Abstract
To ensure replenishment, a refine protocol for micropropagation of Decalepis salicifolia (Bedd. ex Hook.f.) Venter a critically endangered and endemic medicinal plant was developed using mature nodal explants. A high frequency shoot regeneration system was obtained on Murashige and Skoog (MS) medium comprised of 6- benzyladenine (BA) (5.0 µM) + α-naphthalene acetic acid (NAA) (0.5 µM) + adenine sulphate (ADS) (30.0 µM) corresponds to a highest mean number of 9.97 ± 0.01 shoots/explants with maximum shoot length of 6.46 ± 0.1 cm. Successful rooting in microshoots was achieved on half strength MS medium supplemented with indole-3-butyric acid (IBA) (2.5 µM). A maximum of 6.10 ± 0.07 roots/microshoot with average root length of 2.30 ± 0.06 cm was obtained. As much as 90% plantlets survived when Soilrite™ was used as planting substrate and finally established in soil without any casualty and morphological variation. Acclimatized plantlets were screened for pigment content, net photosynthetic rate (PN), stomatal conductance (Gs) and transpiration rate (E) during subsequent days of acclimatization as well as the changes in antioxidant was also evaluated. A steady rise was observed in the activity of superoxide dismutase (SOD) for initial 21 days and then after a decrease was found showing improved acclimatization efficiency of the plant. Similarly, the activities of catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) enzyme shows reliable increase as the days of acclimatization advanced which play their precautionary role against oxidative damage to the plant. The genetic fidelity of the in vitro raised plantlets with that of mother plant was further confirmed using random amplified polymorphic DNA (RAPD) and inters simple sequence repeats (ISSR) analysis. Additionally, the effect of acclimatization on the biosynthesis of 2-hydroxy-4-methoxybenzaldehyde (2H4MB) in the root system was also evaluated in relation to their biomass production. Maximum fresh weight (4.9 g/plant), dry weight (0.65 g/plant) of roots and 2H4MB content (6.8 µg ml−1 of root extract) was obtained after 10 weeks of acclimatization. Accelerated multiplication rate with the stability of genetic virtue, physiological and biochemical parameter assure the efficacy of the protocol developed for the propagation of this critically endangered medicinal plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Decalepis salicifolia (Bedd. ex Hook.f.) Venter (family—Apocynaceae) is a critically endangered and endemic medicinal plant with very restricted distribution in Western Ghats of India. The persent-day position of the plant comes under critically endangered categories as listed by IUCN (International Union for conservation of nature) due to over exploitation and loss in habitat. This species is endemic to Palakkad and Idukki district of Kerala and it is also found in Coimbatore and Dindigul districts in Tamil Nadu, India. The moniliform tuberous root is the most important part of the plant containing 2H4MB which gives vanilla like aroma to the root (George et al. 2011). The plant is used to treat skin diseases, tuberculosis, asthma and intestinal disorders (Radhakrishnan et al. 1998) and shown antiulcer activities (Rao et al. 2004). The compound 2H4MB have larvicidal activities (Akenga et al. 2005), taste modifying activities (Mukonyi and Ndiege 2001), tyrosinase inhibitor activity (Kubo and Kinst-Hori 1999), antimicrobial and antioxidant (Wang et al. 2010). This compound has also been reported in Decalepis hamiltonii (Nagarajan et al. 2001a), D. arayalpathra (Verma et al. 2014) and Hemidesmus indicus (Nagarajan et al. 2001b). Western Ghats being as UNESCO world heritage site and is one of the eight hottest hot-spot of biological diversity in the world (Myers et al. 2000). However, a large number of plant species are being eroded due to various reasons and many of them are poorly understood because of the lack of researches conducted on them. Indiscriminate collection of roots questioned the wild stand of this lactiferous shrub in the past years in the Western Ghats, India. The unprocessed way of regeneration and propagation is obstruct due to poor fruit set and low seed germination. Such kind of orphan plant needs special attention as it represents a restricted geographical distribution.
Decalepis salicifolia is heightening towards vulnerable categories as their pharmacological wealth is known. Although its over-utilization and medicinal inference, there is no description for the reintroduction of this critically endangered plant. Plant tissue cultures techniques offer possible plan for ex vitro conservation, mass scale production and bioactive compound production to meet the industrial demands. George et al. (2011) screened the root of D. salicifolia and establish the presence of 2H4MB as a major constituents. However, improved mass propagation and conservation strategies have been proposed for D. hamiltonii (Sharma et al. 2014) and D. arayalpathra (Ahmad et al. 2017) using biotechnological approaches. The proposed work has been under taken to evolve an efficient and reproducible micropropagation system for D. salicifolia through mature nodal explants. In vitro raised clones were validated for genetic homogeneity by using molecular markers (RAPD, ISSR). Characterization of antioxidant enzymes along with physiochemical parameters during the acclimatization period was also studied. The desired compound (2H4MB) is also identified and quantified in relation to the biomass of root system of micropropagated plantlets.
Materials and method
Plant material, media and culture conditions
The healthy and mature shoots from field grown plant of D. salicifolia were taken from a 3-year-old growing plant in the Department net house, A.M.U. Shoots were assiduously washed for 30 min using tap water followed by treatment with 1% (w/v) Bavistin (Carbendazim Powder, BASF India Ltd.) and 5% (v/v) labolene for 20 min respectively. The explants were washed with sterilized double distilled water (SDDW) followed by surface disinfect under horizontal laminar flow hood using 0.1% (w/v) mercuric chloride (HgCl2) solution for 2.5 min. It was further succeeded by 3–4 times washing with SDDW before the inoculation. Nodal segment with two axiallary buds were taken as explants for inoculation.
MS (Murashige and Skoog 1962) medium augmented with distinct plant growth regulators (PGRs) and 3% sucrose (Qualigens, India) was used throughout the experiments. Agar (Qualigens) at 0.8% was used as solidifying agent, pH was set as 5.8. About 15 ml of culture medium was dispersed in each culture tube (25 × 150 mm, Borosil, India) and Erlyenmeyer flasks (100 ml) and were sealed with non-absorbent cotton plugs. The sterilization of medium was achieved by autoclaving at 121 °C and 1.06 × 105 Nm− 2 (Pa) for 15 min. After inoculation the cultures were incubated at 16 h photoperiod under 50 µmol m− 2 s− 1 of photosynthetic photon flux density (PPFD) furnished by fluorescent tubes (40 W; Philips, India) and 55 ± 5% relative humidity at 24 ± 2 °C during the experimentation.
Shoot induction and multiplication
To standardize the response of different PGRs on axiallary shoot regeneration, the excised nodal segments were inoculated aseptically on full-strength MS medium augmented with cytokinins like 6-benzyladenine (BA), 2-isopentanyladenine (2iP) and kinetin (Kn) at distinct molarity (1.0, 2.5, 5.0 and 7.5 µM) with or without auxins like α-naphthalene acetic acid (NAA), indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) at various concentrations (0.1, 0.5 and 1.0 µM). To improve the shoot regeneration, various growth additives (GAds) viz. glutamine (Glu), phlouroglucinol (PG) and adenine sulphate (ADS) at various molarity (10, 20, 30 and 40 µM) were supplied with optimal cytokinin and auxin combination.
Rooting, acclimation and field relocation
For the induction of root in the microshoots various auxins like indole-3-butyric acid (IBA), α-naphthalene acetic acid (NAA) and indole-3-acetic acid (IAA) and at various concentrations (1.0, 2.5 and 5.0 µM) were supplied with 1/2 MS medium to optimise the best rooting efficiency. MS medium lacking any auxins was served as control. The plantlets with fully developed root and shoot system were isolated from culture flask, washed gently to remove adhered agar particle followed by transferring to thermacol cups (expanded polystyrene) containing autoclaved Soilrite™ (75% Irish peat moss and 25% horticulture grade expanded perlite) (Keltech Energies Ltd., India) and watering was done periodically. To optimize the maximum moisture, the micropropagated plantlets were covered with transparent plastic bag for 14 d and maintained in the culture room at similar physiological conditions as described for multiplication in material and method section. The polythene bag was gradually opened to maximize the plant adaptation towards new environments. After 28 days of plantation, the hardened plants settled to clay pots containing garden soil and green manure (2:1) and kept in the culture room initially for 2 weeks and then they were transferred to green house followed by transfer in natural environment.
Physiochemical analysis
Estimation of chlorophyll and carotenoid content
Methodologies used by Sahai and Shahzad (2013) were used to evaluate the chlorophyll and carotenoid content. Ten micropropagated plantlets were preferred randomly, fully expanded leaves were tabbed at day 0 (control) and after 7, 14, 21 and 28 days of acclimatization and followed by extraction with 80% (v/v) acetone and filtered with whatman No. 1 filter paper. The absorbance for chl a, chl b and carotenoids were monitored at 663, 645 and 466 nm respectively by using Ultraviolet–Visible (UV–Vis) spectrophotometer (UV-1700 Pharma Spec, Shimadzu, Kyoto, Japan).
Net photosynthetic rate and related attributes
PN, Gs and E were investigated by using Infrared gas analyzer (IRGA), a handy photosynthetic system (Li-COR 6400, LI-COR, and Lincoln, NE, USA). The estimation was carried out followed by the methodologies of Ahmad et al. (2017).
Electrolyte leakage (EL) and lipid peroxidation
The permeability of the membrane was evaluated by following the methodologies of Lutts et al. (1995). After gently washing the leaves, leaf disc (2–3 mm2) were made by a punch player. The leaf disc were dipped in 12 ml DDW and incubated for 24 h in a closed culture vial and electrical conductivity of the solution (EC1) was calculated. After that, autoclaved at 15 psi and 121 °C for 18 min followed by cooling at 25 °C and the electrical conductivity (EC2) was calculated. EL was calculated as: EL (%) = EC1/EC2 × 100. Thiobarbituric acid reactive substances (TBARS) content was evaluated following the protocol by Faisal and Anis (2009).
Antioxidant enzyme analysis
2.0 ml of extraction buffer consisting of 1% of polyvinylpyrrolidone (PVP) and Triton X-100, and 0.11 g ethylenediaminetetra acetic acid (EDTA) is used to homogenize the 0.5 g fresh and healthy leaf in mortar and pestle followed by filtration with whatman No. 1 filter paper. The filtrate was centrifuged at 15,000 rpm for 20 min using high-speed centrifuge. The process of extraction will perform in dark and assaying of enzyme was carried out with supernatant.
SOD (EC 1.15.1.1) action was evaluated by following the protocol of Dhindsa et al. (1981). The reaction mixture consisting of 13 mM methionine, 0.5 M phosphate buffer at pH 7.2, 1.2 mM riboflavin, 63.3 mM nitroblue tetrazolium (NBT) 0.1 mM EDTA and 0.1 ml enzyme extract and incubated for 15 min at 25 °C. Tube showing no illumination and equal concentration of reaction mixture served as blank and the absorbance was read at 560 nm. 50% inhibition of NBT reduction corresponds to one unit of SOD activity.
CAT (EC 1.11.1.6) action was evaluated by using the methodologies of Aebi (1984). 50 mM phosphate buffer (pH 7.0) and 100 µl enzymes extract was used to prepare the volume (3 ml) of assay mixture and H2O2 disappearance in the supernatant was regulated by measuring the fall in absorbance at 240 nm. The reaction began with addition of 10 mM H2O2. The coefficient of absorbance 0.036 mM− 1 cm1 was used to calculate CAT activity.
APX (EC 1.11.1.11) action was evaluated by following the methodologies of Nakano and Asada (1981). The fall in absorbance due to disruption of ascorbate with enzyme was monitored at 290 nm The reaction mixture was consisting of 50 mM phosphate buffer (pH 7.5), 0.1 mM H2O2, 0.1 mM EDTA, 0.5 mM ascorbate, and 0.1 ml enzyme extract and activity of enzyme is represented as EU mg− 1 protein min− 1.
GR (EC 1.6.4.2) action evaluated by following the methodologies of Foyer and Halliwell (1976). Enzyme extract (0.1 ml) added to assay mixture contained 50 mM phosphate buffer (pH 7.5), 1.0 mM EDTA, and 0.2 mM NADPH (Nicotinamide adenine dinucleotide phosphate), and 0.5 mM glutathione disulfide (GSSG) to began the reaction. The action of the enzyme was measured by using the coefficient of absorbance of 6.2 mM− 1 cm− 1 indicated as EU mg− 1 protein min− 1.
Genomic DNA extraction
The genetic fidelity was performed by using RAPD and ISSR technique. Nine micropropagated plantlets of D. salicifolia were selected for DNA extraction using CTAB (cetylemethylammonium bromide) method following the protocol of Doyle and Doyle (1990) and extracted DNA was screened for clarity (A260/280 ratio) on UV–vis spectrophotometer. A set of ISSR (UBC, Vancouver, BC, Canada) and RAPD (kit OPL) primers were used for PCR analysis and reaction was executed on a thermocycler (Biometra, T Gradient Thermoblock, Germany). The reaction mixture of 20 μl for PCR amplication was prepared with 10X buffer (2 μl), 10 mM dNTPs (0.4 μl), 25 mM MgCl2 (1.2 μl), 3 Unit Taq polymerase (0.2 μl), 2 μM primers and 40 ng template DNA. PCR amplification cycle was set as 45 cycles consisting of a 94 °C denaturation step for 5 min without any repeat, a 55 °C annealing for 1 min and and elongation step of 72 °C for 1 min. A final extension was achived at 72 °C for 10 min. The amplified products were separated on electrophoresis in 0.8% (w/v) agrose gels with 4 μl ethidium bromide in TAE buffer (pH 8.0) run at 50 V for 2 h and visualized on a UV trans-illuminator (BIO RAD, Hercules, CA, USA).
HPLC analysis of 2H4MB content in the root system
Plant material, extract and standard preparation
The plant material, extract and standard were prepared according to the methodologies followed by Ahmad et al. (2017). The roots were collected from micropropagated plantlets in Soilrite™ after 0, 2, 4, 6, 8 and 10 weeks of acclimatization for qualitative and quantitative analysis of the compound. Fresh weight of roots was taken, and then dried at room temperature, dry weight calculated and squashed in mortar and pestle with liquid nitrogen. n-hexane (150 ml) was added to the dried root powder (1 g) and incubated on shaker at 120 rpm. Solvent was evaporated at 50 °C on water bath and 100 ml of methanol was used to maintain the volume following the filtration with a syringe filter (0.22 μm, Genetix, Bangalore, India). Standard compound 2H4MB (98% purity) (purchased from Sigma Aldrich, New Delhi, India) stock solution of 1000 µg/ml was processed by dissolving 10 mg 2H4MB in 10 ml of methanol. Different concentration gradient was prepared with methanol and all working solution was stored at 4 °C.
Chromatographic conditions
Waters system (Milford, MA, USA) equipped with C18 analytical column (250 × 4.6 mm, 5.0 µm) (Waters) was used for HPLC. 10 µl of sample was injected and quantification was carried out at 280. Data acquisition was achieved by using Empower2 software and reporting on Windows 2000™ platform. Methanol with water (70:30) in combination was preferred for mobile phase, pH adjusted at 5.0 and flow rate was adjusted to 1 ml min−1. The chromatograms were adjusted at 280 nm. UV scan was measured from 210 to 400 nm. The corresponding retention time (RT) of both standard and sample was affirmation of the presence of the compound.
Statistical analysis
For statistical analysis, all the experiment were conducted with 20 replicates and repeated three times. One-way ANOVA using SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used for data analysis of different parameter. The significance of differences among means was attributed using Tukey’s test at 5% level of significance and data represented as mean ± standard error (SE).
Results and discussion
In vitro regeneration system
Direct regeneration is the most relevant approach for clonal propagation in several medicinal plants (Sadeghi et al. 2015). The influence of different cytokinin concentration has been screened for direct shoot regeneration (Fig. 1a; Table 1). Only single shoot emerges on control treatment indicating the application of PGRs. MS medium fortified with different cytokinin like BA, Kn and 2ip brings changes in single nodal explants and bud development was observed within 6–8 days of inoculation. BA was proved to be the best among different cytokinin used in terms of shoot proliferation and shoot length. MS medium with BA (5.0 μM) induced 2.06 ± 0.09 shoots per explant with 2.84 ± 0.09 cm shoot length in 88.6 ± 1.07% of cultures after 6 weeks. BA beyond the optimum concentration did not bring any change in regeneration efficiency while the application of other two cytokinin i.e. Kn and 2 iP was unable to evoke any good response instead exhibited retarded growth in regenerants thereby stunted shoots were produced. Kn and 2 iP at 5.0 μM induced 1.62 ± 0.1 and 1.42 ± 0.05 shoots per explant with 2.60 ± 0.07 cm and 1.93 ± 0.05 cm shoot length in 76.6 ± 1.07 and 62.6 ± 1.00% cultures, respectively in 6 weeks of incubation. The stimulating effect of BA has also been reported in other medicinal plant species such as Pistasia lentiscuss (Kılınc et al. 2015) and Crataegus arotina (Nas et al. 2012). BA being as the first generation synthetic cytokinin elicit an efficient proliferation, bud break, multiple shoot formation and it is might be due to its more permeability across plasma membrane and high cell uptake (Malik et al. 2005).
Establishment of D. salicifolia culture. Nodal segments at MS medium—3 weeks old culture (a), shoot regeneration on MS medium + BA (5.0 µM) + NAA (0.5 µM)—6 weeks old culture (b), shoot multiplication on MS medium + BA (5.0 µM) + NAA (0.5 µM) + ADS (30.0 µM)—6 weeks old culture (c), In vitro rooting on 1/2 MS medium + IBA (2.5 µM)—4 weeks old culture (d), exposed view of above culture (e), an acclimatized plantlet in SoilriteTM-4 weeks old (f)
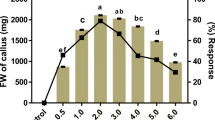
The combination treatment of cytokinin and auxin was also evaluated to boost the regeneration efficiency of the explants. Application of optimum concentration of BA (5.0 μM) with various auxins (NAA, IAA and IBA) at definite concentration produced significant improvement in regeneration and growth in shoots. Combination of BA with NAA (0.5 μM) induced 4.04 ± 0.05 shoots per nodal segments with 4.99 ± 0.05 cm shoot length in 90.2 ± 1.28% of cultures in 6 weeks (Fig. 1b; Table 2). Jaykumar et al. (2015) also found improved results in combination treatment of BA with NAA in Salacia chinessis. Other two auxin IAA and IBA also showed some improvement but lesser effective as compare to BA. However, on the above media tested, poor leaf expansion, abscission of leaf and early leaf fall were observed in the shoots regenerated on cytokinin alone or in combination with auxin. Therefore, to improve the healthy shoot system, another set of experiment was carried out with the treatment of growth additive like ADS, Glu and PG to the MS medium. Significant improvement was obtained when different additives were tried. MS medium augmented with BA (5.0 μM) + NAA (0.5 μM) + ADS (30 μM) was found to be optimal to induce 9.97 ± 0.01 shoots per nodal segment with 6.46 ± 0.1 cm shoot length, after 6 weeks of incubation (Fig. 1c; Table 3). ADS being a rich source of nitrogen might have improved the multiplication rate along with the healthy leaf development and also prevention of early leaf fall. Murashige (1974) has established that the addition of adenine in the form of ADS act as a catalyst on the organization of enhanced shoot bud proliferation. The supremacy of ADS was also observed in other medicinal plant such as Ficus religiosa (Siwach and Gill 2011) and Decalepis arayalpathra (Ahmad et al. 2017).
In vitro rooting and acclimatization
Excised shoot were transfer on full-strength MS medium (control) devoid of auxin declined to promote root initiation in microshoot. 1/2 MS medium fortified with NAA, IAA and IBA was also screened to improve the rooting. Among all the auxins screened, half MS + IBA (2.5 μM) proved to be most effective in comparison to other auxin and corresponds maximum of 6.10 ± 0.07 roots per shoot with 2.30 ± 0.06 cm root length in 91.0 ± 1.0% cultures after 4 weeks of transfer (Fig. 1d, e; Table 4). The root generated on NAA and IAA was fragile, fibrous and devoid of any branching. The demands of half-strength MS medium augmented with IBA have also been found more suitable for induction of root in microshoot of Rhinacanthus nasutus (Cheruvathur and Thomas 2014), Salacia chinesis (Jaykumar et al. 2015) and Pittosporum eriocarpum (Thakur et al. 2016). In contrast to the present finding, optimum rooting was observed in D. arayalpathra (Ahmad et al. 2017) when MS medium was fortified with NAA. Healthy plantlets were well hardened as represented in “Materials and Method” section. The hardened plant in Soilrite™ when transferred to the natural conditions showed healthy growth with 89.3% survival rate (Fig. 1f). In the field, in vitro raised plantlets showed plushy growth and exhibit similar morphological character as that of mother plant, which indicated the stability of plant free from visible abnormalities.
Physiochemical study
Pigments, photosynthesis and related attributes
Micropropagated plantlets when transferred to the ex vitro condition, face high light irradiance and situation demands increased in pigment content to overcome the effect. The reduction in chlorophyll content was found during the initial days of acclimatization for which Pospisilova et al. (1999) has found the reason i.e. disordered granna might be responsible for initial fall. During the first weak, reduction in chlorophyll a (0.64–0.52 mg g− 1), chlorophyll b (0.19–0.1 mg g− 1) and carotenoid content (0.24 − 0.14 mg g− 1) was found (Fig. 2a, b, c). After that steady rise was found in chl a (1.14 mg g− 1), chl b (0.28 mg g− 1), and carotenoid content (0.82 mg g− 1) during 8 weeks of acclimatization which corresponds to increase in photosynthetic efficiency. The increased in photosynthetic pigment content was also reported in D. hamiltonii (Sharma et al. 2014), Plantago algarbiensis Samp. and P. almogravensis Franco (Goncalves et al. 2017).
Pigment content and related characteristics in in vitro raised plantlets of D. salicifolia. Chlorophyll a (a), Chlorophyll b (b), carotenoid (c), net photosynthetic rate (PN) (d), stomatal conductance (Gs) (e), transpiration rate (f). bars represent mean ± SE. Bars represented by same letter within response variable are not significantly different (P = 0.05) according to Tukey’s test at 5% probability
The very first challenge that micropropagated plantlets faces when relocated to the ex vitro condition is related to water loss due to poor stomatal functioning and lack of thick cuticular deposition (Pospisilova et al. 1999). After the initial fall in PN, a gradual rise was found in PN which might be due to the formation of new leaf and photosynthetic pigment. PN becomes 2.31 μmol CO2m− 2s− 1 after 8 weak of acclimatization (Fig. 2d). An incremental practice in photosynthetic rate is not surprising as it has been also reported in several medicinal plants during ex vitro transplantation (Pospisilova et al. 1988; El-Mahrouk et al. 2016).
The plant performance in new environment is greatly influenced by the stomatal activity as its control on gaseous exchange. However, the process of acclimatization is genetically controlled. During in vitro phase the stomata remain open due to high humidity and the initial days of acclimatization (Xiao et al. 2011). Transpiration rate and stomatal conductance are directly related to the water balance in the plant body. The rate of transpiration (Tr) decreased from 0.7 to 0.4 mmol m− 2s− 1 at initial days of acclimatization followed by a gradual increase was found, as the days of acclimatization increase (Fig. 2e). Similarly, stomatal conductance decreased from 0.037 to 0.016 mol m− 2 s− 1 during the initial days and then a gradual increase was noticed (Fig. 2f). The rise and fall in stomatal activities reflects strong regulation under ex vitro environment. The results were in accordance with the findings of Galmés et al. (2007) and Perez-Jimnez et al. (2015).
Electrolyte leakage (EL), lipid peroxidation and antioxidant enzyme activities
The light being as preliminary energy source for the growth of the plant however, excess of light intensities can be harmful for the plant and leads to photoinhibition of photosynthetic light reaction and the incomplete reduction of oxygen responsible for the successive production of ROS (reactive oxygen species) i.e., hydrogen peroxide, superoxide radical and singlet oxygen (Batková et al. 2008). The polyunsaturated fatty acid present in the plasma membrane is more prone to damage by ROS and leads damage to the plasma membrane and production of MDA as consequence of lipid peroxidation. The leakage in electrolytes directly corresponds to the membrane permeability (Gill and Tuteja 2010). During the transfer of in vitro to ex vitro acclimatization, the plantlets feel light stress and a maximum increase was noticed in both EL (51.8%) and TBARS [2.06 µmol g−1 (f.w.)] at 7 days of acclimatization (Fig. 3a, b). After that a gradual decrease was found due to improved acclimatization. Our finding was according to the findings of Faisal and Anis (2009) and Perez-Jimnez et al. (2015) conducted in Rauvolfia tetraphylla and Cynara scolymus respectively. The harmful effects of ROS are generally overcome by the antioxidant enzymes such as SOD, CAT, GR and APX. In this study a steady rise was found for SOD as the days of acclimatization increases, it reaches maximum value of 2.36 Umg-1 (protein) after 21 days of acclimatization and decreases to 1.98 Umg-1 (protein) after 28 days of acclimatization (Fig. 3c). Increase in H2O2 content coresponds the catalytic activity of SOD as it converts superoxide to H2O2 and oxygen (O2) to prevent membrane oxidation and damage to the biological molecules. Moreover, increase in CAT activities (Fig. 3d) and SOD both represents photorespiratory detoxification of H2O2 into O2 and H2O in mitochondrial electron system (Scandalios 1990). CAT scavenges H2O2 by converting it into O2 and H2O and hence reduced level of H2O2 suggest successful acclimatization. The similar kind of behavior of these enzymes was also found in Plantago algarbiensis Samp. and P. almogravensis Franco (Gonçalves et al. 2017), D. arayalpathra (Ahmad et al. 2017).
Both APX and GR carried out chloroplast based detoxification of ROS via Mehler pathway (Foyer and Mullineaux 1998) and the activity of both the enzyme reached highest value of 4.5 mmol mg− 1 (protein) min− 1 and 3.3 mmol mg− 1 (protein) min− 1 respectively after 28 days of acclimatization (Fig. 3e, f). Both performed essential function by scavenging H2O2 in chloroplast, cytosol, vacuoles and apoplastic space (Asada 1999). If the action of SOD, CAT, APX and GR are in coordination, the rate of oxidative damage to the plant with ROS could be controlled and plant can perform better towards acclimatization process in a new environment (Yan 2009). The elevation of APX and GR is not astonished as it is previously reported in D. arayalpathra (Ahmad et al. 2017).
2H4MB quantification during acclimatization
Now a great interest are being developed for separation, identification and quantification of 2H4MB from D. salicifolia root system due to its pharmacological value and it could be possible through HPLC. The chromatogram obtained for the extract was compared with that of standard 2H4MB peak showed similar retention time (RT) for both. Figure 4a, b clearly depict the similarity in RT of 2H4MB (3.686 min) and RT of extract (3.689 min) at same chromatographic condition. Figure 5 represent the relationship between fresh weigh, dry weight and 2H4MB content in root. Maximum fresh weigh and dry weight of root were found to be 4.6 and 0.65 g per plant and the highest accumulation of 2H4MB was recorded 6.8 µg ml−1 after 10 weeks of acclimatization. The results support the hypothesis that accumulation of 2H4MB start only after 2 weeks of acclimatization and increased gradually. Our results are according to the reports of Sharma et al. (2014) and Ahmad et al. (2017) they found similar kind of growth accumulation pattern in D. hamiltoni and D. arayalpathra respectively.
Genetic homogeneity assessment
Nine in vitro raised plants randomly selected including mother plant for the screening of genetic homogeneity by RAPD and ISSR markers. Clear and distinct banding with no reproducible alien bands were observed and manually scored from the gel profiles and included for final analysis (Fig. 6a, b; Tables 5, 6). Both RAPD and ISSR marker are unsophisticated and profitable in screening of genetic uniformness. Out of 10 RAPD primers screened from Kit L, 9 primers produced comprehendible and reproducible bands. Total of 42 scorable bands and highest number of bands were obtained with the primer 3 i.e., 8 and the lowest number of bands i.e., one. In case of ISSR primers, out of 10 primers used, nine primers produced clear band and a total of 50 bands were produced. Maximum number of bands were obtained in the primer UBC-825 i.e., 9 and the lowest number of bands i.e., 3. No polymorphism was detected during the analysis of tissue culture raised plant, confirming the genetic uniformity of the plant. The proposed dendrogram from the analysis also proved over 99% similarity with mother and nine randomly selected in vitro raised plantlets showing high level of monomorphism (Fig. 7). The present findings also suggest that micropropagation of D. salicifolia using nodal explants maintains genetic uniformity even after prolonged period under in vitro conditions. Our results are in accordance to the earlier report on genetic uniformity of in vitro derived plantlets of Curcuma longa (Nayak et al. 2011), Helicteres isora (Mariappan et al. 2016) and Salacia chinenssis (Jaykumar et al. 2015).
Conclusions
Proposed findings account a holistic and reliable protocol for micropropagation of D. salicifolia. This is the first productive efforts to set up a micropropagation protocol for the plant using nodal explants, which imparts reintroduction, large-scale propagation, commercialization and ex situ conservation. DNA based markers proved to evaluate genetic fidelity and true-to-type nature of micropropagated plant. Considering the importance of the plant adjustment during acclimatization, different physiochemical parameters and biochemical characterization were also studied for the first time in this species. The results obtained with high rate of plantlets survivability could be utilized in making micropropagation and conservation of this endangered species more advantageous and profitable. The identification and quantification of 2H4MB in relation to biomass accumulation during acclimatization was also encouraging and reported for the first time. The developed protocol can be used for the conservation of D. salicifolia by providing valuable resource material and might play a significant role in the delisting of species from Red Data List.
References
Aebi H (1984) Catalase in vitro methods. Enzymol 105:112–121
Ahmad Z, Shahzad A, Sharma S (2017) Enhanced multiplication and improved ex vitro acclimatization of Decalepis arayalpathra. Biol Plant. https://doi.org/10.1007/s10535-017-0746-3
Akenga TO, Lwande W, Ndiege IO (2005) 2-Hydroxy- 4-methoxybenzaldehyde: larvicidal structure-activity studies. Bull Chem Soc Ethiop 19:61–68
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Batková P, Pospíšilová J, Synková H (2008) Production of reactive oxygen species and development of antioxidative systems during in vitro growth and ex vitro transfer. Biol Plant 52:413–422
Cheruvathur MK, Thomas TD (2014) High frequency multiple shoot induction from nodal segments and rhinacanthin production in the medicinal shrub Rhinacanthus nasutus (L.) Kurz. Plant Growth Regul 74:47–54
Dhindsa RS, Plumb-Dhindsa P, Thorp TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–110
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
El-Mahrouk ME, Dewir YH, Murthy HN, Rihan HZ, Al-Shmgani HS, Fuller MP (2016) Effect of photosynthetic photon flux density on growth, photosynthetic competence and antioxidant enzymes activity during ex vitro acclimatization of Dieffenbachia cultivars. Plant Growth Regul 79:29–37
Faisal M, Anis M (2009) Changes in photosynthetic activity, pigment composition, electrolyte leakage, lipid peroxidation, and antioxidant enzymes during ex vitro establishment of micropropagated Rauvolfia tetraphylla plantlets. Plant Cell Tiss Organ Cult 99:125–132
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Mullineaux PM (1998) The presence of dehydroascorbate and dehydroascorbate reductase in plant tissues. FEBS Lett 425:528–529
Galmés J, Flexas J, Savé R, Medrano H (2007) Water relations and stomatal characteristics of Mediterranean plants with deferent growth forms and leaf habits: responses to water stress and recovery. Plant Soil 290:139–155
George S, Sulaiman CT, Balachandran I, Augustine A (2011) Decalepis salicifolia (Bedd. Ex Hook.f.) Venter (Apocynaceae)—a new source for 2-hydroxy-4-methoxybenzaldehyde. Med Plant 3:259–260
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Goncalves S, Martins N, Romona A (2017) Physiological traits and oxidative stress markers during acclimatization of micropropagated plants from two endangered Plantago species: P. algarbiensis Samp. and P. almogravensis Franco. In Vitro Cell Dev Biol Plant. https://doi.org/10.1007/s11627-017-9812-y
Jaykumar JC, Dhanaji MG, Ashok SB, Suraj DU (2015) Micropropagation, molecular profiling and RP-HPLC determination of mangiferin across various regeneration stages of Saptarangi (Salacia chinensis L.). Ind Crops Prod 76:1123–1132
Kılınc FM¸ Suiizerer V, Cifeic YZ, Onay A, Yıldırım H, Uncuog˘lu AA, Tilkat E, Koc I¸ Akdemir OF, Metin OK (2015) Clonal micropropagation of Pistacia lentiscus L. and assessment of genetic stability using IRAP markers. Plant Growth Regul 75:75–88
Kubo I, Kinst-Hori I (1999) 2-Hydroxy-4- methoxybenzaldehyde: a potent tyrosinase inhibitor from African medicinal plants. Planta Med 65:19–22
Lutts S, Kinet JM, Bouharmont J (1995) Changes in plant response to NaCl during development of rice (Oryza sativa L.) Varieties differing in salinity resistance. J Exp Bot 46:1843–1852
Malik SK, Chaudhury R, Kalia RK (2005) Rapid in vitro multiplication and conservation of Garcinia indica: a tropical medicinal tree species. Sci Hort 106:539–553
Mariappan M. Thiruppathi SK, Mandali VR (2016) Organogenesis and evaluation of genetic homogeneity through SCoT and ISSR markers in Heliteres idora L., a medicinally important tree. S Afr J Bot 106:204–210
Mukonyi KW, Ndiege IO (2001) 2-Hydroxy-4- methoxybenzaldehyde: aromatic taste modifying compound from Mondia whytei Skeels. Bull Chem Soc Ethiop 15:137–141
Murashige T (1974) Plant propagation through tissue culture. Annu Rev Plant Physiol 25:135–166
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Myers N, Mittermeier RA, Mittermeier CG, Gustavo ABF, Kent J (2000) Biodiversity hotspots for conservation on priorities. Nature 403:853–858
Nagarajan S, Rao LJM, Gurudutt KN (2001a) Chemical composition of the volatiles of Decalepis hamiltonii (Wight & Arn). Flavour Fragr J 16:27–29
Nagarajan S, Rao LJM, Gurudutt KN (2001b) Chemical composition of the volatiles of Hemidesmus indicus R. Br. Flavour Fragr J 16:212–214
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nas NN, Gokbunar L, Sevgin N, Aydemir M, Dagli M, Susluoglu Z (2012) Micropropagation of mature Crataegus aronia L., a medicinal and ornamental plant with rootstock potential for some fruit. Plant Growth Regul 67:57–63
Nayak S, Kaur T, Mohanty S, Ghosh G, Choudhury R, Acharya L, Subudhi E (2011) In vitro and ex vitro evaluation of long-term micropropagated turmeric as analyzed through cytophotometry, phytoconstituents, biochemical and molecular markers. Plant Growth Regul 64:91–98
Perez-Jimnez M, Lopez-Perez AJ, Otalora-Alcon G, Marin-Nicolas D, Pifiero MC, Amor FM (2015) A regime of high CO2 concentration improves the acclimatization process and increase plant quality and survival. Plant Cell Tiss Organ Cult 121:547–557
Pospisilova J, Solarova J, Catsky J, Ondrej M, Opatrny Z (1988) The photosynthetic characteristics during micropropagation of tobacco and potato plants. Photosynthetica 22:205–213
Pospisilova J, Ticha I, Kadlecek P, Haisel D, Plazakova S (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497
Radhakrishnan K, Pandurangan AG, Pushpangadan P (1998) Utleria salicifolia—a new ethnobotanical record from Kerala, India. Fitoterapia 69:403–405
Rao CHV, Ojha SK, Radhakrishnan K, Govindarajan R, Rastogi S, Mehrotra S, Pushpangadan P (2004) Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol 91:243–249
Sadeghi F, Yadollahi A, Kermani MJ, Eftekhari M (2015) Optimizing culture media for in vitro proliferation and rooting of Tetra (Prunus empyrean 3) rootstock. J Genet Eng Biotechnol. https://doi.org/10.1016/j.jgeb.2014.12.006
Sahai A, Shahzad A (2013) High frequency in vitro regeneration system for conservation of Coleus forsklohlii: a threatened medicinal herb. Acta Physiol Plant 35:473–481
Scandalios JG (1990) Response of plant antioxidant defense genes to environmental stress. Adv Genet 28:1–41
Sharma S, Shahzad A, Ahmad A, Anjum L (2014) In vitro propagation and the acclimatization effect on the synthesis of 2-hydroxy-4-methoxy benzaldehyde in Decalepis hamiltonii Wight and Arn. Acta Physiol Plant 36:2331–2344
Siwach P, Gill RA (2011) Enhanced shoot multiplication in Ficus religiosa L. in the presence of adenine sulphate, glutamine and phloroglucinol. Physiol Mol Biol Plant 17:271–280
Thakur J, Dwivedi MD, Sourabh P, Uniyal PL, Pandey AK (2016) Genetic homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle—an endemic and end-ngered medicinal plant. PLoS ONE 11(7):e0159050
Verma RS, Mishra P, Kumar A, Chauhan A, Padalia RC, Sundaresan V (2014) Chemical composition of root aroma of Decalepis arayalpathra (J. Joseph and V. Chandras.) Venter, an endemic and endangered ethano-medicinal plant from Western Ghats, India. Nat Prod Res 28:1202–1205
Wang J, Liu H, Zhao J, Gao H, Zhou L, Liu Z, Chen Y, Sui P (2010) Antimicrobial and antioxidant activities of the root bark essential oil of Periploca sepium and its main component 2-hydroxyl-4- methoxybenzaldehyde. Molecules 24:5807–5817
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult 105:149–158
Yan L (2009) Physiological responses of tomato seedlings (Lycopersicon esculentum) to Salt Stress. Mod Appl Sci 3:171–176
Acknowledgements
Financial support by the Department of Science and Technology (DST), India, is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration among four authors. Author Dr. AS conceived and designed the experiments. Author Dr. SS and Dr SP helped for the quantitative estimation of the chemical compound and writing of the manuscript. Author ZA performed all the experimental work, analyzed the data and wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors proclaimed no conflict of interest.
Additional information
Communicated by Ali R. Alan.
Rights and permissions
About this article
Cite this article
Ahmad, Z., Shahzad, A., Sharma, S. et al. Ex vitro rescue, physiochemical evaluation, secondary metabolite production and assessment of genetic stability using DNA based molecular markers in regenerated plants of Decalepis salicifolia (Bedd. ex Hook.f.) Venter. Plant Cell Tiss Organ Cult 132, 497–510 (2018). https://doi.org/10.1007/s11240-017-1345-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1345-x