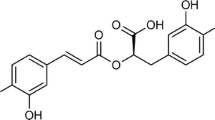
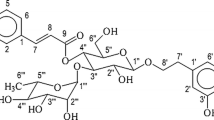
Abstract
A protocol has been standardized for establishment and characterization of cell suspension cultures of Stevia rebaudiana in shake flasks, as a strategy to obtain an in vitro stevioside producing cell line. The effect of growth regulators, inoculum density and various concentrations of macro salts have been analyzed, to optimize the biomass growth. Dynamics of stevioside production has been investigated with culture growth in liquid suspensions. The callus used for this purpose was obtained from leaves of 15-day-old in vitro propagated plantlets, on MS medium fortified with benzyl aminopurine (8.9 μM) and naphthalene acetic acid (10.7 μM). The optimal conditions for biomass growth in suspension cultures were found to be 10 g l−1 of inoculum density on fresh weight basis in full strength MS liquid basal medium of initial pH 5.8, augmented with 2,4-dichlorophenoxy acetic acid (0.27 μM), benzyl aminopurine (0.27 μM) and ascorbic acid (0.06 μM), 1.0× NH4NO3 (24.7 mM), 3.0× KNO3 (56.4 mM), 3.0× MgSO4 (4.5 mM) and 3.0× KH2PO4 (3.75 mM), in 150 ml Erlenmeyer flask with 50 ml media and incubated in dark at 110 rpm. The growth kinetics of the cell suspension culture has shown a maximum specific cell growth rate of 3.26 day−1, doubling time of 26.35 h and cell viability of 75 %, respectively. Stevioside content in cell suspension was high during exponential growth phase and decreased subsequently at the stationary phase. The results of present study are useful to scale-up process and augment the S. rebaudiana biological research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vitro technology offers the opportunity to develop new germplasm, better adapted to the changing demands especially for stress tolerance and production of medicinally important metabolites (Shekhawat et al. 2009, 2010). Stevia rebaudiana (Bertoni) is a perennial herb that belongs to family Asteraceae characterized by a very limited range of natural habitats and is an endemic plant native to the regions between 22–24°S and 53–56°W in Paraguay and Brazil. The leaves of Stevia are the source of diterpene steviol glycosides, which are estimated to be 300–400 times sweeter than sucrose at their concentration of 4 % w/v (Geuns 2003). These glycosides are nontoxic, nonmutagenic, low caloric maintain heat stability at 100 °C, features a lengthy shelf life and unlike traditional sugar substitutes, such as xylitol, sorbitol and aspartame. They are not susceptible to any acquired tolerance (Matsui et al. 1996). The material does not induce tooth decay and could be successfully used as a possible sugar substitute for the patients suffering from diabetes and other diseases related to the disturbance in carbohydrate metabolism. In addition, Stevia extracts have captured interest in food industry as a potential source of natural sweeteners for diet conscious people (JECFA 2005). Cell suspension cultures offer an in vitro system that can be used as an efficient tool for various studies in S. rebaudiana. They can be used in experiments involving mutant selection, mass propagation, protoplast isolation, gene transfer, and to study cellular traits. It is now accepted that plants and cultured cells metabolize foreign compounds in qualitatively similar ways (Hellwig et al. 2004). Stevia cell suspensions could be used for examining the idiosyncrasy of steviol glycoside metabolism and aids in understanding the way these processes may function in bioreactor and such investigations are of great importance for practice because cultured cells of Stevia might be used for large scale production of noncaloric sugar substitute. At present, diterpenoid glycoside production in Stevia callus and suspension cultures is poorly understood, and the reports of the earlier results highly contradictory. Nabeta et al. (1976) and Suzuki et al. (1976) did not provide any confirmation for the presence of steviol glycosides in callus and suspension cultures of S. rebaudiana. Simultaneously, only few attempts have been made to determine the peculiarities of Stevioside production in in vitro suspension cultures of Stevia. Striedner et al. (1991) reported the maximum concentration of 0.4 % of cell dry weight, where the media contained 100 g/l sucrose after 49 days of incubation. Bondarev et al. (2001) reported a maximal content of steviosides of 103 g g−1 DW on the 14th day of cultivation at the end of exponential phase. Moreover, the reports dealing with the establishment and maintenance of suspension cultures for growth and stevioside production are practically not available. In the present study, we have described establishment of cell suspension culture of S. rebaudiana with leaf callus as an initial inoculum and have optimized various components of the nutrient medium that are capable of exerting profound effect on growth and maintenance of Stevia cells in the shake flask. The culture growth kinetics, morphology and production dynamics of steviosides, with growth phases of cell cultures are also assessed with an objective to provide an opportunity, to gain further insights into the potential applications of S. rebaudiana cell suspension cultures in enhanced production of steviosides.
Materials and methods
Plant material and propagation of experimental plant
Intact plants of St. rebaudiana were procured from Sanjeevani medicinal plant garden, Rishikesh, Uttaranchal. An in vitro multiplication protocol for S. rebaudiana was standardized as an efficient and reproducible micro propagation system through shoot tip segments (data not shown). For in vitro plant propagation, fresh shoot tips were collected from 6-month-old mother plant of S. rebaudiana grown at Botanical garden, Banasthali University and washed under running tap water for 20 min, surface sterilized with 50 % (v/v) ethanol for 5 min and then under aseptic conditions explants were treated with 0.01 % HgCl2 solution (2–3 min). After each step, the explants were rinsed (5 min per rinse) in autoclaved distilled water. About 0.5–0.8 cm shoot tips were prepared aseptically and were implanted vertically on MS medium (Murashige and Skoog 1962) fortified with 0.02 mM thiamine hydrochloride. The pH of the medium was adjusted to 5.8 prior to sterilization and the culture conditions were maintained at 25 ± 2 °C and humidity at 55 ± 5 % under cool white fluorescent light at irradiance of 150 μmol m−2 s−1 and 16 h light/8 h dark photoperiod. Stevia plantlets were raised in vitro after 10 days of incubation and subcultures were performed at every 15-day interval.
Callus induction and culture conditions
Leaf explants obtained from in vitro grown plants were cultured on MS medium supplemented either with BA alone or in combination with NAA at a concentration of (2.2–22.2 μM) and were used for callus induction. The MS basal media consisted of MS macro and micro salts, 3 % sucrose and 0.8 % (w/v) agar (all chemicals are procured from HI-MEDIA Laboratories, Merck, Germany). The pH of the medium was adjusted to 5.8 before adding agar. The medium was autoclaved at 121 °C, ~105 kPa for 20 min. All aseptic manipulations were carried out under a laminar airflow chamber. All cultures were incubated at 25 ± 2 °C, under 40 W cool white fluorescent light (Philips, India), under 16 h photoperiod at a photosynthetic photon flux density (PPFD) of 50 μmol−2 s−1 with 55 ± 5 % humidity of culture room.
Establishment of suspension culture
S. rebaudiana cell suspension cultures were initiated using 15-day-old fresh friable callus obtained from leaf segments by transferring 5–20 g l−1 callus as initial inoculum to 150 ml Erlenmeyer flasks containing 50 ml of modified MS liquid medium, supplemented with either BA (0.09–0.89 μM) alone or in combination with 2,4-D (0.07–0.45 μM); NAA (0.32–0.80 μM) and ascorbic acid (0.03–0.06 μM). The pH of the media was adjusted to 5.8 before autoclaving. Cultures were incubated under constant dark with continuous agitation at 110 rpm in an orbital shaker and incubated at 24 ± 2 °C and 60–65 % relative humidity. The effect of various concentrations of macro salts (NH4NO3, KNO3, MgSO4 and KH2PO4) and different initial inoculum densities (5, 10 and 20 g l−1) were evaluated for optimal growth and biomass accumulation in cell suspension culture by keeping the other parameters constant (growth regulators, pH, and culture volume). Packed cell volume (PCV) for each flask was calculated after every 7 days up to 4 weeks with 5 ml cell culture, centrifuged at 12,000 rpm for 10 min to analyze the cell growth index in suspension culture (Verma et al. 1976).
Characterization of suspension culture
The cultures were maintained during 6 months in the growth chamber on the culture media standardized for optimal growth of cells. Subcultures were performed every 12–16 days using a cell inoculums size of 10 % (v/v) in 150 ml Erlenmeyer flasks, containing 50 ml of cultured medium. To establish the growth kinetics and steviol glycoside production, individual flasks were sacrificed every 7 days over 28 days period and used to determine biomass accumulation, cell viability and stevioside content. Cells were separated from the medium by filtration using Whatman No. 1 filter paper and weighed as fresh weight. The dry weight of the cells was recorded after drying them to a constant weight at 60 °C for 24 h.
Cell viability
The cells viability was determined by the Evan’s blue staining test (Rodríguez-Monroy and Galindo 1999). Two milliliter sample from each flask was incubated into 0.25 % Evan’s blue stain for 5 min and then at least 500 cells were counted, and this was repeated twice (n = 6).
Cytological examination
To observe the cells in suspension culture, one drop of (10 μl) liquid suspension was transferred directly to the slide and observed Olympus CH20i compound microscope (Olympus, India).
Extraction and HPLC analysis
Extraction of stevioside was carried out by following the method of Ahmed et al. (1980). Stevioside standard was purchased from Chromadex™ (CDXC.OB) USA. 10 μl of methanol extract of experimental samples or standard samples were injected to C18 column for high-performance liquid chromatography (HPLC) analysis and run at isocratic condition using solvent mixture of acetonitrile:water (3:2) with a flow rate of 0.5 ml min−1, wavelength set at 258 nm. Quantitative estimation of stevioside was done based on the peak area of specific concentrations of the sample and the standard.
Statistical analysis
All the data are presented as mean ± standard error mean (SEM). Fifteen replicates of each concentration were taken. All the experiments were repeated thrice. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test for inter-group comparisons, using the SPSS 16.0 (Statistical program for Social Sciences) program. The level of significance was set at P < 0.05.
Results and discussion
Callus induction
In vitro propagated plantlets were maintained in culture chamber for 15 days and then used in callus induction experiments. Leaf explants cultured on MS basal medium without plant growth regulators did not show any response (Table 1). Callus was induced from the leaf explants of S. rebaudiana on MS basal medium supplemented with BA either alone or in combination with NAA (Table 1). The explants swelled up and calli started growing from the cut surface. The calli were green, nodular compact on medium containing BA singly, while combination with NAA induces green friable calli (Fig. 1b). The highest frequency of leaf disc showing callus formation was 100 % with BA (8.9–13.3 μM) and NAA (10.7 μM), respectively (Table 1). Lower concentrations of BA inhibited callus induction. Similar observations with BA and NAA, at different concentrations to support callus induction and proliferation were reported earlier in S. rebaudiana (Bondarev et al. 1998) and in other plant species (Jana and Shekhawat 2011; Mathur et al. 2002a, b; Sharma et al. 2006; Shekhawat et al. 2002). However, optimal concentration of these compounds may depend on many factors, such as a genotype of original plant, explants origin, peculiarities of the strain etc.
Establishment of Stevia rebaudiana cell suspension culture. a A 15-day-old propagated plantlet, b callus from leaf, c Stevia rebaudiana suspension culture grown in flasks, d cell aggregates at the bottom of flask, e photomicrograph of cell suspension culture showing viable cells (×) and non viable cells (z) (×10), f photomicrograph of cell suspension culture (×10), g photomicrograph of cell suspension culture stained with safranin (×10)
Establishment of suspension culture
Callus cultured on MS basal liquid medium without growth regulators showed no growth initiation response (data not shown) indicating that the cell growth was not supported by the endogenous growth regulators and for this reason require exogenous plant growth regulators for their proliferation. Suspension initiation and growth was observed either on BA singly or in combination with either 2,4-D or NAA (Table 2). The loosening of callus clumps started and small cell clusters appeared after 7 days in liquid medium (Fig. 2a). The cultures were yellow/white in color and showed slow growth on medium containing BA and NAA, while a combination of 2,4-D and BA enhanced growth response and biomass accumulation but browning was observed after 3 weeks of culture initiation. The addition of ascorbic acid in combination with 2,4-D and BA leads to improved growth and reduce browning (Table 3). This can be attributed to the antioxidant property of ascorbic acid that causes complete blockage of phenolic compounds leaching into the medium. Similar results were reported in Anola (Verma and Kant 1999). The optimal growth of Stevia suspension cultures with a maximum growth rate (μ) 2.61 day−1 was observed on media augmented with 2,4-D (0.27 μM), BA (0.27 μM) and ascorbic acid (0.06 μM) as revealed by maximum PCV at different time intervals (Table 2). Critical supportive role of 2,4-D in suspension culture initiation and biomass accumulation were reported earlier in cell suspension cultures of other plant species (Sakamoto et al. 1993; Meyer and Van Staden 1995). Moreover, our results support the postulation of the important role played by plant growth regulators in plant tissue culture.
The optimum concentration and proportion of mineral salts are a critical determinant in controlling the growth of cells in suspension cultures (Rao and Ravishankar 2002). Table 4 depicts how growth responses of Stevia cell suspension culture have been affected by the concentrations of macro salts in the MS medium. Optimum growth response (0.57 PCV on 14th day) was observed on MS medium supplemented with 1× NH4NO3 (24.7 mM) concentration but much higher concentrations, 2× NH4NO3 (49.4 mM) and 3× NH4NO3 (74.1 mM) resulted in reduced growth response. Similar results with NH4NO3 in growth and biomass accumulation of adventitious shoots were reported in B. monnieri (Naik et al. 2011). In contrast, on the media containing 1× KNO3 (18.8 mM) very low growth response was attained (0.11 PCV on 14th day), but the cell growth increased significantly to 0.30–0.64 PCV (on 14th day) when concentration is raised to 2× KNO3 (37.6 mM) and 3× KNO3 (56.4 mM), indicating the supportive role of KNO3 in cell growth. Optimum growth responses were obtained on the medium with 3× KNO3 (56.4 mM) concentrations as revealed by 4.6-fold increase in PCV after 14 days (Table 4). Similarly at 1× MgSO4 (1.5 mM) concentration low growth (0.35 PCV on 14th day) was observed, but the higher concentrations, 2× (3.0 mM) MgSO4 and 3× (4.5 mM) MgSO4 favored cell growth in suspension culture as indicated by the marked increase in PCV by 1.6-folds at 3× MgSO4 (4.5 mM) concentration. At the same time at 1× KH2PO4 (1.25 mM) slow growth was observed, but at higher concentrations 2× KH2PO4 (2.5 mM) and 3× KH2PO4 (3.75 mM) a significant increase in cell growth was observed. Cell growth was significantly increased by 3.1-folds at 3× KH2PO4 (3.75 mM) concentrations after 14 days of culture, with respect to PCV at the time of initiation. The composition of macro- and microelements in most standard media has been developed through manipulation of one or more combinations of existing formulations and evaluating the effects on callus growth of certain model plant species. Our results reveal that 1× NH4NO3 (24.7 mM), 3× KNO3 (56.4 mM), 3× MgSO4 (4.5 mM) and 3× KH2PO4 (3.75 mM) concentrations of macro salts are required for the optimal growth responses of S. rebaudiana cell suspension cultures. Supportive role of high mineral salts concentrations was also reported in Stevia by earlier authors (Bondarev et al. 1997; Naik et al. 2011). In contrast low salts strength favored the biomass accumulation in adventitious root cultures of Withania somnifera (Praveen and Murthy 2010).
One of the factors that determine the productivity in plant tissue cultures is the optimal inoculum density (Lee and Shuler 2000). Cell suspension cultures were significantly affected by the initial inoculum densities (5, 10 and 20 g FW l−1) tried. 10 g FW l−1 concentration yielded optimal growth response (Table 5) as indicated by a significant increase in PCV (0.89 after 14 days) of cell culture. Poor results obtained at low (5 g FW l−1) and high (20 g FW l−1) inoculums density, in the present study confirms the assumption that the stimulatory influence of inoculums density affects the cell growth kinetics in plant cell cultures (Su and Lei 1993; Lee and Shuler 2000).
Growth kinetics of cell culture
Stevia cell suspension cultures have been established (Fig. 1c). The growth curve of S. rebaudiana cell suspension culture is shown in Fig. 3. The cell suspension culture was characterized by 7 days lag phase, during which biomass reached only 7.29 g DM l−1. Subsequently, the cells entered into the exponential growth phase, which continues until day 14 of culture. During this phase, the cultured cells attained maximum growth and a 4.9-fold increase in biomass accumulation (35.39 g DM l−1) was observed. The stationary phase was followed by a gradual reduction in cell density (Fig. 3). The calculated doubling time was 26.35 h and the observed growth rate was 3.26 day−1 on dry weight basis. A similar behavior was previously reported in the establishment of other suspension cultures of Cleome rosea (Simões et al. 2011). Furthermore, the cell viability remained around 75 % throughout the 18 days of culture (Table 6). When cell viability remained around 50 %, it is considered that the suspension culture establishment has failed (Qui et al. 2009). These results confirm that the S. rebaudiana cell suspension culture has been successfully established.
Morphology of S. rebaudiana cell suspension
A light yellow colored S. rebaudiana cell suspension cultures were established. A high degree of aggregation was observed in the cultures. The culture comprised mostly of uniform cell masses of small size and dense friable aggregates settled at the bottom of the flask (Fig. 1d). Morphological changes in cell culture at different growth phases are shown in Fig. 2. The growth color and texture of the culture to a certain extent depends on the duration of feeding the cells. It was visually apparent that the culture became markedly viscous and pale yellow in color after 3 weeks of feeding the cells. During the exponential phase, cultured cells grew at a faster rate and hence the viscosity of the culture increased markedly after 21 days of establishment (Fig. 2c). Viscosity of cell suspension culture might be related to secreted polysaccharide or pectinaceous substances from cells (Conrad et al. 1982). Cytological analysis using Olympus CH20i microscope demonstrated distinct morphological features between the various phases of cell culture. Initially, cells were present as small but compact aggregates (Fig. 2a, b). It has been noted that the suspension consisted of two types of cells, round and elongated shaped (Fig. 1g). Usually in durations longer than 7 days exponential phase), the number of large round shaped cells in the culture increased (Fig. 1f). These results point out that cells have changed their shape from elongated to round during the culture time, and this fact has important implication on the establishment of S. rebaudiana cell suspension culture when scaling up to bioreactor level. These results are in corroboration with Curtis and Emery (1993) and Trejo-Tapia and Rodríguez-Monroy (2007) who have reported that the morphology of different plants cell suspension culture affects the rheology of plant cell broths during bioreactor culture.
Dynamics of stevioside production in suspension culture
Dynamics of stevioside accumulation in S. rebaudiana suspension culture, during its cultivation cycle is shown in Fig. 3 (additional data are given in online resource 1). The maximal content of stevioside (about 381.03 μg g−1 DW) in the cells was observed on the 7th day of cultivation cycle, i.e. at the beginning of exponential growth phase. Stevioside content remained unchanged (about 380.3 μg g−1 DW) on the 14th day of cultivation cycle, i.e. at the end of exponential phase suggesting a constant behavior of stevioside accumulation during culture growth phase. At the same time, stevioside content in cells decreased significantly to 345 μg g−1 DW on 21st day of cultivation cycle, i.e. at the end of stationary phase, indicating a positive correlation between active cell growth and steviol glycoside synthesis in suspension culture. These results are in corroboration with the earlier results (Bondarev et al. 2002), who reported a decline in the stevioside content at the beginning of the stationary phase. Thus, our findings lend further support to the previous results. Taking into consideration, the results already reported by certain authors, in this study an attempt has been made to elucidate the dependence of stevioside synthesis cell disaggregation. To validate this assumption, a Stevia callus used as a starting material has been used for the analyses to determine its glycoside content. A significant decrease in the content of synthesized stevioside has been observed in suspension culture when compared with stevioside content of Stevia callus (415 μg g−1 DW) indicating that the production of stevioside has been influenced by disaggregation of cells. Our results are in corroboration with Rajasekaran et al. (2008) who has also reported more amounts of steviosides in Stevia callus than in suspension cultures. The contradictory results reported by several authors (Bondarev et al. 2001; Striedner et al. 1991; Swanson et al. 1992; Nabeta et al. 1976) concerning the biosynthesis and accumulation of steviosides in Stevia cell and callus cultures may be simply explained by the variation in nutrient media and culture conditions used for initiation of Stevia cell suspension, difference in genotype of explants and the unstable level of these compounds during prolonged plant maintenance.
To conclude, the cell suspension cultures of S. rebaudiana have been successfully established as an efficient tool towards stevioside biotechnological production. The growth of cell cultures has been found to be dependent on the type and salt concentration of culture medium, growth regulators and inoculums density. S.rebaudiana cell suspension cultures produce stevioside at different concentrations during its growth cycle. Our study demonstrates the possibilities of production of stevioside in large scale bioreactors using S. rebaudiana suspension cultures. Further studies are required to investigate its potential for enhanced production of steviosides through precursor feeding, elicitation and biotransformation which is of potential research and development value in the field of pharmaceutical and functional foods.
Author contribution
G.S. Shekhawat designed, conceptualized the study and Shaifali Mathur executed the experiments.
Abbreviations
- MS:
-
Murashige and Skoog
- NAA:
-
Naphthalene acetic acid
- 2,4-D:
-
2,4-Dichloro phenoxyacetic acid
- BA:
-
Benzyl adenine
- PPFD:
-
Photosynthetic photon flux density
- PCV:
-
Packed cell volume
- Asc. A:
-
Ascorbic acid
- ANOVA:
-
Analysis of variance
- HPLC:
-
High-performance liquid chromatography
- DM:
-
Dry mass
References
Ahmed MS, Dobberstein RH, Farnsworth NR (1980) Use of p-bromophenacyl bromide to enhance ultraviolet detection of water-soluble organic acids (Stevioside and Rebaudioside-B) high performance liquid chromatography analysis. J Chromatogr 192:387–393
Bondarev NI, Nosov AM, Kornienko AV (1997) Influence of several cultural factors on the growth and efficiency of Stevia callus and suspension cultures. Biotechnology 7(8):30–37
Bondarev NI, Nosov AM, Kornienko AV (1998) Effects of exogenous growth regulators on callusogenesis and growth of cultured cells of Stevia rebaudiana Bertoni. Russ J Plant Physiol 45:888–892
Bondarev NI, Reshetnyak OV, Nosov AM (2001) Peculiarities of diterpenoid steviol glycoside production in in vitro cultures of Stevia rebaudiana Bertoni. Plant Sci 161:155–163
Bondarev NI, Reshetnyak OV, Nosov AM (2002) Effects of nutrient medium composition on development of Stevia rebaudiana shoots cultivated in the roller bioreactor and the production of steviol glycosides. Plant Sci 165:845–850
Conrad PA, Binari LLW, Racusen RH (1982) Rapidly-secreting cultured oat cells serve as a model system for the study of cellular exocytosis, characterization of cells and isolated secretary vesicles. Protoplasma 112:196–204
Curtis W, Emery A (1993) Plant cell suspension culture rheology. Biotechnol Bioeng 42:520–526
Geuns JMC (2003) Molecules of interest: stevioside. Phytochemistry 64:913–921
Hellwig S, Drossard J, Twyman RM, Fischer R (2004) Plant cell cultures for the production of recombinant proteins. Nat Biotechnol 22:1415–1422
Jana S, Shekhawat GS (2011) Plant growth regulators, adenine sulfate and carbohydrates regulate organogenesis and in vitro flowering of Anethum graveolens. Acta Physiol Plant 33:305–311
JECFA (Joint FAO/WHO Expert Committee on Food Additives) (2005) Evaluation of certain food additives. Sixty-third Report of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, Switz. WHO Technical Report Series No. 928, 34–39 and 138
Lee CWT, Shuler ML (2000) The effect of inoculums density and conditioned medium on the production of ajmalicine and catharanthine from immobilized Catharanthus roseus cells. Biotechnol Bioeng 67:61–71
Mathur S, Shekhawat GS, Batra A (2002a) Micropropagation of Salvadora persica via cotyledonary nodes. J Biotech 1:197–200
Mathur S, Shekhawat GS, Batra A (2002b) An efficient in vitro method for mass propagation of Salvadora persica via apical meristem. J Biochem Biotechnol 11:125–127
Matsui M, Matsui K, Kawasaki Y, Oda Y, Noguchi T, Kitagawa Y, Sawada M, Hayashi M, Nohmi M, Yoshihira K, Ishidate M, Sofuni T (1996) Evaluation of the genotoxicity of stevioside and steviol using six in vitro and one in vivo mutagenicity assays. Mutagenesis 11:573–579
Meyer HJ, van Staden J (1995) The in vitro production of anthocyanin from callus culture of Oxalis linearis. Plant Cell Tiss Org Cult 40:55–58
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–495
Nabeta K, Kasai T, Sugisawa H (1976) Phytosterol from the callus of Stevia rebaudiana Bertoni. Agric Biol Chem 40:2103–2104
Naik PM, Manohar SH, Murthy HN (2011) Effects of macro elements and nitrogen source on biomass accumulation and bacoside A production from adventitious shoot cultures of Bacopa monnieri (L.). Acta Physiol Plant 33:1553–1557
Praveen N, Murthy NH (2010) Production of withanolide-A from adventitious root cultures of Withania somnifera. Acta Physiol Plant 32:1017–1022
Qui JA, Castro-Concha LA, García-Sosa K, Peña-Rodríguez LM, Miranda-Ham ML (2009) Differential effects of phytotoxic metabolites from Alternaria tagetica on Tagetes erecta cell cultures. J Gen Plant Pathol 75:331–339
Rajasekaran T, Ramakrishna A, Sankar KU, Giridhar P, Ravishankar GA (2008) Analysis of predominant steviosides in Stevia rebaudiana Bertoni by liquid chromatography/electrospray ionization-mass spectrometry. Food Biotechnol 22:179–188
Rao RS, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotech Adv 20:101–153
Rodríguez-Monroy M, Galindo E (1999) Broth rheology, growth and metabolite production of Beta vulgaris suspension culture: a comparative study between cultures grown in shake flasks and in stirred tank. Enz Microb Technol 24:687–693
Sakamoto K, Iida K, Sawamura K, Hajiro K, Asada Y, Yoshikawa T, Furuya T (1993) Effects of nutrients on anthocyanin production in cultured cells of Aralia cordata. Phytochemistry 33:357–360
Sharma A, Kansal N, Shekhawat GS (2006) In Vitro culture and plantlet regeneration of economically potent plant species Jatropha Curcas. Biochem Cell Arch 6:165–170
Shekhawat GS, Batra A, Mathur S (2002) A reliable in vitro protocol for rapid mass propagation of Azadirachta indica Juss. J Plant Biol 29:109–112
Shekhawat GS, Mathur S, Batra A (2009) Role of phytohormones and various nitrogen inorganic and organic nutrients in induction of somatic embryogenesis in cell culture derived from leaflets of Azadirachta indica A. Juss. Biol Planta 53:707–710
Shekhawat GS, Verma K, Jana S, Singh K, Teotia P, Prasad A (2010) In vitro biochemical evaluation of cadmium tolerance mechanism in callus and seedlings of Brassica juncea. Protoplasma 239:31–38
Simões C, Cordeiro L, Castro TC, Callado H, Albarello N, Mansur E (2011) Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-9945-3
Striedner J, Gutjahr E, Czygan FE, Braunegg G (1991) Contributions to the biotechnological production of sweeteners from Stevia rebaudiana Bertoni II. Induction of stevioside accumulation in cell cultures by variation in the nutrient medium and analysis of small amount of stevioside. Acta Biotechnol 11:501–504
Su WW, Lei F (1993) Rosmarinic acid production in perfused Anchusa officinalis culture: effect of inoculum size. Biotechnol Lett 15:1035–1038
Suzuki H, Ikeda T, Matsumoto T, Noguchi M (1976) Isolation and identification of rutin from cultured cells of Stevia rebaudiana Bertoni. Agric Biol Chem 40:819–820
Swanson SM, Mahady GB, Beecher CWW (1992) Stevioside biosynthesis by callus, root, shoot and rooted shoot cultures in vitro. Plant Cell Tissue Organ Cult 28:151–157
Trejo-Tapia G, Rodríguez-Monroy M (2007) Cellular aggregation in secondary metabolite production in in vitro plant cell cultures. Interciencia 32:669–674
Verma B, Kant U (1999) Propagation of Emblica officinalis Gaertn through tissue culture. Adv Plant Sci 12:21–25
Verma DC, Tavares J, Loewus FA (1976) Effect of benzyladenine, 2,4-dichlorophenoxy acetic acid and d-glucose on myo-inositol metabolism in Acer pseudoplatanus L. cells grown in suspension culture. Plant Physiol 57:241–244
Acknowledgments
G. S. Shekhawat acknowledges financial support from the University Grants Commission (UGC), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K.-Y. Paek.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mathur, S., Shekhawat, G.S. Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: an in vitro approach for production of stevioside. Acta Physiol Plant 35, 931–939 (2013). https://doi.org/10.1007/s11738-012-1136-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1136-2