study can aid us to develop our information about the mechanism(s) of the undifferentiated plant cells reactions to SMF. It too may aid us to induce the construction and secretion of the valuable flavonoid metabolites in undifferentiated plant cells.
Materials and methods
Plant material
Matricaria chamomilla L. plants were gathered in July 2018 from Dizin Mountains in the north-east of the Tehran province of Iran. The plants were identified with a voucher number to the Herbarium in the Department of Biological Science of Tehran University, Tehran, Iran.
Callus induction for cell suspension culture
Seeds were put in sodium hypochlorite solution (10%) for 10 min and then washed three times with sterile distilled water. Surface sterilized seeds were placed in the Murashige and Skoog (MS) medium containing sucrose (3%) and agar (7%), temperature of 25 ± 2 °C (day/night), a 16-h photoperiod per day, 65% relative humidity and white fluorescent light (46 µmol−1 m−2 s−1) (Murashige and Skoog 1962).
For callus induction, small pieces (1–1.5 cm) of hypocotyls were put on the MS medium culture with different 2,4-dichlorophenoxyacetic acid (2,4-D) (0, 0.5, 1.5 and 2 mg l−1) levels and kinetin (Kin) (0, 0.5, 1 and 1.5 mg l−1) at pH of 5.7. The cultured samples were placed in darkness at 25 ± 2 °C. Four explants were put on to the Petri dish, with five replicates, and all experiments were performed at least twice independently. The calli were emerged from the hypocotyl explants and were subcultured on MS medium supplemented with the same plant growth regulators after 3 weeks, and callus induction rate, fresh weight, callus index, callus status, and apigenin content were measured. The best friable callus derived from hypocotyl was selected for cell suspension culture. Callus induction rate of 30 replicates per treatment was evaluated based on Zeng et al. (2009).
For the determination of the callus index, callus induction rate was multiplied with growth score which was assessed by visual rating (poor = 1, medium = 2, good = 3, and profuse = 4). The mean score was expressed as a growth score (Hazeena and Sulekha 2008).
Cell suspension culture was initiated by transferring 2.5 g of friable-cream calli into 100 ml flasks containing 25 ml of MS liquid medium supplemented with Kin (0.5 mg l−1), 2,4-D (1.5 mg l−1) and sucrose (30 g l−1) at pH 5.7 on a rotary shaker (120 rpm, 25 °C), and kept in darkness. The suspension cultures were subcultured in the fresh MS liquid medium at a two-week interval. The cells were homogenized after 2 months. For determination of cell suspension growth curve, fresh cells (1 g) from stock were separated from the medium by filtration under suction and then, were inoculated into 20 mL of the fresh liquid medium in a 100 mL Erlenmeyer flask. Growth of cell suspension culture and cell viability were measured with sets of flasks harvested at 2 day-intervals in a period of 21 days. Readings were taken from three flasks for each parameter. Cells were separated from the medium by filtration using nylon mesh and weighed as fresh weight.
Static magnetic field (SMF) treatment
Exposure to SMF was performed using locally designed homogenous SMF generator (MFG-13971, OFOGH, Iran) with a 220 V DC power supply (MP-6010, MEGATECH, Iran) for producing various SMF intensities in the range of 0.5 µT-15 millitesla (mT) (Mansourkhaki et al. 2019). This system consisted of a copper wire (1.1 mm in diameter), wrapped 1300 rounds around a polyethylene tube in 12 cm diameter and 50 cm length. Containers containing cell suspension culture of M. chamomilla (Erlenmeyer flask) were placed in the middle of the tube to get uniform intensity at all points of the container, and magnetic field intensities were measured by the Tesla meter (MG-3002) with a B-probe type of hall sound. The M. chamomilla cells in their exponential growth phase (from day 5 to 7 after subcultures) were treated with different intensities of the static magnetic field (0, 2, 4 and 6 mT) for one hour during three days. The cells were harvested for biochemical analyses on day 13 of the subculture. Different intensities of SMF were selected based on some literature studies (Aleman et al. 2014).
Cell viability
Cells viability was determined by Evan’s blue dye with a new method. Cell suspension (500 µL) from each flask was centrifuged at 1000 rpm at 25 °C for 5 min. The supernatant was separated, and 400 µL distilled water was added to the plate and centrifuged again. Then, 80 µL of 0.25% Evan’s blue stain for 4 min was added to the tube, centrifuged, and the stain was separated. Finally, 200 µL distilled water was added to the tube and the cells were observed by ECLIPSE Nikon microscope, E-200. At least 550 cells were counted, and the experiment was repeated twice (n = 6). For cell leaching, cells were observed by a Nikon inverted microscope, MA100N after SMF treatment on day 10.
DPPH radical scavenging
Reducing of 2,2-diphenyl-2-picryl hydrazyl (DPPH) solution was determined based on Patro et al. (2005). The fresh cells (100 mg) were homogenized in 2 ml of 80% methanol and then were centrifuged at 5000 rpm for 5 min. The 30 µL of extract solution was mixed with 1 mL of DPPH (0.1 mM in methanol). The absorbance was read at 517 nm at room temperature after 30 min incubation. The radical scavenging activities were calculated as the percent inhibition of the DPPH radical (%) = 100(A − B)/A, where A and B are the absorbance of the control and the absorbance of the sample reaction mixture.
Total phenolic and flavonoid contents
Total phenolic content was determined by a modified Folin-Ciocalteu procedure (Singleton and Rossi 1965). The 100 μL of the diluted extract was added to the 500 μL Folin–Ciocalteu reagent [previously diluted with water 1:9 (v/v)] and 400 μL sodium carbonate (0.7 mol l−1) and then were incubated 90 min at room temperature in the dark. The absorbance was measured spectrophotometrically at 765 nm. The total phenolic content was measured by a calibration curve of gallic acid and expressed as per mg gallic acid equivalent.
Flavonoid content was estimated by using the aluminum chloride method with a minor modification (Hatamnia et al. 2014). Briefly, 500 µL of the plant cell extract, 50 µL of sodium nitrate solution (5%), 50 µL of aluminum chloride solution (10%) and 250 µL of sodium hydroxide solution (4%) were mixed and then, samples were put at room temperature for 30 min. The absorbance of the mixture was immediately measured at 415 nm. Total flavonoid content was expressed as mg of rutin equivalents in 1 g FW.
Determination of apigenin by HPLC
Apigenin was extracted by callus or cell samples (0.5 g of dried samples) in methanol aqueous solution (20 ml), with bath ultrasonic and overnight incubation. The homogenated solution was centrifuged at 5000 rpm and then, the supernatant was collected, dried and dissolved in methanol (500 µL). For the quantitative and qualitative determination of apigenin and derivate in methanol, a HPLC program equipped with a UV–Vis photodiode-array detector (Agilent Technologies 1260 infinity, Santa Clara, CA). The chromatographic separation was obtained by a C18 column (MZ Analysentechnik, Mainz, Germany). The mobile phase (A solvent) consisted of deionized water with 0.1% (v/v) phosphoric acid and (B solvent) consisted of acetonitrile. The gradient system consisted of 18% B (0–30 min), 67% B (30–60 min) and 18% B (60–65 min). The flow rate was 1 mL min−1at 25 °C, and the injection volume was 20 μL. The flavonoid apigenin was characterized by recording their absorbance peaks at 330 nm. The compounds were identified by comparing its retention time and UV λmax of peaks with those of standards, and its percentage was identified based on its standard calibration curve (Barreca et al. 2016). Peak identity was confirmed by comparing their retention times and absorption spectra with those of pure (> 99%) standards of apigenin and apigenin-7-glucoside from Sigma-Aldrich.
H2O2 level
The content of H2O2 was measured using the method of Velikova et al. (2000). Samples were homogenized in an ice bath with 0.1% (w/v) trichloroacetic acid (5 mL). The homogenate was centrifuged at 12,000 rpm for 15 min and the supernatant (0.5 mL) was added to 10 mM potassium phosphate buffer (0.5 mL, pH 7.0) and 1 M potassium iodide (1 mL). The H2O2 level was evaluated by comparison of its absorbance at 390 nm with a standard calibration curve.
Protein content and antioxidant enzyme activity
Soluble protein content was evaluated using the Bradford method (Bradford 1976) and bovine serum albumin as a standard. Fresh cells (0.2 g) were homogenized with 1 M Tris–HCl (pH 6.8) at 4 °C. Then, samples were centrifuged at 10,000 rpm for 15 min at 4 °C. The obtained supernatant was put at − 70 °C and used for enzyme assays.
The activity of superoxiddismutase (SOD; EC 1.15.1.1) was determined by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT) as described by Giannopolitis and Ries (1977). The 80 μL of enzyme extract was added to the reaction mixture containing 75 μM NBT, 75 μM riboflavin, 50 mM sodium phosphate buffer (pH 7.5), 0.1 mM EDTA, and 13 mM methionine. The reaction mixture was irradiated for 14 min, and then, absorbance was measured at 560 nm against the non-irradiated blank.
Peroxidase (POX; E.C.1.11.1.7) activity was evaluated using the method of Abeles and Biles (1991). The 100 μL enzyme extract was added to the reaction mixture containing 0.2 mL H2O2 (3%), 2 mL of acetate buffer (0.2 M, pH 4.8), and 0.1 mL benzidine (40 mM). The absorbance was read at 530 nm.
Ascorbate peroxidase (APX; EC 1.11.1.11) activity was evaluated by the method of Jebara et al. (2005). The 10 μL of enzyme extract was added to the reaction mixture containing H2O2 (0.1 mM), potassium phosphate buffer (50 mM, pH 7.0) and ascorbic acid (0.5 Mm) in a total volume of 1 mL. The content of oxidized ascorbate was determined at 290 nm, and calculated using the extinction coefficient (ε = 2.8 mM−1 cm−1).
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) using SPSS. Duncan’s multiple range test was used to determine statistical differences between treatments and control. Each experiment was repeated three to five times at the P ≤ 0.5 level of confidence.
Results
Calli induction
Plant growth regulators are used to induce callus formation and growth in various plant species. In the present work, various concentrations of 2,4-D and Kin were applied in MS medium to detect the optimum concentration of plant growth regulators in hypocotyl explants of M. chamomilla (Table 1). Callus formation was successfully induced in all combination of 2,4-D and Kin (Fig. 1). No callus induction obtained in medium without hormone and showed that exogenous hormone was necessary to callus formation of M. chamomilla. Most calli were cream in color for 8 days, but some treatments started to get brownish and light green in color after 14 days which happened in treatments with high Kin concentration (1.5 mg l−1). The lowest concentration of Kin (0.5 mg l−1) showed the optimum concentration for M. chamomilla callus induction. Kin at 0.5 mg l−1combined with 2,4-D (1 and 1.5 mg l−1) show the highest fresh weight, fragile and cream calli in color and callus index of 390–400 after 3 weeks.
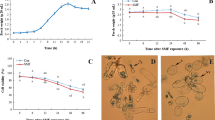
Establishment of M. chamomilla callus and cell suspension culture. a Callus induction (scale bar 1 cm), b optimization of callus culture (scale bar 1 cm), c cell culture on a shaker (scale bar 8 cm), d cell suspension culture (scale bar 1 cm), e and f cells on day 8 and 14 (scale bars 60 µm), and also g cell fresh weight and h cell viability after 21 days. V Viable cell, NV non-viable cell
Calli apigenin content
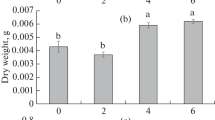
The content of apigenin and apigenin-7-glucoside were various between different levels of 2,4-D and Kin. A trace amount of apigenin was defined at an equal concentration of 2,4-D/Kin, and with raise of 2,4-D concentration, apigenin production increased in the M. chamomilla calli (Fig. 2). The highest amount of apigenin (82%) and apigenin-7-glucoside (1.57%) were detected on the treatment of 2,4-D (1.5 mg l−1) and Kin (0.5 mg l−1). This treatment was selected for cell suspension culture and SMF treatment.
Cell suspension culture, viability and SMF treatment
Friable and cream callus clumps with optimum growth and apigenin content were used for starting the cell suspension culture. Cell growth was evaluated in liquid MS medium supplemented with 2,4-D (1.5 mg l−1) and Kin (0.5 mg l−1) by measuring the fresh weight of the cells every 2 days (Fig. 1). The growth curve of suspension cultures showed that the growth rate was primarily slow during the first 3 days (lag phase). However, as the cultures continued it presented a noticeable rise from day 7 and accumulated a great amount of fresh weight over for 13 days (exponential phase) (Fig. 1g). Maximum growth in fresh weight was obtained on days 13 and 15 which was about fivefold over the primary fresh weight. The speed of growth was steady for 5 days (stationary phase). The stationary phase was followed by a decrease in cell growth (Fig. 1g). According to the growth curve, the time for subculturing to new fresh media was between days 13–15 of incubation, the finish of the exponential growth phase. Moreover, the cell viability as shown in Fig. 4b was around 84.5% throughout the 13 days of culture. Two kinds of cells (round and elongated shape) were identified in the cell suspension (Fig. 1e and f). Commonly in durations longer than 14 days (exponential phase), the number of round-shaped cells in the culture was augmented.
Different intensities of SMF changed the cell fresh weight, viability, and morphology of M. chamomilla. Observation of cells using an inverted microscope on day 10 of subculture appeared cell leaching with more round shape at 6 mT as compared to control. Cell fresh weight increased at 4 mT, but decreased at 2 and 6 mT SMF (Fig. 3a). Cell viability under SMF decreased as comparison to control, but there was no significant difference between treatments (Fig. 3b). The lowest cell viability was identified at 6 mT (71%) on day 13 of culture.
Protein content
Cells treated to SMF showed different protein values under SMF (Fig. 3c). Protein content slightly decreased at 2 mT, and then significantly increased at 4 and 6 mT. The maximum protein content was observed at 4 mT with a 41.9% increase as compared to control.
H2O2 level and antioxidant enzyme activity
The H2O2 level of SMF treated cells was more content than the control group. The maximum H2O2 content was witnessed at 6 mT (16.1 µmol g−1 FW) (Fig. 4). The activities of antioxidant enzymes involved in reactive oxygen species (ROS) detoxification induced under different SMF treatments as compared to control. SOD as a scavenging enzyme of superoxide radicals increased with increasing of SMF intensity, and the highest activity was observed at 6 mT. POX and APX activities performed scavenging H2O2 in the cells were significantly increased at 4 and 6 mT, and their maximum activities were identified at 4 mT. The activity of antioxidant enzymes was similar to DPPH activity under SMF.
DPPH radical scavenging
Overall, all treatments considerably improved the activity of DPPH relative to the control (Table 2). Cells treated with 4 and 6 mT SMF showed the highest value of the DPPH radical scavenging about 68 and 76%, respectively.
Phenol, flavonoid and apigenin contents
Total phenolic and flavonoid contents of the M. chamomilla cells that were exposed to 0, 2, 4 and 6 mT significantly increased under SMF (Table 2). Cells treated with 4 mT displayed the highest phenolic content (9.51 ± 0.21 mg GAE g−1 DW) which showed a 1.73 fold rise as compared to control.
Cell responses to flavonoid production were various between different SMF treatments. Flavonoid content slightly decreased at 2 mT, but increased its content at 4 and 6 mT as compared to control. The highest value of total flavonoid content (3.54 mg RE g−1 DW) was witnessed at 6 mT which showed a 1.3 fold increase compared to control (Table 2).
Treated cell to 4 and 6 mT showed a significant increase in apigenin and apigenin-7-glucoside as comparison to control (Fig. 5). The maximum apigenin (1.36%) and apigenin-7-glucoside (2.14%) induced in cell treated to 4 mT.
Discussion
Growth regulators are essential for callus induction and stimulate cell lines growth. Various plant species require various phytohormones for callus formation and metabolites generation. Therefore, it is vital to choose the suitable growth regulators and identification of their optimal levels. Other investigators have performed callus induction in M. chamomilla previously. Sayadi et al. (2014) presented that the maximum callus formation was obtained on MS medium contained NAA (1 mg l−1), Kin (1 mg l−1) from axillary buds (89.68%), leaf (93.26%), and stem discs (80.75%) explants. In this study, callus formation successfully happened from hypocotyls explants, and the optimum levels of growth regulators were obtained at Kin (0.5 mg l−1) and 2,4-D (1.5 mg l−1) with maximum callus induction (100%), fresh weight, cream and friable calli (Table 1). 2,4-D causes the differentiation, elongation and cell division and belongs to phenoxy acetic acid herbicides. It commonly used for callus induction (Venkov et al. 2000). Cytokinin influences on the formation and function of mitotic apparatus and stimulation of cell division by regulation of proteins synthesis (Chawla 2002; George et al. 2008). In this study, the upper concentration of Kin (1.5 mg l−1) changed the color from cream to green and brownish after 2 weeks (Table 1) which may be correlated to the effect of the high level of Kin on chlorophyll synthesis (George et al. 2008). Our data showed that exogenous hormone was necessary for callus induction of M. pulegium, and callus was not formed on basal MS medium. Several works showed that the formation of callus did not occur on MS medium without hormones (Mathur and Shekhawat 2013; Ray et al. 2011). Among the leaf, shoot and hypocotyl explants used, hypocotyl was more responsive for callus formation as a comparison to leaf and shoot explants (data not published).
Optimization of callus induction and cell suspension culture is the first stage to create the high contents of bioactive metabolites. Our results point out that MS medium with Kin (0.5 mg l−1) and 2,4-D (1.5 mg l−1) showed the maximum percentage of callus formation, callus growth and bioactive compounds including apigenin (0.82% w/w) and apigenin-7-glucoside (1.57% w/w) contents in M. chamomilla calli (Fig. 2), and it can be selected for cell suspension culture. Apigenin content in M. chamomilla has been previously reported in wild and in vitro plants, but not in callus tissue and cell suspension culture. Apigenin-7-glucoside content was 72.96 mg kg−1 and 13.44 mg kg−1 in in vivo and in vitro conditions in M. chamomilla, respectively (Banaz 2018). Haghi et al. (2014) presented the content of apigenin [0.74% (w/w)] and apigenin-7-glucoside [1.11% (w/w)] in wild M. chamomilla aerial part. There is a lack information about apigenin content in M. chamomilla callus. However, some reports have shown the impact of plant growth regulators on secondary compounds generation in callus tissues. Combination of 2,4-D and BAP increased saponin (4.8 mg g−1 DW) content in Centella asiatica calli (Rao et al. 2015). Çolgecen et al. (2018) reported that 2,4-D and BAP induced aucubin (3.06 mg kg−1) and verbascoside (0.74 mg kg−1) in Camptotheca acuminate. Types and concentrations of plant growth regulators influence the in vitro creation of secondary compounds in medicinal plants (Palacio et al. 2012). Application of 2,4-D can induce some genes encoding stress, defense proteins, and also changes hormone hemostasis in the plant cells (Pazmiño et al. 2012). It should mention that the increase of apigenin and apigenin-7-glucoside might relate with the influence of a high level of 2,4-D on enzyme activities from flavonoid biosynthetic pathways.
Cell growth measurements of M. chamomilla showed the progression of cell growth over a 21-day culture period, and the subculture for new fresh media was performed between days 13 and 15 (Fig. 1g). The typical sigmoidal growth curve consists of three distinct phases including the lag phase, exponential phase, and stationary phase. The cells at the termination of the exponential stage are reliable to subculture, and after the logarithmic growth phase, the nutrients become limited or/and toxic materials are created by cells and increase the cell death (Stafford and Warren 1991; Bhojwani and Razdan 1983). In this study, cell viability reached to 84.5% throughout the 13 days of culture and then decreased to 66% after 21 days (Fig. 1h). The 50% cell viability considered that the suspension culture creation has unsuccessful (Qui et al. 2009). Our findings showed that the cell shapes in liquid medium changed from length to round during the culture (Fig. 1e and f). Changes of cell shape and size can relate to the production of secondary metabolites in medicinal plant cell suspension cultures (Forni et al. 1998). Based on our findings, cells treated to SMF showed various responses to growth and protein content. In contrast to 4 mT treatment, SMF at 2 and 6 mT decreased the fresh weight and protein content (Fig. 3a and c). Also, cell viability didn’t show a significant response between different SMF intensities on day 7 of the subculture (Fig. 3b), while cell leaching induced with the increase of SMF intensity especially at 6 mT SMF on day 10 of subculture. It seems that SMF can induce ROS production and oxidative stress in cells, and following may decrease reaching time to stationary phase with more round shape cells especially at 6 mT (Fig. 3a and b). The first reaction of the cell to MF is varying the electrical conduct and permeability of cell membrane (Zhang et al. 2015). Moreover, MF affects the spins of paramagnetic molecules in cells and induces formation intracellular paramagnetic ROS (Durmuset al. 2015). So, cell proliferation and growth are affecting by SMF. However, the various impacts of SMF on cell proliferation and growth can be related to cell type, MF intensity, cell density and plant species (Zhang et al. 2017).
Plant cells treated to MF were stimulated to the formation of ROS and oxidative damage (Maffei 2014; Payez et al. 2013; Sahebjamei et al. 2007). ROS can influence gene expression, cellular membrane integrity, and activity of enzymes (Serrano et al. 1999; Jithesh et al. 2006). Plant cells comprise various types of antioxidant enzymes that detoxify or inhibit the production of the destructive ROS, which maintain cells against oxidative stress (Matamoros et al. 2003). SOD, POX, APX, and CAT are commonly considered as the main components of the antioxidant mechanism of the plants (Xue et al. 2008). APX and CAT decompose H2O2 into oxygen and water. POX detoxifies H2O2 by oxidation of compounds include phenolic compounds and/or antioxidants (Apel and Hirt 2004; Demir and Kocaliskan 2001).
Based on our findings, SMF through excessive production of ROS such as H2O2 induced oxidative stress in SMF treated cells (Fig. 4a) and defense mechanisms for suppression of the harmful effects of ROS including SOD, POX and APX enzymes markedly increased in M. chamomilla cells. The maximum activity of POX and APX was identified at 4 mT, whereas SOD activity was more at 4 and 6 mT. It has also been previously shown that oxidative stress and some scavenging enzymatic mechanisms induced by MF treatment in suspension-cultured plant cells (Rajabbeigi et al. 2013; Çelik et al. 2009). It seems that SMF at especial intensity (4 mT) could protect M. chamomilla cells of oxidative damage by more induction of antioxidative enzymes, and promote cell growth.
The activity of DPPH-radical scavenging is one of the simplest manners used for the detection of antioxidant ability of plant cells in the short time (Khan et al. 2013). In this work, the DPPH activity markedly induced under different SMF intensities. Increased DPPH activity may be due to more accumulation of phenolic compounds in cell treated to SMF. Sharma and Ramawat (2013) proved a positive correlation between established DPPH concentration and phenolics in Salvadora persic callus tissue.
Our study indicated that SMF treatments at 4 and 6 mT increased the contents of flavonoid, total phenolic compounds and apigenin against the oxidative hurt induced by SMF (Table 2, Fig. 5). The positive correlation between the metabolism of secondary metabolites and defense processes has been presented in the other researches (Çelik et al. 2009; Maffei 2014). The oxidative stress induced by SMF can perform like an endogenous sign on increasing the defense mechanism of plants via alternation in bioactive compounds including phenol and flavonoid compounds which have multiple roles in plant cells, such as removing free radicals, inhibiting enzymatic systems producing free radicals, inducing the gene expression responsible for inhibition of oxidative damage, and enhancing the antioxidant enzymes activity. It has been published that various environmental stresses can improve the content of these metabolites in plants (Taghizadeh et al. 2019; Primiano et al. 1997; Złotek et al. 2014).
Conclusion
In conclusion, this study is the first study about the formation of callus tissue and cell suspension culture of M. chamomilla, and identification of apigenin as an important flavonoid under different growth regulators and SMF treatments. The best callus growth and cell suspension culture, as well as the highest apigenin production were identified in MS medium with 2,4-D (1.5 mg l−1) and Kin (0.5 mg l−1). Overall, SMF-treated cells show an increase of total phenolics, flavonoids, and H2O2 levels. SMF at the higher level increase the cell leaching and decrease cell growth. The result of this study provides a new and efficient way to cell suspension culture and bioactive compounds production in M. chamomilla, and SMF can be a suitable tool for induction of antioxidative compounds in cell suspension culture.
References
Abeles FB, Biles CL (1991) Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol 95:269–273. https://doi.org/10.1104/pp.95.1.269
Aladjadjiyan A (2010) Influence of stationary magnetic field on lentil seeds. Int Agrophys 24:321–324
Aleman EI, Mbogholi A, Boix YF, Gonzalez-Ohnedo J, Chalfun A (2014) Effects of EMFs on some biological parameters in coffee plants (Coffea arabica L.) obtained by in vitro propagation. Pol J Environ Stud 23:95–101
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Banaz M (2018) In vivo, in vitro micropropagation and chemical characterization of medicinal compounds in chamomile and yarrow species (Asteraceae). University of Plymouth Research Theses. https://pearl.plymouth.ac.uk.
Barreca D, Laganà G, Leuzzi U, Smeriglio A, Trombetta D, Bellocco E (2016) Evaluation of the nutraceutical, antioxidant and cytoprotectiveproperties of ripe pistachio (Pistaciavera L., variety Bronte) Hulls. Food Chem 196:493–502
Bhojwani SS, Razdan MK (1983) Plant tissue culture: theory and practice. Elsevier, Amsterdam
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Çelik Ö, Büyükuslu N, Atak Ç, Rzakoulieva A (2009) Effects of magnetic field on activity of superoxide dismutase and catalase in Glycine max (L.) Merr. Roots Pol J Environ Stud 18:175–182
Chawla HS (2002) Introduction to plant biotechnology, 2nd edn. Science Publishers INC, New Hampshire, USA, 528 p
Çolgecen H, Atar H, Toker G, Akgul G (2018) Callus production and analysis of some secondary metabolites in Globulariatrichosantha subsp. trichosantha. Turk J Bot 42:559–567. https://doi.org/10.3906/bot-1712-13
Demir Y, Kocaliskan I (2001) Effects of NaCl and proline on polyphenol oxidase activity in bean seedlings. Biol Plant 44:607–609
Durmus NG, Tekin HC, Guven S, Sridhar K, ArslanYildiz A, Calibasi G, Ghiran I, Davis RW, Steinmetz LM, Demirci U (2015) Magnetic levitation of single cells. PNAS 112(28):E3661–E3668
Estrada-Zuniga ME, Cruz-Sosa F, Rodrıguez-Monroy M, Verde-Calvo JR, Vernon-Carter EJ (2009) Phenylpropanoid production in callus and cell suspension cultures of Buddleja cordata Kunth. Plant Cell Tissue Organ Cult 97:39–47
Forni C, Frattarelli A, Damiano C (1998) Different size, shape and growth behavior of cells in suspension cultures of strawberry (Fragaria x ananassa Duch.). Plant Biosys 133(2):205–212
Galland P, Pazur A (2005) Magnetoreception in plants. J Plant Res 118:371–389
George EF, Hall MA, De Klerk GJ (2008) Plant propagation by tissue culture, vol 1. 3rd edn. Springer, Dordrecht, The Netherlands, p 501
Giannopolitis CN, Ries SK (1977) Superoxide dismutases II. Purification and quantitative relationship with water-soluble protein in seedlings. J Plant Physiol 59:315–318
Giri A, Narasu ML (2000) Production of podophyllotoxin from Podophyllumhexandrum: a potential natural product forclinically useful anticancer drugs. Cytotech 34:17–26. https://doi.org/10.1023/A:1008138230896
Haghi G, Hatami A, Mehran M (2014) Analysis of phenolic compounds in Matricaria chamomilla and its extracts by UPLC-UV. Res Pharma Sci 9(1):31–37
Hazeena MS, Sulekha GR (2008) Callus induction and plantlet regeneration in Aegle marmelos (L.) Corr. using cotyledon explants. J Trop Agr 46:79–84
Hatamnia AA, Abbaspour N, Darvishzadeh R (2014) Antioxidant activity and phenolic profile of different parts of Bene (Pistaciaatlantica subsp. kurdica) fruits. Food Chem 145:306–311
Hellwig S, Drossard J, Twyman RM, Fischer R (2004) Plant cell cultures for the production of recombinant proteins. Nat Biotech 22:1415–1422. https://doi.org/10.1038/nbt1027
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. Plant Physiol 162:929–936
Jithesh MN, Prashanth SR, Sivaprakash KR, Ajayk P (2006) Antioxidative response mechanisms in halophytes: their role in stress defense. J Genetics 85:237–254
Khan MA, Abbasi BH, Ahmed N, Ali H (2013) Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Indust Crop Product 46:105–110
Maffei ME (2014) Magnetic field effects on plant growth, development, and evolution. Front Plant Sci 5:445
Mansourkhaki M, Hassanpour H, Hekmati M (2019) Effect of static magnetic field on growth factors, antioxidant activity and anatomical responses of Silybum marianum seedlings. J Plant Proc Func 7(28):9–15
Matamoros MA, Dalton DA, Ramos J, Clemente MR, Rubio MC, Becana M (2003) Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiol 133:499–509
Mathur S, Shekhawat GS (2013) Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: an in vitro approach for production of stevioside. Acta Physiol Plant 35:931–939. https://doi.org/10.1007/s11738-012-1136-2
Miean KH, Mohamed S (2001) Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) content of edible tropical plants. J Agric Food Chem 49(6):3106–3112. https://doi.org/10.1021/jf000892m
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Palacio L, Cantero JJ, Cusidóc RM, Goleniowski ME (2012) Phenolic compound production in relation to differentiation in cell and tissue cultures of Larreadi varicata (Cav.). Plant Sci 193–194:1–7
Patel D, Shukla S, Gupta S (2007) Apigenin and cancer chemoprevention: progress, potential and promise. Int J Onco 30:233–245
Patro BS, Bauri AK, Mishra S, Chattopadhyay S (2005) Antioxidant activity of Myristica malabarica extracts and their constituents. J Agri Food Chem 53:6912–6918
Pauling L (1979) Diamagnetic anisotropy of the peptide group. PNAS 76(5):2293–2294
Payez A, Ghanati F, Behmanesh M, Abdolmaleki P, Hajnorouzi A, Rajabbeigi E (2013) Increase of seed germination, growth and membrane integrity of wheat seedlings by exposure to static and a 10-KHz. Electromagne Biol Med 32:417–429
Pazmiño DM, Romero-Puertas MC, Sandalio LM (2012) Insights into the toxicity mechanism of and cell response to the herbicide 2,4-D in plants. Plant Signal Behav 7:1–3
Primiano T, Sutter RT, Kensler WT (1997) Redox regulation of genes that protect against carcinogens. Comp Biochem Physiol 118(4):487–497
Qui JA, Castro-Concha LA, García-Sosa K, Peña-Rodríuez LM, Miranda-Ham ML (2009) Differential effects of phytotoxic metabolites from Alternaria tageticaon Tageteserecta cell cultures. J Gen Plant Pathol 75:331–339
Rajabbeigi E, Ghanati F, Abdolmaleki P, Payez A (2013) Antioxidant capacity of parsley cells (Petroselinum crispum L.) in relation to iron-induced ferritin levels and static magnetic field. Electromagn Biol Med 32:430–441. https://doi.org/10.3109/15368378.2012.736441
Ranpariya V, Parmar S, Sheth N, Chandrashekhar V (2011) Neuroprotective activity of Matricaria recutita against fluoride-induced stress in rats. Pharma Biol 49:696–701
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotech Adv 20:101–153. https://doi.org/10.1016/S0734-9750(02)00007-1
Rao S, Usha K, Shetty A (2015) Production of secondary metabolites from callus cultures of Centella asiatica (L.) Urban Srinath. Ann Phytomed 4(1):74–78
Ray BP, Hassan L, Nasiruddin KM (2011) In vitro regeneration of BRINJAL (Solanum melongena L.). Bangladesh J Agric Res 36:397–406. https://doi.org/10.3329/bjar.v36i3.9268
Rezaei A, Ghanati F, Behmanesh M (2010) Static magnetic field improved salicylic acid effect on taxol production in suspension cultured hazel (Corylus avellana) cells. In: 6th International workshop on biological effects of electromagnetic fields pp 70–71.
Sahebjamei H, Abdolmaleki P, Ghanati F (2007) Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnet 28:42–47
Sharma V, Ramawat KG (2013) Salinity-induced modulation of growth and antioxidant activity in the callus cultures of miswak (Salvadora persica). Biotechnol 3(1):11–17
Sayadi V, Mehrabi AA, Saidi M, Nourollahi K (2014) In vitro culture and callus induction of chamomile (Matricaria chamomilla L. ) explants under different concentrations of plant growth regulators. Int J Biosci 10:206–211. https://doi.org/10.12692/ijb/4.10.206-211
Sebai H, Jabri M-A, Souli A, Rtibi K, Selmi S, Tebourbi O, El-Benna J, Sakly M (2014) Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J Ethnophar 152:327–332. https://doi.org/10.1016/j.jep.2014.01.015
Serrano R, Mulet J, Rios G, Marquez J, Larrinoa I, Leube M, Mendizabal I, Pascual-Ahuir A, Proft M, Ros R, Montesinos C (1999) A glimpse of the mechanisms of ion homeostasis during salt stress. J Exp Bot 50:1023–1036
Silva N, Barbosa L, Seito L, Fernandes Junior A (2012) Antimicrobial activity and phytochemical analysis of crude extracts and essential oils from medicinal plants. Nat Pro Res 26:1510–1514. https://doi.org/10.1080/14786419.2011.564582
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Srivastava JK, Gupta S (2007) Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem 55:9470–9478. https://doi.org/10.1021/jf071953k
Stafford A, Warren G (1991) Plant cell and tissue culture. Open University Press, Buckingham
Taghizadeh M, Nasibi F, Kalantari KM, Ghanati F (2019) Evaluation of secondary metabolites and antioxidant activity in Dracocephalum polychaetum Bornm. cell suspension culture under magnetite nanoparticles and static magnetic field elicitation. Plant Cell Tiss Organ Cult 136:489–498
Tahsili J, Sharifi M, Safaie N, Esmaeilzadeh-Bahabadi S, Behmanesh M (2014) Induction of lignans and phenolic compounds in cell culture of Linum album by culture filtrate of Fusarium graminearum. J Plant Interact 9:412–417. https://doi.org/10.1080/17429145.2013.846419
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Venkov P, Topashka-Ancheva M, Georgieva M, Alexeiva V, Karanov E (2000) Genotoxic effects of substituted phenoxyacetic acid. Arch Toxicol 74:560–566
Xue FF, Liu L, Liu ZP, Mehta SK, Zhao GM (2008) Protective role of Ca against NaCl toxicity in Jerusalem artichoke by up-regulation of antioxidant enzyme. Pedos 18:766–774
Zhang L, Yang XX, Liu JJ, Luo Y, Li ZY, Ji XM, Wang WC, Zhang X (2015) 1 T moderate intensity static magnetic field affects Akt/mTOR pathway and increases the antitumor efficacy of mTOR inhibitors in CNE-2Z cells. Sci Bull 60(24):2120–2128
Zhang L, Ji X, Yang X, Zhang X (2017) Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Onco 8(8):13126–13141. https://doi.org/10.18632/oncotarget.14480
Zemestani M, Rafraf M, Asghari-Jafarabadi M (2016) Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nut 32:66–72. https://doi.org/10.1016/j.nut.2015.07.011
Zeng FS, Wang WW, Zhan YG, Xin Y (2009) Establishment of the callus and cell suspension culture of Elaeagnusan gustifolia for the production of condensed tannins. Afric J Biotech 8:5005–5010
Złotek U, Świeca M, Jakubczyk A (2014) Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.). Food Chem 148:253–260
Acknowledgements
The financial support of this study was provided using research grant 95841966 from the Iran National Science Foundation (INFS).
Author information
Authors and Affiliations
Contributions
HH and VN designed the project and discussed the results. HH participated in the bench experiments, and organized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Konstantin V. Kiselev.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassanpour, H., Niknam, V. Establishment and assessment of cell suspension cultures of Matricaria chamomilla as a possible source of apigenin under static magnetic field. Plant Cell Tiss Organ Cult 142, 583–593 (2020). https://doi.org/10.1007/s11240-020-01885-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01885-4