Abstract
The effects of culture media and cytokinin types on micropropagation of mature Crataegus aronia L. were investigated. Using single-axillary bud explants, the growth of cultures on MS, WPM, DKW and NRM containing 4.44 μM benzyladenine (BA) plus 0.05 μM indole-3-butyric acid (IBA), and on NRM containing thidiazuron, meta-Topolin (mT) or BA at 1.25, 2.5, 5.0 or 7.5 μM plus 0.05 μM IBA were compared. The culture medium had significant effects on shoot number and length. In comparison with MS, DKW and WPM, shoot production was greater on NRM (5.7 shoots per explant). Shoot production on MS, DKW and WPM (4.2, 4.2 and 4.1, respectively) were statistically similar to each other. Thidiazuron was detrimental to shoot formation and caused formation of rosette shoots and/or large callus to form on explants. In the presence of mT, only some of the explants developed into shoots. Benzyladenine was the only cytokinin that promoted both shoot proliferation and shoot elongation. Higher shoot numbers were obtained at 5.0 and 7.5 μM BA compared to lower concentrations of BA. Over 80% of microshoots rooted and rooted shoots were successfully acclimatized to ex vitro conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Crataegus, commonly known as hawthorn or thornapple contains some important multi-function medicinal and horticultural shrubs and small trees (Phipps et al. 2003). Hawthorn is a long known etnopharmaceutical plant that has been consumed for 2,500 years (Dai et al. 2007). The flowers, leaves and fruit of hawthorn are rich in antioxidant and polyphenolics (Zhang et al. 2002; El-Saleh 2005), and they have become popular herbal medicine in the treatment of congestive heart failure (Long et al. 2006). In addition, hawthorn fruit are consumed fresh or processed into sauces, jam and juice. In Turkey’s local markets the demand for fresh hawthorn fruit is increasing. Large-sized fruit are more preferred and they are three to five times more expensive than apples and pears.
Hawthorns are attractive landscape and hedgerow plants (Cummins and French 1994; c.i. Croxton and Sparks 2002), provide habitats and winter food for wildlife and they are an important nectar source for bees (Croxton and Sparks 2002).
Hawthorn plants are highly drought tolerant and are recommended for water conserving horticultural practices. In Turkey, wild-grown hawthorn plants are often topworked with pears and less frequently with apples. In sandy and calcareous soils and in arid zones, hawthorn can be used as dwarfing rootstocks for pears (Al-Junaidee 1977; Ozbek 1978). The bud take ratios of ‘Williams’ pear and ‘Golden Delicious’ apple grafted on Crataegus azaroulus L. seedlings were 85 and 75%, respectively (Qrunfleh 1993).
Considering all current and the potential uses of hawthorn, it is necessary to develop effective propagation methods. Hawthorn is usually seed propagated but because of parthenocarpic fruit development and/or development of stones without fertilization the true seed (stones containing embryos) ratio of Crataegus aronia L. may be as low as 10–60% (unpublished data). Also, depending on fruit maturity, seed lot, seed storage conditions and stratification treatment seed germination ratio of Crataegus monogyna varies (10–95%) and it may take 1, 2 or 3 years for seeds to germinate (Bujarska-Borkowska 2002). Although clonal propagation of superior genotypes is preferred, there were no reports of hawthorn propagation by cuttings or by grafting. Tissue culture of Crataegus species, especially micropropagation from mature plants, is also limited. Shoot tip culture (Marks and Simpson 1999) and re-growth of encapsulated buds (Piccioni and Standardi 1995) of Crataegus oxyacantha, and cell suspension culture (Rakotoarison et al. 1997) of Crataegus monogyna were reported. However, none of the above cited studies reported rooting of microshoots. Iapichino and Airo (2009) reported 3.5 mean shoot production and 52% rooting of Crataegus monogyna and Dai et al. (2007) reported 33% mean regeneration ratio from cotyledon and leaf explants, and 50% rooting of regenerated shoots of Crataegus pinnatifida.
To our knowledge, there is only one study (Caboni et al. 2010) that reports micropropagation of Crataegus azarolus L. in the presence of benzyladenine (BA) and CPPU. There is still a need for a complete and readily available protocol for micropropagation of mature Crataegus aronia L. (syn. Crataegus azarolus var. aronia L.). In the present study, using various culture media and cytokinins, micropropagation of Crataegus aronia L. from mature plants was investigated. The results and a complete micropropagation protocol reported herein may be useful for clonal propagation of superior mature hawthorn genotypes selected for fruit, ornamental, and pharmaceutical values or rootstock potentials.
Materials and methods
Plant materials
Dormant cuttings of three wild grown Crataegus aronia L. genotypes (Genotype 1, 2 and 3) taken from over 15-year old non-pruned plants were used as the explant source. These genotypes were chosen because they produce large fruit. Following leaf fall, twigs were cut from stock plants, wrapped with moist papers, placed in plastic bags and stored at 4°C for 5 months. New shoot growth was forced by immersing the basal ends of 25–35 cm long cuttings in forcing solution (Read and Yang 1987) containing 200 mg L−1 8-hydroxyquinoline sulfate + 20 g L−1 sucrose + 10 mg L−1 gibberellic acid (GA3, Phytotechnology Laboratories G500). The forcing solution was replaced twice a week and newly developed shoots were harvested within 2–3 weeks when shoots had 6 or more leaves. New shoots were defoliated and surface disinfested with 70% (v/v) ethanol for 20 s, rinsed twice with sterile distilled water, soaked in 20% (v/v) commercial bleach solution (1% sodium hypochlorite) containing 10 drops of Tween-20 L−1 for 15 min and rinsed three times with sterile distilled water.
Unless mentioned otherwise, in all experiments the media were supplemented with 30 g L−1 sucrose, the pH was adjusted to 5.5, then 6 g L−1 agar was added and the media were autoclaved at 121°C and 1.2 kgf cm−2 for 15 min.
In vitro cultures were initiated using shoot tips and nodal explants containing one axillary bud. Nas and Read (2004a) medium (NRM) containing 0.05 μM indole-3-butyric acid (IBA, Sigma I5386), 4.44 μM benzyladenine (BA, Sigma B3408) and gelled with 6 g L−1 agar (Sigma A7921) was used as the culture initiation medium. The combination of plant growth regulators for initiating the cultures was based on preliminary studies. Individual shoot-tip and nodal explants cultured in 15 × 250 mm glass test tubes containing 15 mL medium were subjected to 16 h light [under cool-white fluorescent light (Tekfen TLD Day Light) at 80 μmol m−2 s−1] and 8 h dark photoperiod in a growth chamber (23–25°C) for 3 weeks. Every 4–5 weeks, microbial contamination-free explants were subcultured on fresh medium in magenta containers. To increase rooting of micropropagated shoots, explants were subcultured over 2 years (see “Results”). To find the best culture medium, cytokinin type and concentration for micropropagation, using single-axillary bud explants (from cultures regularly subcultured over 2 years), two independent experiments were conducted.
The effect of culture media
Using 0.5–1.0 cm long single-axillary bud explants, the growth of cultures on MS (Murashige and Skoog 1962) medium, WPM (Lloyd and McCown 1980), DKW (Driver and Kuniyuki 1984) medium and NRM (Nas and Read 2004a) were compared. All media were supplemented with 4.44 μM BA, 0.05 μM IBA and gelled with 6 g L−1 Merck microbiological agar. After autoclaving, filter-sterilized vitamins (of each medium) were added to the media and 75 mL medium was distributed into magenta containers.
The effect of cytokinins
Explants were produced on NRM containing 0.05 μM IBA, 4.44 μM BA and gelled with 6 g L−1 Merck microbiological agar. After autoclaving, 1.25, 2.5, 5.0 or 7.5 μM of filter-sterilized thidiazuron (TDZ) (Riedel-de Haen 45686), mT (Phytotechnology Laboratories T41) or BA and vitamins were added to the medium, and then 75 mL medium was distributed into magenta containers.
Rooting and acclimatization
Microshoots obtained on NRM were transferred to a root induction medium (½ NRM) containing 2.5, 5.0, 7.4 or 10 μM IBA for 3 weeks. The rooting and acclimatization procedures were according to Nas et al. (2010).
Experimental design, collection of data and statistical analysis
The experiments were set up as completely randomized designs. Four replications (magenta containers each containing 4 axillary buds) of each genotype were randomly applied to each culture medium or cytokinin level and containers were randomly placed on the growth shelves. Cultures were subjected to a 16/8 h (light/dark) photoperiod under cool-white fluorescent light at 80 μmol m−2 s−1 and at 23–25°C. At the end of a 35-day culture period, shoot length and shoot numbers were recorded, and the appearance of cultures was visually evaluated. Experiments were conducted twice by using explants randomly taken from shoots grown on each medium and cytokinin level tested, and culturing explants on the same fresh media for another 35-day subculture period.
Data for explants in a culture vessel were divided by the number of explants, and the mean shoot length and shoot numbers were used for statistical analysis. The analysis of variance was completed using the SAS PROC GLM (SAS Institute Inc., Cary, NC, 2000). Separation of means was conducted by Fisher’s least significant difference (LSD) test at p ≤ 0.05.
Results
Microbial contamination was low and explants were obtained from the three wild-grown hawthorn genotypes. However, browning of explants was a serious problem. Because of phenolic oxidation of explants, cultures of Genotype 3 could not be established. Thus, the study was carried out using cultures of Genotype 1 and 2. When contamination-free explants were cultured on NRM medium containing 0.05 μM IBA and 4.44 BA multiple shoots were obtained (Fig. 1a). However, the rooting of micropropagated shoots that were subcultured up to 10 times was usually below 10% (data not shown). This was probably because explants were still physiologically mature and were not rejuvenated enough. When these microshoots were left intact and transferred to a fresh medium, floral buds developed and following a 2-month chilling period microshoots flowered in vitro (Fig. 1b). Therefore, to enhance explant rejuvenation and increase rooting (Nas and Read 2004b) explants were repeatedly subcultured every 4–5 weeks over 2 years. Then, culture medium and cytokinin were investigated.
a Multiple microshoots obtained on NRM containing 0.05 μM IBA and 4.44 μM BA, b in vitro flowering microshoots as an indication of physiological maturity retained in vitro, c shoot development and callus formation from hawthorn nodal explants cultured on various concentrations of TDZ, d in vitro rooted microshoots e successfully acclimatized to ex vitro conditions
The effect of culture medium on shoot number and on shoot length
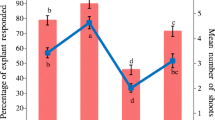
Culture medium was the main factor that significantly (p = 0.027) affected shoot production. When Genotype 1 and Genotype 2 were combined, the highest shoot production (5.7 shoots) was obtained when NRM was used. The mean numbers of shoots obtained on MS (4.2 shoot), DKW (4.2 shoots) and WPM (4.1 shoots) were statistically similar. The effects of genotype and genotype × medium interaction on the number of shoots were not statistically significant. Across all media, the mean number of shoot production was 4.6 for Genotype 1 and 4.4 for Genotype 2 (Fig. 2).
With respect to shoot length, the effect of genotype was significant (p = 0.032). When the culture media were DKW and NRM, Genotype 2 produced longer shoots compared to Genotype 1. However, when MS and WPM were used the mean shoot lengths of both genotypes were similar indicating no significant effects of medium and medium × genotype interaction on shoot length (Fig. 2).
The effect of cytokinin on shoot number and shoot length
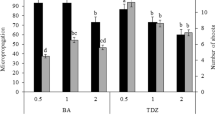
Thidiazuron had inhibitory effects on both shoot multiplication and on shoot elongation. When TDZ was added to the medium large calli were produced at the base of explants, and parallel with its increasing concentration the detrimental effects of TDZ increased. At the lowest level, 1.25 μM TDZ, short shoots with a large amount of callus were obtained. At higher TDZ levels explants produced only rosette shoots and/or large amounts of callus (Fig. 1c) and the cultures could not be sustained. Thus, when the experiment was repeated TDZ was not used and it was excluded from statistical analysis.
The cytokinin type and interaction of cytokinin type × concentration (p = 0.001) had great influences on the mean number of shoots produced per cultured explant. Benzyladenine was much more effective on shoot proliferation than mT. Higher mean shoot numbers (8.0–8.2 shoots per cultured explant) were obtained using 5.0 and 7.5 μM BA compared to lower concentrations of BA. Across all BA levels, the mean numbers of shoots were 5.3 for Genotype 1 and 5.7 for Genotype 2. However, in the presence of mT (at any level used) some of the explants did not develop into shoots, and as a result less then one shoot was obtained when all cultured explants were considered (Fig. 3). The cytokinin type had also a great influence (p = 0.001) on shoot length, but all the other interactions (genotype, cytokinin and cytokinin concentration) effects were not significant. BA produced longer shoots compared to mT. At all levels of BA tested shoots were longer than 1.2 cm, mean shoot lengths were 1.6 cm for Genotype 1 and 2.0 cm for Genotype 2. At higher levels (5.0 and 7.5 μM) of BA shoot length slightly decreased but this reduction was not statistically significant. On the other hand, when mT was used mean shoot lengths never exceeded 0.7 cm (Fig. 3).
When ≥1.5 cm long microshoots were transferred to the root induction medium containing 2.5 or 5.0 μM IBA, over 80% of shoots developed roots within 3 weeks. Shoots cultured at higher IBA levels (7.4 or 10 μM) did not root but produced callus at their bases (data not shown). Rooted shoots (Fig. 1d) were successfully acclimatized to ex vitro conditions (Fig. 1e) using the procedures reported by Nas et al. (2010).
Discussion
In horticulture and forestry, micropropagation of mature plants that have been tested in the field and proven to be superior genotypes is preferred. But, when explants from mature plants are used the majority of woody plant species are recalcitrant to in vitro culture (McCown 2000; Nas and Read 2004b). To ease the establishment of cultures and improve explant response, various treatments that enhance tissue rejuvenation level, such as severe pruning or spraying stock plants with plant growth regulators and the use of forcing solution are frequently employed (Preece and Read 2003). The forcing solution we used was an effective method to obtain clean explants from cuttings of wild grown mature hawthorn plants.
Once the cultures were established, with an average multiplication rate of ≥5 that was obtained in the current study, theoretically 9.8 million (510) shoots could be propagated from a single explant in 1 year. In micropropagation rooting of microshoots is also important. Hawthorn is a difficult to root species and rooting of microshoots propagated from mature explant source might also be unsatisfactory. This could partially be explained by explants’ physiological maturity that is retained in vitro for several months or even years (Nas et al. 2003). It is well known that subculturing enhances explant rejuvenation level and this in turn increases rooting capacity of micropropagated shoots (Nas and Read 2004b). In our study, subculturing explants over 2 years, then culturing microshoots on half strength NRM containing 2.5 or 5.0 μM IBA provided a high rooting ratio.
The culture medium affects all types of morphogenic responses (Nas and Read 2004a; Preece 1995) and for successful micropropagation “the best” culture medium need to be determined. For micropropagation of hawthorn NRM and DKW were found superior to MS and WPM. Using NRM or DKW, a high number of microshoots long enough (≥1.5 cm long) to be rooted could be propagated in 1 year.
Plant growth regulators also have great influences on shoot proliferation and shoot elongation. In micropropagation of woody species, BA is the cytokinin used most, while TDZ has been used less and mT has rarely been used (Amoo et al. 2011; Aracama et al. 2010; Nas et al. 2010). TDZ had detrimental effects, caused formation of short shoots and/or large amounts of callus. The detrimental effects of TDZ observed on shoot formation could be related to that even the lowest concentration (1.25 μM) that was used might have been too high. BA is blamed to have negative carryover effects that hamper rooting and ex vitro acclimatization of micropropagated plants. In micropropagation of Spathiphyylum floribundum the main derivative of BA was [9G]BA which accumulated at the plant base. The slow release of BA from [9G]BA inhibited rooting and caused heterogeneity in growth of plants during acclimatization in the greenhouse (Werbrouck et al. 1995). Taking into account the reported carryover effects of BA, some post vitro problems due to the long subculturing period of 2 years in the presence of BA could be expected. Apparently, 4.44 μM BA used during continues subculturing of Crataegus aronia L. explants for 2 years was not too high to cause any post vitro problem. To alleviate BA-induced micropropagation problems substitution of BA with mT has been suggested (Werbrouck et al. 1996; Aracama et al. 2010; Amoo et al. 2011). To our knowledge, there is no study that reports the use of mT for micropropagation of hawthorn to compare the results reported herein. Nonetheless, our findings indicated that mT was ineffective for shoot proliferation and shoot elongation. This could be related to that even the highest concentration (7.5 μM) of mT that was used might have been not high enough. Unlike the main derivative of BA which was highly stable and located at the plant base, the derivatives of mT were located at all plant parts and their breakdown was relatively fast (Werbrouck et al. 1996), and this in turn may have caused a low (ineffective) concentration of mT in a specific plant part. BA was the only cytokinin that provided a reasonable shoot number and shoot length. The results indicated that 5.0 μM would be the best BA concentration to obtain the highest number of shoots long enough to be rooted. Although the effects of species and genotypes could not be excluded, in hawthorn micropropagation BA could be used as the choice cytokinin more effective than mT and TDZ.
Conclusion
This micropropagation protocol provides a reasonable shoot production, rooting rate and describes the best culture medium, cytokinin type and concentration of those here tested. The results presented herein could be used for clonal micropropagation of superior hawthorn genotypes selected for their fruit, ornamental and pharmaceutical values and rootstock potentials. For micropropagation of mature Crataegus aronia L., the use of NRM and/or DKW as the culture medium and BA as the cytokinin more effective than mT and TDZ could be suggest.
Abbreviations
- BA:
-
Benzyladenine
- DKW:
-
Driver and Kuniyuki Walnut Medium
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog Medium
- mT:
-
Meta-Topolin
- NRM:
-
Nas and Read Medium
- TDZ:
-
Thidiazuron
- WPM:
-
Woody Plant Medium
References
Al-Junaidee MJ (1977) Flora in Jordan and ecological distribution. Ministry of Agriculture, Amman
Amoo SO, Finnie JF, Staden JV (2011) The role of meta-topolins in alleviating micropropagation problems. Plant Growth Regul 63:197–206
Aracama CV, Kane ME, Wilson SB, Philman NL (2010) Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult- and easy-to-acclimatize sea oats (Uniola paniculata L.) genotypes. Plant Growth Regul 60:43–49
Bujarska-Borkowska B (2002) Breaking of seed dormancy, germination and seedling emergence of the common hawthorn (Crataegus monogyna Jacq.). Denbdrobiology 47:61–70
Caboni E, Meneghini M, Tonelli M (2010) Improved micropropagation of Azarole (Crataegus azarolus L.). Prop Orn Plants 1:9–13
Croxton PJ, Sparks TH (2002) A farm-scale evaluation of the influence of hedgerow cutting frequency on hawthorn (Crataegus monogyna) berry yields. Agric Ecos Environ 93:437–439
Cummins RP, French DD (1994) Floristic diversity, management and associated land use in British hedgerows. In: Watt TA, Buckley GP (eds) Hedgerow management and nature conservation. Wye College Press, Wye, pp 95–106
Dai H, Zhang Z, Guo X (2007) Adventitious bud regeneration from leaf and cotyledon explants of Chinese hawthorn (Crataegus pinnatifida Bge. var. major N.E.Br.). In Vitro Cell Dev Biol Plant 43:2–8
Driver JA, Kuniyuki AH (1984) In vitro propagation of paradox walnut rootstock. HortScience 19:507–509
El-Saleh SC (2005) Characterization of antioxidant activities of wild hawthorn fruits growing in Lebanon. J Food Tech 3:456–459
Iapichino G, Airo M (2009) Multiplication of Crataegus monogyna by in vitro culture of nodal segments. Acta Hortic 812:135–140
Lloyd G, McCown B (1980) Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc Int Plant Prop Soc 30:421–427
Long SR, Carey RA, Crofoot KM, Proteau PJ, Filtz TM (2006) Effect of hawthorn (Crataegus oxycantha) crude extract and chromatographic fractions on multiple activities in a cultured cardiomyocyte assay. Phytomedicine 13:643–650
Marks TR, Simpson SE (1999) Effect of irradiance on shoot development in vitro. Plant Growth Regul 28:133–142
McCown BH (2000) Recalcitrance of woody and herbaceous perennial plants: dealing with genetic predetermination. In Vitro Cell Dev Biol Plant 36:149–154
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Nas MN, Read PE (2004a) A hypothesis for the development of a defined tissue culture medium of higher plants and micropropagation of hazelnuts. Sci Hortic 101:189–200
Nas MN, Read PE (2004b) Improved rooting and acclimatization of micropropagated hazelnut shoots. HortScience 37:1688–1690
Nas MN, Read PE, Miller V, Rutter PA (2003) In vitro rejuvenation of woody species is temporary. Acta Hortic 625:211–215
Nas MN, Bolek Y, Sevgin N (2010) The effects of explant and cytokinin type on regeneration of Prunus microcarpa. Sci Hortic 126:88–94
Ozbek S (1978) Özel Meyvecilik. Çukurova Ü. Ziraat Fak. Yayınları, No:128 (in Turkish)
Phipps JB, O’Kennon RJ, Lance RW (2003) Hawthorns and medlars. Royal Horticultural Society, Cambridge
Piccioni E, Standardi A (1995) Encapsulation of micropropagated buds of six woody species. Plant Cell Tiss Org Cult 42:221–226
Preece JE (1995) Can nutrient salts partially substitute for plant growth regulators? Plant Tissue Cult Biotech 1:26–37
Preece JE, Read PE (2003) Novel methods in micropropagation. Acta Hortic 616:71–76
Qrunfleh MM (1993) Studies on the hawthorn (Crataegus azarolus L.) III. A potential rootstock for ‘Golden Delicious’ apple and ‘Williams’ pear. J Hort Sci 68:983–987
Rakotoarison DA, Gressier B, Trotin F, Brunet C, Dine T, Luyckx M, Vasseur J, Cazin M, Cazin JC, Pinkas M (1997) Antioxidant activities of polyphenolic extracts from flowers, in vitro callus and cell suspension cultures of Crataegus monogyna. Pharmazie 52:60–64
Read PE, Yang Q (1987) Novel growth regulator delivery systems for in vitro culture of horticultural crops. Acta Hortic 212:55–59
Werbrouck SPO, van der Jeugt B, Dewitte W, Prinsen E, Van Onckelen HA, Debergh PC (1995) The metabolism of benzyladenine in Spathiphyllum floribundum Schott ‘Petite’ in relation to acclimatisation problems. Plant Cell Rep 14:662–665
Werbrouck SPO, Strnad M, Van Onckelen HA, Debergh PC (1996) Meta-topolin, an alternative for benzyladenine in tissue culture? Physiol Plant 98:291–297
Zhang Z, Ho WKK, Huang Y, James AE, Lam LW, Chen ZY (2002) Hawthorn fruit is hypolipidemic in rabbits fed a high cholesterol diet. J Nutr 32:5–10
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nas, M.N., Gokbunar, L., Sevgin, N. et al. Micropropagation of mature Crataegus aronia L., a medicinal and ornamental plant with rootstock potential for pome fruit. Plant Growth Regul 67, 57–63 (2012). https://doi.org/10.1007/s10725-012-9662-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9662-x