Abstract
Copper oxide (CuO) nanoparticles (NPs) synthesized through co-precipitation method were employed in MS media during in vitro culture of Stevia rebaudiana. Physiological characteristics, production of steviol glycosides, and antioxidative parameters were investigated in regenerated plants. CuO NPs had crystalline monoclinic cubic cuprous oxides with average size 47 nm. The NPs were applied at 0, 0.1, 1.0, 10, 100 and 1000 mg/L in MS media for direct organogenesis of S. rebaudiana from nodal segments. Shoot organogenesis was found highest (88.5%) at 10 mg/L CuO and average shoot length, mean number of shoot per explant, and fresh weight were also found significantly higher at the same concentration. High performance liquid chromatography (HPLC) illustrated significant rise of bioactive major steviol glycosides (rebaudioside A and stevioside) at 10 mg/L CuO NPs in MS media. The oxidative stress produced by CuO nanoparticles on S. rebaudiana was affirmed by antioxidant activities i.e. total antioxidant activity (TAC), total reducing power (TRP) and 2,2-diphenyl-1-picryl hydrazyl (DPPH)-free radical scavenging activity. The oxidative stress generated by NPs involved production of antioxidative molecules total phenolic content (TPC), total flavonoid content (TFC) depending on NPs concentration. The study concludes that copper oxide nanoparticles functions as a stimulator of bioactive components productions, and can be employed in in vitro batch cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticles having 1–100 nm size possess large surface area as compared to their bulk counterparts. The physical and chemical nature of nanoparticles make them highly reactivity therefore are employed in industrial scale and also have biological properties (Yadav 2013; Kołodziejczak-Radzimska and Jesionowski 2014). Nanoparticles have positive as well as negative influence on biological systems (Boczkowski and Hoet 2010). In agriculture perspective, nanoparticles have been elucidated including biocide in plants, nano-fertilizer and nano-pesticide formulations, soil condition sensors, and targeted gene delivery in transformation (Aslani et al. 2014; Perreault et al. 2014).
Stevia rebaudiana belongs to family Asteraceae and is a perennial herb native to South America. The secondary metabolites such as steviol glycosides (rebaudioside A, stevioside, rebaudioside C, etc.) are well known as anti-diabetic, anti-bacterial and anti-cancerous (Dey et al. 2013) which produce in leaves at higher level. Different kinds of abiotic and biotic stresses have been employed to S. rebaudiana during in-vitro propagation to enhance the steviol glycosides production (Javed et al. 2017a).
Metal oxide nanoparticle stress elicitors such as zinc oxide (ZnO) and copper oxide (CuO) have gained enormous importance in recent years (Javed et al. 2017b). The reactivity and toxicity of metallic oxide NPs depend on their size, surface, structure, concentration, dissolution and exposure routes (Franklin et al. 2007; Jiang et al. 2009; Chang et al. 2012). CuO nanoparticles belong to the group of nanoparticles that are used for both household and industrial purposes. However, the CuO toxicity has largely been demonstrated in aquatic organisms such as algae and zebrafish (Aruoja et al. 2009). CuO intracellular oxidative stress involves the release of Cu ions (Cu+2) causing toxicity after exceeding the maximum physiological tolerance range, hence disturb the balance between oxidation and anti-oxidation processes. CuO has largely been found phytotoxic because of generation of reactive oxygen species (ROS) and necrotic lesions, ultimately leading to the cell death (Chang et al. 2012).
The effect of CuO nanoparticles on the growth, photosynthesis and oxidative response has recently been studied in crop plant, Oryza sativa, Brassica napus (Da Costa and Sharma 2016; Zafar et al. 2016), Lemna minor (Duckweed), (Song et al. 2016; Perreault et al. 2014), Landolti apunctata (Shi et al. 2011), Elodea nuttallii (Regier et al. 2015). The production of steviol glycosides has been accomplished in the presence of abiotic stress i.e. metal (Pal et al. 2013; Jain et al. 2009), nutrient application (Allam et al. 2011; Utumi et al. 1999), osmotic stress (Vives et al. 2017); biotic stress i.e. endophytic fungi, genetic transformation (Pandey et al. 2016; Kilam et al. 2017).Copper is required for normal plant growth and development; however, it is toxic at higher levels. Due to its prominent role in development and stimulatory effect on secondary metabolites production, copper has received noticeable attention. However, despite of ions, to determine role of nanoparticles in secondary metabolite production, copper oxide nanoparticles were applied during in vitro propagation and for metabolite production of S. rebaudiana. Physiological characteristics of micro-propagated shoots, steviol glycosides production, and non-enzymatic anti-oxidant activities in leaves of regenerated S. rebaudiana are evaluated.
Materials and methods
Synthesis of CuO nanoparticles
CuO nanoparticles were synthesized by co-precipitation method as described by Javed et al. (2017a, b). It involved addition of 30 mL of 6 M aqueous sodium hydroxide (NaOH) solution to 600 mL of 0.2 M copper acetate monohydrate [Cu (CH3COO)2·H2O; 98%, Sigma-Aldrich) solution and 2 mL of glacial acetic acid (CH3COOH) solution in a drop-wise manner under continuous stirring at 100 °C. Blue color of solution changed to green and then brown. Finally, a black solution was obtained at pH 6–7 that was subsequently filtered, washed and then heated at 100 °C. Later on, the dried grinded powder was subjected to calcination at 500 °C for 4 h.
Characterization of CuO nanoparticles
Different analytical methods including X-ray diffraction (XRD), Fourier-transform infra-red (FTIR) spectra, scanning electron microscopy (SEM) and Energy dispersive X-ray (EDX) spectra were performed for characterization of CuO nanoparticles. The crystalline phase of the prepared sample was identified by X-ray diffraction (XRD) technique using a PANalytical Empyrean system. X-ray powder diffraction was performed at room temperature using Cu Kα radiation (λ = 1.5406 Å). The Fourier transform infrared (FTIR) spectra were recorded using FTIR spectrometer (Tensor 27 Bruker Germany) in the wave number ranging from 4000 to 500 cm−1. Morphological studies of CuO NPs were done by field emission scanning electron microscope (FESEM, Nova NanoSEM 450) and operated at an accelerating voltage of 10 kV. Energy dispersive X-ray (EDX, Oxford Aztec) was utilized to determine the elemental composition (purity) of prepared CuO nanoparticles.
Preparation of medium having CuO nanoparticles
Murashige and Skoog (MS) (1962) medium was used as basal medium for shoot organogenesis. In MS media solution CuO nanoparticles were added at 0, 0.1, 1, 10, 100 or 1000 mg/L. Sucrose (30 g/L) was added and pH was adjusted to 5.7–5.8 using 0.1 N NaOH. The media was sonicated in water bath for 30 min for thorough dispersion of nanoparticles followed by addition of agar (8 g/L). The agar was dissolved by heating and media was dispensed as 30/100 mL conical flask while thorough mixing. The culture media was then autoclaved for 15 min at 121 °C and 1.06 kg/cm2 pressure. The media was allowed to cool at room temperature before inoculation of explant.
Growth conditions of shoot organogenesis
The seeds of S. rebaudiana were purchased from POLISAN Tarim, Istanbul, Turkey. The seeds were cultured on plain MS medium after being disinfected with 0.1% (w/v) mercuric (II) chloride (HgCl2) for 3 min. The axillary shoot nodes were excised from 4 weeks-old seedlings and incubated in media treatments having different concentrations of CuO nanoparticles. The cultured flasks were kept in growth room chamber having 16 h light/8 h dark photoperiod, provided by cool-white fluorescent light of 35 μmol/m/s irradiance and 24 ± 1 °C temperature at 55–60% rate of relative humidity. Each treatment had 15 nodal explants that were cultivated for 4 weeks. Finally, the physiological parameters involving percentage of shooting, mean length of shoots, mean number of leaves, and fresh weight of shoots produced by direct shoot organogenesis were revealed.
Extraction and analysis of steviol glycosides
Steviol glycosides were extracted from the leaves of in vitro regenerated shoots grown under CuO nanoparticles stress. The shoots propagated were carefully washed with sterile distilled water, and soaked on filter paper to remove excess water and dried in oven at 60 °C for 48 h.
The analysis of steviol glycosides was performed through high performance liquid chromatography (HPLC) according to method described by Yücesan et al. (2016). The dried plant material was grinded and 20 mg was suspended in 1 mL of 70% (v/v) methanol in a micro-centrifuge tube. After incubation in an ultrasonic bath at 55 °C for 15 min, samples were centrifuged at 25 °C and 12,000 rpm for 10 min. The pellet was discarded and supernatant was filtered using 0.22 µm PTFE Millipore syringe filters.
Chromatography was performed using an autosampler (WPS-3000-SL Dionex Semi Prep Autosampler) injecting 10 µL of each sample, a binary pump (LPG 3400SD Dionex) solvent delivery system working at a flow rate of 0.8 mL/min, and a dual wavelength absorbance detector operating at 210 and 350 nm (MWD-3100 Dionex UV–VIS Detector). The column, Inertsil® ODS-3 (GL Sciences Inc., Japan) with 150 × 4.6 mm in length and 5 μm particle size, was kept warm at 40 °C in a column oven system (TCC-3000SD Dionex). At the end, isocratic flow was performed using acetonitrile and 1% (w/v) phosphoric acid buffer mixture (68:32) for 20 min.
Preparation of extract and anti-oxidant assays
The leaf extracts of S. rebaudiana were prepared by drying the leaves, and then taking 0.1 g of their fine powder obtained under different CuO concentrations. 500 μL of methanol was used for the dissolution of powder. It was vortexed for 5 min and then sonicated for 30 min followed by 15 min centrifugation at 10,000 rpm. The pellet was discarded and supernatant was stored to perform different antioxidant activities.
Determination of total phenolic content
Method of Ali et al. (2017) was performed after slight modifications, to estimate the total phenolic content in leaf extracts of Stevia. The process involved transfer of an aliquot of 20 µL (4 mg/mL) dimethyl sulfoxide (DMSO) stock solution of each sample to the respective well of 96 well plate, and then the addition of 90 µL of Folin–Ciocalteu reagent in it. The plate was kept for 5 min and later on, 90 µL of sodium carbonate was added to the reaction mixture. All samples were run in triplicate and their absorbance was obtained at 630 nm using microplate reader. Gallic acid was used as standard and the results were expressed as mg Gallic acid equivalent (µg GAE/mg).
Determination of total flavonoid content
Aluminum chloride colorimetric method of Zafar et al. (2017) was performed after slight modification, to determine total flavonoid content of different leaf extracts of Stevia. 10 µL of 10% aluminum chloride, 10 µL of 1.0 M potassium acetate and 160 µL of distilled water were added to the aliquot of 20 µL (4 mg/mL) DMSO stock solution of each sample contained in the respective well of 96 well plate. It was kept at room temperature for 30 min. The samples were run in triplicate and their absorbance was measured at 630 nm using microplate reader. Quercetin was used as standard and the results were expressed as mg quercetin equivalent (µg QE/mg).
Determination of total antioxidant capacity
Total antioxidant capacity was evaluated by the procedure of Ali et al. (2015) after slight modification. An aliquot of 100 μL from stock solution of each sample (4 mg/mL in DMSO) was mixed with 900 µL reagent solutions containing 0.6 M sulfuric acid, 4 mM ammonium molybdate and 28 mM sodium phosphate. The reaction mixture was incubated at 95 °C for 90 min, and later on cooled at room temperature. All samples were run in triplicate and their absorbance was measured at 695 nm using microplate reader. Ascorbic acid was used as standard and the results were expressed as mg ascorbic acid equivalent (µg AA/mg).
Determination of total reducing power
Total reducing power of samples was calculated according to the procedure of Rehman et al. (2014) after slight modifications. 100 µL of each sample (4 mg/mL in DMSO) was mixed with 200 µL of phosphate buffer (0.2 M, pH 6.6) and 250 µL of 1% w/v potassium ferricyanide. The resulting mixture was incubated at 50 °C for 20 min. Thereafter, the reaction was acidified with 200 µL of 10% w/v trichloroacetic acid and centrifugation was performed at 3000 rpm for 10 min. The pellet was discarded and the supernatant (150 µL) obtained was mixed with 50 µL of 0.1% w/v ferric chloride solution. All samples were run in triplicate and their absorbance was measured at 630 nm. Ascorbic acid was used as standard and the results were expressed as mg ascorbic acid equivalent (µg AA/mg).
Determination of DPPH free radical scavenging activity
Since the over-production and accumulation of free radicals is damaging to plant cells, the ability of antioxidants produced to prevent oxidative damage was elucidated by 2,2-diphenyl-1-picryl hydrazyl (DPPH) reagent. This assay was performed according to the protocol of Haq et al. (2012) after slight modifications. 10 µL (4 mg/mL) of Stevia leaf extracts was mixed with 190 µL of DPPH (0.004% w/v in methanol). The resulting reaction mixture was incubated in darkness for a period of 1 h. All samples were run in triplicate and their absorbance was measured at 515 nm of wavelength using microplate reader. Ascorbic acid was used as positive control while DMSO as negative control.
where Abs indicates the absorbance of DPPH solution with sample, and Abc is the absorbance of only DPPH solution. The IC50 was calculated by using Table curve software 2D Ver. 4.
Statistical analysis
The design of experiments was randomized and the statistical analysis of data was performed using SPSS, Version 17.0 (SPSS Inc., Chicago, IL, USA). Statistical difference was determined using ANOVA, and the significance of difference between means ± SE (standard error) values was obtained using Duncan’s multiple range tests performed at p < 0.05.
Results and discussion
XRD results
The powder patterns were recorded with the use of Empyrean PANalytical X-ray diffractometer with Bragg–Brentano geometry using Cu Kα radiation (λ = 1.54 Å). The step-scan covered the angular range 20–80° with the step of 0.02°. Figure 1a shows the XRD pattern of CuO nanoparticle. The diffraction data revealed that the material was composed of crystalline monoclinic cubic cuprous oxides (Fig. 1a). The peak positions are in good agreement with the PCPDFWIN data card 895899. The crystallite size determination was carried out using the Scherrer equation (Eq. 2) (Cullity 1978).
where D is the crystallite size, k is a constant (~0.94 assuming that the particles are spherical), λ is the wavelength of the X-ray radiation, β is the line width at half maximum intensity of the peak and θ B is the angle of diffraction. The particles size obtained from the XRD data for CuO, is 47 nm. The lattice parameter of CuO monoclinic structure and the plane spacing d is related to the lattice constant and the Miller indices (hkl) by Eq. 3 (Cullity 1978)
Using above equation along with the Bragg’s law (2d hkl sinθ = nλ), we calculate the values of lattice parameters. Monoclinic CuO crystal have the lattice constants a = 4.69 Å, b = 3.418 Å and c = 5.122 Å and angle β = 99.57°.
FTIR results
Figure 1b shows the FTIR spectrum of CuO nanoparticles. Various well-defined peaks were observed at 535, 594, 869, 1052, 1411, 2094, 2847, 2920 and 3419 cm−1. The appearance of the peaks at 535, 594 corresponds to the characteristic stretching vibrations of Cu–O bonds in the monoclinic crystal structure of CuO (Zheng and Liu 2007; Ethiraj and Kang 2012) and 869 cm−1 corresponds well to Cu–O–H vibration (Yu et al. 2012; Park and Kim 2004). The bond at 1052 cm−1 is due to the C–O stretching vibration (Zhao et al. 2012). Origin of two well-defined absorption bands at 1411 cm−1 is due to the CH3 group and CH3 asymmetrical stretching mode present on the surface of CuO nanostructures. The bands at 2847 and 2920 cm−1 are assigned to –CH2 and C–H stretching mode. The absorption peak around 2094 cm−1 is due to the existence of CO2 molecules in air, and the peak around 3419 cm−1 is assigned to the O–H stretching vibration.
SEM and EDX analysis
Morphology of the sample was investigated using field emission scanning electron microscope (FESEM). Specimens were prepared by sticking CuO nanoparticles to the carbon tape, and blow away the excess of powder with compressed air. This specimen was sputter coated with a thin Au–Pd layer of about 3 nm thick in vacuum to avoid the charging. Typical SEM micrograph for prepared CuO nanoparticles is shown in Fig. 2. The SEM micrograph clearly showed irregular shaped morphologies of CuO nanoparticles. The SEM observation showed the presence of agglomerated nanoparticles with an average size of 40–100 nm.
The EDX spectrum of CuO nanoparticles is given in Fig. 3. The EDX results show that there are no other elemental impurities present in the prepared CuO nanoparticles.
Estimation of physiological parameters and steviol glycosides
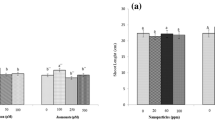
The results (Table 1 and Fig. 4a, b) show that the growth of S. rebaudiana shoots reached to maximum at 10 mg/L of CuO nanoparticles. However, after attaining this threshold level, further increase in NPs concentration resulted in toxicity to the plant. Table 1 indicates that the highest amount of shooting (~90%) occurred in MS medium supplemented with 10 mg/L of CuO nanoparticles. In contrary, the least shooting frequency (~41%) was obtained in MS medium containing 1000 mg/L of CuO nanoparticles. Maximum shoot length (4.9 cm) was achieved with MS medium augmented with 10 mg/L of CuO nanoparticles, followed by MS containing 1 mg/L (4.5 cm). Similarly, highest number of nodes (4.9) and leaves (16.1) were also recorded by 10 mg/L CuO nanoparticles treatment. Fresh weight of shoots was also measured to be highest in MS medium fortified with 10 mg/L of CuO nanoparticles (0.44 g).Direct shoot organogenesis is a method used to perform in vitro shoot growth of S. rebaudiana and presence of nanoparticles stimulates metabolic pathways for production of active constituents (Dimkpa et al. 2013). Mineral nutrients are the basic components of tissue culture media and the explant responds based on type and concentration of nutrient. Various Cu-containing enzymes involved in electron transport, protein and carbohydrate biosynthesis also play a role in plant regeneration (Niedz and Evens 2007). However, the threshold level of each component is critical. Copper has been investigated as stimulatory component for induction of in vitro culture of many plant species i.e. Eleusine, Cucumis, Sorghum, Lepidium, Capsicum, Triticale, barley, wheat, rice, Tinospora (Garcia-Sago et al. 1991; Purnhauser and Gyulai 1993; Dahleen 1995; Sahrawat and Chand 1999; Pande et al. 2000; Kumar et al. 2003; Nirwan and Kothari 2003; Kothariet al. 2004; Tahiliani and Kothari 2004). Based on these findings Cu nanoparticles were used in this study that reflects that CuO NPs also has stimulatory effect on in vitro shoot regeneration and production of secondary metabolites.
A Shoot organogenesis of Stevia in MS basal medium containing a no CuO, b 0.1 mg/L CuO, c 1 mg/L CuO, d 10 mg/L CuO, e 100 mg/L CuO, f 1000 mg/L CuO nanoparticles. B Comparison of different-sized shoots of Stevia obtained in MS medium having a no CuO, b 0.1 mg/L CuO, c 1 mg/L CuO, d 10 mg/L CuO, e 100 mg/L CuO, f 1000 mg/L CuO nanoparticles
Figure 5 shows the production of steviol glycosides (rebaudioside A and stevioside) in S. rebaudiana in the presence of up to 10 mg/L of CuO nanoparticles, thereafter, it causes phytotoxicity. The amount of rebaudioside A enhanced from 2.07% in control MS treatment to 4.17% in MS treatment supplemented with 10 mg/L of CuO nanoparticles. On gradual increasing the CuO nanoparticles concentration, decrease in rebaudioside A amount was observed. Similarly, HPLC spectra for quantity of stevioside reveales that the amount of stevioside was 0.73% obtained in control group that increased up to 1.19–2.06% in treatments having 1 and 10 mg/L of CuO nanoparticles, respectively. CuO nanoparticles have been considered as abiotic stress elicitors that positively effect the growth parameters, and enhance the quantity of steviol glycosides as well as non-enzymatic antioxidant activities found specifically in the Stevia leaves. This positive influence had been observed in the start, but after crossing a threshold barrior of 10 mg/L of CuO nanoparticles, a sudden decline was investigated as a result of continued addition of CuO nanoparticles to the growth medium. Zafar et al. (2017) have reported that at some level CuO NPs have beneficial role and it is mainly due to release of Cu ions from the nanoparticles that are taken up by the cells and play a pivotal role in plant biochemistry. Therefore, significant variation in plant biomass, steviol glycoside and antioxidants are observed. Allam et al. (2001) showed a significant effect of nitrogen to enhance the concentration of stevioside in the stevia leaf while deficiency of some nutrients like potassium and calcium decreased the concentration of stevioside on a dry weight basis (Utumi et al. 1999). The accumulation of steviol glycoside in cells of S. rebaudiana relates to the extent of the development of the membrane system of chloroplast and the content of photosynthetic pigments (Ladygin et al. 2008).
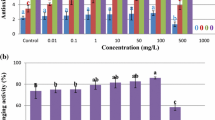
Evaluation of antioxidant activities
Table 2 illustrates that a premier quantity of total phenolic content (6.22 µg GAE/mg), total flavonoid content (7.49 µg QE/mg), total anti-oxidant capacity (11.9 µgAAE/mg), total reducing power (11.5 µg AAE/mg)and % DPPH inhibition (74.8%) was assessed from Stevia leaves obtained from MS medium containing 10 mg/L of CuO nanoparticles. However, the lowest total phenolic content (3.99 µg GAE/mg), total flavonoid content (2.11 µg QE/mg), total anti-oxidant capacity (9.16 µgAAE/mg), total reducing power (10.3 µg AAE/mg) and % DPPH inhibition (58.5%) were found out from extracts containing 1000 mg/L of CuO nanoparticles employed in MS medium.The enzymatic and non-enzymatic antioxidants naturally present in plants actually help them cope with an oxidative stress of metal ions or free radicals (Klaper et al. 2009). Hence, the imbalance of endogeneous plant growth regulators and an accumulation of reactive oxygen species (ROS) causes depletion of plant cells and their activities (Choi and Hu 2008). Based on our study, CuO nanoparticles implicated an intracellular oxidative stress by the release of metal ions (Cu+2) or free radicals into MS culture medium (Gajewska and Skłodowska 2007; Dimkpa et al. 2014), and as a consequence of oxidative damage at 1000 mg/L, an impaired growth, reduced steviol glycoside quantity, and mitigation of antioxidant activities was observed.
Conclusion
In conclusion, it has been demonstrated from these findings that CuO nanoparticles confer positive effect for in-vitro Stevia growth dynamics and steviol glycoside production. In this regard, a significantly enhanced amount of secondary metabolites and antioxidant activities have been obtained at 10 mg/L of CuO nanoparticles concentration employed in MS basal medium. In the meanwhile, phytotoxic effects of CuO nanoparticles have also been observed, and the highest level of phtotoxicity has been achieved by CuO nanoparticles at 1000 mg/L concentration. Our research opens up new avenues for the study of metabolic pathways in context of an interaction between different concentrations of nanoparticles and in vitro grown medicinal plants.
References
Ali A, Phull AR, Zia M, Shah AMA, Malik RN (2015) Phytotoxicity of River Chenab sediments: in vitro morphological and biochemical response of Brassica napus L. Environ Nanotechnol Monit Manag 4:74–84
Ali JS, Haq ul, Ali I, Ahmed A, Zia M, M (2017) Onosma bracteatum wall and Commiphora stocksiana Engl extracts generate oxidative stress in Brassica napus: an allelopathic perspective. Cogent Biol 3(1):1283875
Allam AI, Nassar AM, Besheit SY (2001) Itrogen fertilizer requirements of Stevia rebaudiana bertoni, under Egyptian conditions. Egypt J Agri Res 79:1005–1018
Allam A, El-Ghareeb AA, Abdul-Hamid M, Baikry A, Sabri MI (2011) Prenatal and perinatal acrylamide disrupts the development of cerebellum in rat: biochemical and morphological studies. Toxicol Ind Health 27(4):291–306
Aruoja V, Dubourguier HC, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO 2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407(4):1461–1468
Aslani F, Bagheri S, Muhd Julkapli N, Juraimi AS, Hashemi FSG, Baghdadi A (2014) Effects of engineered nanomaterials on plants growth: an overview. Sci World J 2014. doi:10.1155/2014/641759
Boczkowski J, Hoet P (2010) What’s new in nanotoxicology? Implications for public health from a brief review of the 2008 literature. Nanotoxicol 4(1):1–14
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5(12):2850–2871
Choi O, Hu Z (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42(12):4583–4588
Cullity BD (1978) Elements of X-ray diffraction, Second edn. Addison-Wesley Publishing Company, Inc., Reading
Da Costa MVJ, Sharma PK (2016) Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54:110–119
Dahleen LS (1995) Improved plant regeneration from barley callus cultures by increased copper levels. Plant Cell Tiss Organ Cult 43(3):267–269
Dey A, Kundu S, Bandyopadhyay A, Bhattacharjee A (2013) Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. CR Biol 336:17–28
Dimkpa CO, Latta DE, McLean JE, Britt DW, Boyanov MI, Anderson AJ (2013) Fate of CuO and ZnO nano- and microparticles in the plant environment. Environ Sci Technol 47(9):4734–4742
Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, Boyanov MI, Anderson AJ (2014) CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14:1–15
Dimkpa CO, McLean JE, Britt DW, Anderson AJ (2015) Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology 24(1):119–129
Ethiraj AS, Kang DJ (2012) Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res Lett 7:1–5
Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS (2007) Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 41:8484–8490
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activitiesin wheat leaves. Biometals 20:27–36
Garcia-Sago B, Roig LA, Moreno V (1991) Enhancement of morphogenetic response in cotyledon-derived explants of Cucumismelo induced by copper ions. Acta Hort 289:229–230
Haq IU, Mannan A, Ahmed I, Hussain I, Jamil M, Mirza B (2012) Antibacterial activity and brine shrimp toxicity of Artemisia dubia extract. Pak J Bot 44:1487–1490
Jain P, Kachhwaha S, Kothari SL (2009) Improved micropropagation protocol and enhancement in biomass and chlorophyll content in Stevia rebaudiana (Bert.) Bertoni by using high copper levels in the culture medium. Sci Hortic 119:315–319
Javed R, Usman M, Yücesan B, Zia M, Gürel E (2017a) Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol Biochem 110:94–99
Javed R, Ahmed M, Haq ul, Nisa I, Zia S, M (2017b) PVP and PEG doped CuO nanoparticles are more biologically active: antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mat Sci Eng C 79:108–115
Jiang W, Mashayekhi H, Xing B (2009) Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ Pollut 157:1619–1625
Kilam D, Saifi M, Abdin MZ, Agnihotri A, Varma A (2017) Endophytic root fungus Piriformospora indica affects transcription of steviol biosynthesis genes and enhances production of steviol glycosides in Stevia rebaudiana. Physiol Mol Plant Pathol 97:40–48
Klaper R, Crago J, Barr J, Arndt D, Setyowati K, Chen J (2009) Toxicity biomarker expression in daphnids exposed to manufactured nanoparticles: changes in toxicity with functionalization. Environ Pollut 157:1152–1156
Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Materials 7:2833
Kothari SL, Agarwal K, Kumar S (2004) Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millets-Eleusine coracana (L.) Gaertn. In Vitro Cell Dev Biol-Plant 40:515–519
Kumar S, Narula A, Sharma M, Srivastava PS (2003) Effect of copper and zinc on growth, secondary metabolite content and micropropagation of Tinospora cordiofolia: a medicinal plant. Phytomorphol 53:79–91
Ladygin VG, Bondarev NI, Semenova GA, Smolov AA, Reshetnyav OV, Nosov AM (2008) Chloroplast ultrastructure, photosynthetic apparatus activities and production of steviol glycosides in Stevia rebaudiana in vivo and in vitro. Biol Plant 52:9–16
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Niedz RP, Evens TJ (2007) Regulating plant tissue growth by mineral nutrition. In Vitro Cell Dev Biol-Plant 43:370–381
Nirwan RS, Kothari SL (2003) High copper levels improve callus induction and plant regeneration in Sorghum bicolar (L.) Moench. In Vitro Cell Dev Biol-Plant 39:161–164
Pal PK, Prasad R, Pathania V (2013) Effect of decapitation and nutrient applications on shoot branching, yield, and accumulation of secondary metabolites in leaves of Stevia rebaudiana Bertoni. J Plant Physiol 170(17):1526–1535
Pande SD, Iqbal M, Srivastava PS (2000) Effect of ZnSO4 and CuSO4 on regeneration and lepidine content in Lepidium sativum L. Biol Plant 43:253–256
Pandey H, Pandey P, Pandey SS, Singh S, Banerjee S (2016) Meeting the challenge of stevioside production in the hairy roots of Stevia rebaudiana by probing the underlying process. Plant Cell Tiss Org Cult 126:511–521
Park SH, Kim HJ (2004) Unidirectionally aligned copper hydroxide crystalline nanorods from two-dimensional copper hydroxy nitrate. J Am Chem Soc 126:14368–14369
Perreault F, Samadani M, Dewez D (2014) Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicol 8:374–382
Purnhauser L, Gyulai G (1993) Effect of copper on shoot and root regeneration in wheat, triticale, rape and tobacco tissue cultures. Plant Cell Tiss Org Cult 35(2):131–139
Regier N, Cosio C, von Moos N, Slaveykova VI (2015) Effects of copper-oxide nanoparticles, dissolved copper and ultraviolet radiation on copper bioaccumulation, photosynthesis and oxidative stress in the aquatic macrophyte Elodea nuttallii. Chemosphere 128:56–61
Rehman R, Chaudhary M, Khawar K, Lu G, Mannan A, Zia M (2014) In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia 69(3):341–349
Sahrawat AK, Chand S (1999) Stimulatory effect of copper on plant regeneration in indica rice (Oryza sativa L.). J Plant Physiol 154(4):517–522
Shi J, Abid AD, Kennedy IM, Hristova KR, Silk WK (2011) To duckweeds (Landoltiapunctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut 159:1277–1282
Song G, Hou W, Gao Y, Wang Y, Lin L, Zhang Z, Niu Q, Ma R, Mu L, Wang H (2016) Effects of CuO nanoparticles on Lemna minor. Bot Stud 57:1–8
Tahiliani S, Kothari SL (2004) Increased copper content of the medium improves plant regeneration from immature embryos derived callus of wheat (Triticum aestivum). J Plant Biochem Biotechnol 13:85–88
Utumi MM, Monnerat PH, Pereira PRG, Fontes PCR, Godinho VD (1999) Macronutri-ent deficiencies in Stevia: visual symptoms and effects on growth, chemical composition, and stevioside production. PesquiAgropecu Bras 34:1039–1043
Vives K, Andújar I, Lorenzo JC, Concepción O, Hernández M, Escalona M (2017) Comparison of different in vitro micropropagation methods of Stevia rebaudiana B. including temporary immersion bioreactor (BIT®). Plant Cell Tiss Organ Cult 131(1):195–199
Yadav V (2013) Nanotechnology, big things from a tiny world: a review. AEEE 3:771–778
Yu Q, Huang H, Chen R, Wang P, Yang H, Gao M, Peng X, Ye Z (2012) Synthesis of CuO nanowalnuts and nanoribbons from aqueous solution and their catalytic and electrochemical properties. Nanoscale 4:2613–2620
Yücesan B, Mohammed A, Büyükgöçmen R, Cevher A, Kavas Ö, Gürel S, Gürel E (2016) In-vitro and ex-vitro propagation of Stevia rebaudiana Bertoni with high Rebaudioside-A content—a commercial scale application. Sci Hortic 203:20–28
Zafar H, Ali A, Ali JS, Haq IU, Zia M (2016) Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci 7:535
Zafar H, Ali A, Zia M (2017) CuO nanoparticles inhibited root growth from Brassica nigra seedlings but induced root from stem and leaf explants. App Biochem Biotechnol 181:365–378
Zhao Y, Song X, Song Q, Yin Z (2012) A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant. Cryst Eng Commun 14:6710–6719
Zheng L, Liu X (2007) Solution-phase synthesis of CuO hierarchical nanosheets at near-neutral pH and near-room temperature. Mater Lett 61:2222–2226
Acknowledgements
Authors are grateful to The Scientific and Technological Research Council of Turkey, TUBİTAK (Program # 2216) for providing financial support. We are also thankful to the Department of Biology and Seed Science and Technology Abant Izzet Baysal University, Bolu, Turkey; Department of Biotechnology and Department of Physics, Quaid-i-Azam University, Islamabad, Pakistan for providing all the research facilities.
Author information
Authors and Affiliations
Contributions
RJ designed the study and wrote manuscript. AM and RJ performed experimental work. BY and RJ analyzed the data. MZ and EG critically reviewed the manuscript and added to its technical part. All authors have contributed, seen and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Silvia Moreno.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Javed, R., Mohamed, A., Yücesan, B. et al. CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant Cell Tiss Organ Cult 131, 611–620 (2017). https://doi.org/10.1007/s11240-017-1312-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1312-6