Abstract
A change in the environmental conditions through CO2 enrichment was used to improve acclimatization of Cynara scolymus tissue-cultured plants. Plants were transferred from tissue culture conditions to a climatic chamber, where the acclimatization was carried out at 800 μmol mol−1 CO2 and at the ambient CO2. The experiment lasted 3 weeks and physiological measurements—such as plant water status and growth, electrolyte leakage, net CO2 assimilation, internal CO2, stomatal conductance, transpiration, chlorophyll fluorescence, free amino acids, photosynthetic pigments and lipid peroxidation—were taken every 4 days. During the experiment plants cultured under CO2 enrichment exhibited earlier stomatal control and a rapid increase in the photosynthetic rates. As a result, the water potential increased rapidly and the relative water content and the water use efficiency were higher than in plants cultured at ambient CO2. At the end of the experiment the plant survival was completely successful under the elevated CO2, which enhanced the acclimatization process—diminishing plant mortality through attenuation of plant damage and allowing better adaptation of plants to the environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acclimatization has been described as the environmental adaptation of tissue-cultured plants to ex vitro conditions (Kozai and Zobayed 2000), considering that leaves need gradual changes in order to adapt to photoautotrophic conditions. Plants cultured in vitro grow under special conditions; namely, extremely high relative humidity, low CO2, low level of light, and a culture medium with high concentrations of carbohydrates and nutrients. The continuous exposure to this unique environment, which has been selected to provide minimal stress and optimum conditions for plant multiplication, produce physiological and morphological disorders in in vitro cultured plants (Hazarika 2006).
Due to a continuous supply of exogenous sucrose and nutrients and poor light conditions, in vitro plants do not need the normal development of photosynthetic apparatus, resulting in an almost complete absence of photosynthesis (Rodriguez et al. 2008). Therefore, in vitro cultured plants are either poor in chlorophyll, thus producing low carbon assimilation. During the first days of acclimatization, this malfunction of the photosynthetic apparatus forces tissue-cultured plants to use the reserves obtained during in vitro culture until the production of sucrose is enough for normal plant development and biomass production (Rodriguez et al. 2008). The duration of the change from heterotrophic to autotrophic metabolism is a key factor and it may result in a high percentage of plant loss or damage during the acclimatization to ex vitro conditions.
Although the low carbon assimilation limits the sugar production and plant growth and development, the major disorder in in vitro plants is the poor mechanism to control water loss. The high relative humidity produces abnormally functioning stomata and thin cuticle (Dias et al. 2014) in leaves of tissue-cultured plants, thus causing desiccation through foliar water loss when in vitro plants are transferred to ex vitro conditions. Stomata of most mesophytic plants close in response to certain environmental changes, while stomata in in vitro plants remain open (Brainerd and Fuchigami 1982). This alteration produces a drop on water potential causing water loss and damages. In commercial acclimatization, methods that increase the relative humidity are used, to avoid collapse and mortality; notwithstanding, they promote bacterial, fungal and insect infestations (Toler et al. 2003). Thus, there is an urgent necessity to develop methods that accelerate a normal functioning of the photosynthetic apparatus and stomata closure preventing plant loss or damage.
It is well known that high CO2 increases the photosynthetic carbon fixation rates, which stimulates plant growth and development. In addition, it has been reported that plants generally respond to atmospheric CO2 enrichment by mitigating water stress (Wall et al. 2006; Vu and Allen 2009) through increased water use efficiency (WUE) (Chaves 1994). This responsiveness of plants to elevated CO2 is being used in commercial farms to accelerate plant growth and production. The use of high CO2 during in vitro plant propagation has given an improvement in the subsequent stage of acclimatization (Rodriguez et al. 2008; Shin et al. 2014), but there is little information about the effects of CO2 applied during acclimatization.
The objective of this work was to evaluate the role played by CO2 in plant acclimatization and its capacity to shorten the period of adaptation from in vitro to ex vitro conditions through the induction of the production of plants better adapted to the environment. The study has been focused on relevant processes and parameters such as fresh and dry weight, leaf area, relative water content, water potential, electrolyte leakage, net CO2 assimilation, transpiration, internal CO2, stomatal conductance, chlorophyll fluorescence, free amino acids, quantification of photosynthetic pigments, lipid peroxidation, starch, and soluble sugars.
Materials and methods
Plant material and experimental conditions
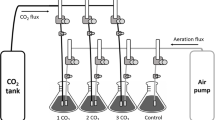
Micropropagated in vitro plants of Cynara scolymus L. cv. ‘Romanesco’, approximately 4 cm long and with at least one root primordium, were transplanted to trays with 40 0.1–l pots containing peat. Immediately after ex vitro transfer, the plants were watered with a commercial nutrient solution (Bayfolan, BAYER®) and then covered with a transparent polythene membrane. The membrane was maintained for 8 days; then the front part was raised and 8 days later the membrane was finally removed, as shown in Fig. 1. Acclimatization was performed in a controlled climate chamber designed by our department specifically for plant research purposes (del Amor et al. 2010), with fully-controlled environmental conditions: 75 % relative humidity, 16/8 h day/night, 25 ± 1 °C and a photosynthetically active radiation of 250 μmol m−2 s−1—provided by a combination of fluorescent lamps (TL-D Master reflex 830 and 840, Koninklijke Philips Electronics N.V., Eindhoven, the Netherlands) and high-pressure sodium lamps (Son-T Agro, Philips). The experiment was carried out at 800 μmol mol−1 CO2 and at control CO2 (ambient CO2, 380 ± 40 μmol mol−1 CO2).
Samples were collected at the moment of the transfer from in vitro conditions and every 4 days of treatment up to 21 days, when the acclimatization process was complete (Fig. 1). The fresh and dry weights, leaf area (LA), relative water content (RWC), gas exchange parameters, chlorophyll fluorescence, water potential and cell membrane permeability were determined. At the same time, leaf samples were collected, immediately frozen in liquid nitrogen and stored at −80 °C for further quantification of photosynthetic pigments, free amino acids, lipid peroxidation, soluble carbohydrates and starch.
The number of plants in active growth (new shoots), in a steady state (no new shoots and no growth) or dead were recorded at the end of the experiment.
Plant water status and growth
Plant weight was measured as well as the number of leaves. The LA was also recorded, using a portable area meter (LI-COR Model LI-3000A; Li-Cor, Lincoln, Nebraska, USA).
The RWC was determined using the equation: RWC = 100[(FW − DW)/(TW–DW)], where FW is the fresh weight, TW is the turgid weight and DW is the dry weight of leaf discs, respectively. To obtain full turgor, the leaf discs were placed in darkness for 24 h in vials containing water—to allow complete rehydration. The DW was determined after drying the leaf discs at 65 °C for 72 h.
The biggest intact leaf was chosen for measurement of the leaf water potential (Ψw), using the Scholander pressure-equilibration technique (Scholander et al. 1965).
Soluble sugars and starch
Soluble sugars were extracted according to Walker et al. (2008), by incubating 40 mg of lyophilised leaf tissue twice in 5 ml of 60 % ethanol, for 30 min each time, at 35 °C. Each extract was centrifuged at 3,500×g for 10 min, at 20 °C, and the two supernatants were combined. Chloroform (5 ml) was added and the mixture shaken before centrifugation at 2,700×g for 10 min, at 20 °C. The upper, colourless layer (20 % ethanol) was diluted four-fold with absolute ethanol, to produce an extract in 80 % ethanol for measurement of soluble sugars according to Buysse and Merckx (1993).
The residual material from the extraction with 60 % ethanol was hydrolysed with 3 % HCl, for 3 h at 125 °C, and the soluble sugars released were measured as an estimate of the starch content.
Electrolyte leakage
The method described by McKay (1992), following the modifications reported by Mena-Petite et al. (2001), was performed to assess solute leakage from plant tissue and study the membrane competence during the acclimatization process. Thus, 0.1 g of leaf discs were weighed and introduced in plastic vials (previously washed in abundant purified water) containing 16 ml of water. The vials were closed and the discs were allowed to hydrate for 24 h at room temperature. Following this, the samples were autoclaved at 115 °C for 10 min, causing membrane rupture and the escape of all the electrolytes. The conductivity of the water was measured before introducing the leaf discs in the water (Co), after hydration for 24 h (C24) and after autoclaving, when the samples were at room temperature (Ct). The percentage of electrolyte leakage was determined using the equation: Electrolyte leakage (EL) (%) = 100 [(C24 − Co)/(Ct − Co)].
Lipid peroxidation
Lipid peroxidation was measured as the amount of thiobarbituric acid-reactive substances (TBARS), as determined by the thiobarbituric acid (TBA) reaction (Heath and Packer 1968). Lyophilised samples (0.1 g) were homogenised in 3 cm3 of 20 % (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 3,500×g for 20 min. To a 1.5-cm3 aliquot of the supernatant, 1.5 cm3 of 20 % (w/v) TCA containing 0.5 % (w/v) TBA and 0.15 cm3 of 4 % (w/v) BHT in ethanol were added. The mixture was heated at 95 °C for 30 min and then quickly cooled on ice. The contents were centrifuged at 10,000×g for 15 min, and the absorbance was measured at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The concentration of TBARS was calculated using an extinction coefficient of 155 mM−1 cm−1 (Balestrasse et al. 2006).
Gas exchange
The net CO2 assimilation (ACO2), internal CO2 (Ci), transpiration rate (E) and stomatal conductance (gs) were measured in the youngest fully expanded leaf of each plant, using a CIRAS-2 (PP system, Amesbury, MA) with a PLC6 (U) Automatic Universal Leaf Cuvette, measuring both sides of the leaves. The cuvette provided light (LED) with a photon flux of 1,300 m−2 s−1, 360 or 800 μmol mol−1 CO2, a leaf temperature of 25 °C and 75 % relative humidity.
Chlorophyll content and fluorescence
Chlorophylls a, b and a + b were extracted from samples of the youngest leaf with N,N–dimethylformamide, for 72 h, in darkness at 4 °C. Subsequently, the absorbance was measured in a spectrophotometer at 750, 664 and 647 nm, and the quantities were calculated according to the method of Porra et al. (1989).
On the leaf used for gas exchange, the dark-adapted maximum fluorescence (Fm) and minimum fluorescence (Fo) and the light-adapted, steady-state chlorophyll fluorescence (F) and maximum fluorescence (Fm′) were measured with a portable modulated fluorometer, model OS-30P (Opti-Science, USA). The ratio between the variable fluorescence from a dark-adapted leaf (Fv) and the maximal fluorescence from a dark-adapted, youngest fully expanded leaf (Fm)—called the maximum potential quantum efficiency of photosystem II (Fv/Fm)—was calculated. A special leaf clip holder was allocated to each leaf to maintain dark conditions for at least 30 min before reading.
Proline determination
Proline colorimetric determination proceeded according to Bates et al. (1973), based on proline’s reaction with ninhydrin. For this, a 1:1:1 solution of proline, acid ninhydrin and glacial acetic acid was incubated at 100 °C for 1 h. The reaction was arrested in an ice bath and the chromophore was extracted with 4 ml of toluene; its absorbance at 520 nm was determined in a BioMate spectrophotometer (Thermo Spectronic).
Free amino acids
Free amino acids were extracted from leaves frozen at −80 °C at the beginning and end of the experiment. Sap was extracted after vortexing at 5,000 rpm (10 min, 4 °C) and filtering (nylon membrane filter, 0.2 μm), and the free amino acids were determined by the AccQ·Tag-ultra performance liquid chromatography (UPLC) method (Waters 2006). For derivatisation, 70 μl of borate buffer were added to the hydrolysed sample or to 10 μl of the fruit sap. Following this, 20 μl of reagent solution were added. The reaction mixture was mixed immediately and heated at 55 °C for 10 min. After cooling, an aliquot of the reaction mixture was used for UPLC injection. The UPLC was performed on an Acquity system (Waters) equipped with a fluorescence detection system. The column used was a BEH C18 100 mm × 2.1 mm × 1.7 m (Waters). The flow rate was 0.7 ml min−1 and the column temperature was kept at 55 °C. The injection volume was 1 µl. Wavelength excitation (ex) and emission (em) were set at 266 and 473 nm, respectively. The solvent system consisted of two eluents: (A) AccQ·Tag-ultra eluent A concentrate (5 %, v/v) and water (95 %, v/v); (B) AccQ·Tag ultra eluent B. The following elution gradient was used: 0–0.54 min, 99.9 % A–0.1 % B; 5.74 min, 90.9 % A–9.1 % B; 7.74 min, 78.8 % A–21.2 % B; 8.04 min, 40.4 % A–59.6 % B; 8.05–8.64 min. 10 % A–90 % B; 8.73–10 min, 99.9 % A–0.1 % B. Empower 2 (Waters) software was used for system control and data acquisition. External standards (Thermo scientific) were used for quantification of (ala) alanine; (arg) arginine; (asp) aspartic acid; (glu) glutamic acid; (gly) glycine; (his) histamine; (ile) isoleucine; (leu) leucine; (lys) lysine; (met) methionine; (phe) phenylalanine; (pro) proline; (ser) serine; (thr) threonine; (tyr) tyrosine; (val) valine.
Data collection and statistical analysis
Two treatments with 160 plants each and 10 replications per sampling were studied. Samples were taken every 4 days, as explained in Fig. 1. The data were tested first for homogeneity of variance and normality of distribution. Significance was determined by analysis of variance (ANOVA), and the significance (P < 0.05) of differences between mean values was tested by Duncan’s New Multiple Range Test, using Statgraphics Centurion® XVI (StatPoint Technologies, Inc.).
Results
Artichoke plants were acclimatized at 800 μmol mol−1 CO2 under controlled conditions in order to test their survival and the plant quality before, during and at the end of the acclimatization process. To compare the differences, the experiment was repeated under control conditions at the ambient CO2. After the experiment, the plants were classified in three groups: active growth, steady state and dead plants. As shown in Table 1, the survival percentage during acclimatization was 100 % in plants cultured at 800 μmol mol−1 CO2 and all of them had active growth with at least one new shoot. In contrast, 11.54 % of the plants used as controls died in the process and only 42.31 % were growing actively.
Plant water status and growth
Plant water status and growth were evaluated immediately after ex vitro transfer and every 4 days of treatment up to 21 days, when the acclimatization process was finished. Plant fresh weight (Table 2) increased gradually from the first day of the process in both CO2 regimes. Nevertheless, the fresh weight of the plants cultured at high CO2 was significantly higher after day 9, when it became double that of the control plants. Similar results were obtained for dry weight (Table 2): the weight of the plants acclimatized at high CO2 was significantly higher than that of the control plants from day 9 until the end of the experiment. The LA increased in both treatments as the process of acclimatization advanced (Table 2). Notwithstanding, as for fresh weight, the LA was higher in plants cultured at high CO2 —being double that of the control plants from day 9.
The RWC (Fig. 2a) showed significant differences between plants acclimatized with high CO2 and control plants. Plants grown in CO2 enrichment conditions had a RWC that ranged from 63.83 % on day 1 to 82.39 % on day 5. In contrast, control plants presented a maximum of 67.65 % on day 1. The Ψw (Fig. 2b) increased in both treatments. However, the rise in Ψw was markedly higher and significant from day 9 in the plants treated with high CO2, when compared with the control plants; the Ψw increased slightly to a maximum of −0.257 MPa in control plants, while plants acclimatized under elevated CO2 reached a maximum of −0.130 MPa at the end of the experiment.
Soluble sugars and starch
The soluble sugars content did not seem to be affected by the CO2 increase or the acclimatization process (Table 2). Similar results were obtained for starch in plants cultured with high CO2. Notwithstanding, starch increased as the acclimatization process advanced in control plants (Table 2).
Electrolyte leakage
Cell membrane permeability was studied through the EL, which increased significantly after ex vitro transfer (Fig. 3a) in plants treated with high CO2 compared with data obtained in control plants. Cell membrane permeability was lower in control plants, except on day 13 when no significant differences were found between control plants and plants under elevated CO2.
Lipid peroxidation
The TBARS increased in CO2 treated plants and decreased in control plants after ex vitro transfer (Fig. 3b). However, no significant differences were found between them at the end of the experiment, when the plants were fully acclimatized.
Gas exchange
The ACO2 increased after in vitro transfer in plants acclimatized under high CO2 and was significantly higher than ACO2 in control plants from day 4 and until the end of the experiment (Fig. 4a). The high CO2 induced an increase in ACO2: from 4.46 μmol CO2 m−2 s−1 on day 1 to 12.39 μmol CO2 m−2 s−1 on day 21. In contrast, the increment in control plants was only from 3.10 to 5.49 μmol CO2 m−2 s−1. The CO2 enrichment produced a significant drop in gs from day 1 to day 5 and the decreasing continued up to day 13 (Fig. 4b) when gs started to stay stable. This stability was also reached on day 13 in control plants. However, the gs was much higher in control plants than in plants treated with high CO2 during the first 2 weeks of the experiment. No significant differences between treatments were found for E, which decreased significantly after the ex vitro transfer in plants grown in elevated CO2 and in control plants from day 5; differences were only found on day 5, when the control plants showed an E of 3.48 mmol m−2 s−1 and the plants cultured in high CO2 had a value of 2.42 mmol m−2 s−1 (Fig. 4c). The Ci decreased gradually during the acclimatization process (Fig. 4d); the Ci in plants cultured in high CO2 was always significantly higher than that of the control plants.
Net CO2 assimilation rate (ACO2) (a), stomatal conductance (gs) (b), transpiration rate (E) (c), internal CO2 concentration (Ci) (d), water use efficiency (WUE) (ACO2/E) (e) and the internal CO2 concentration/ambient CO2 ratio (f) in Cynara scolymus before, during and after acclimatization at 800 μmol mol−1 CO2 (800) and at the ambient concentration of CO2 (control). Values are mean ± SE (n = 10) (P < 0.005)
With regard to the WUE (ACO2/E), the levels increased as the acclimatization was accomplished being always significantly higher in plants under elevated CO2 than in control plants. The WUE increased drastically in plants cultured with high CO2, reaching 13.30 in fully acclimatized plants, while the WUE ratio in control plants was 6.48 (Fig. 4e). By contrast, the Ci/Ca ratio decreased in both regimes, with differences only on day 9 (Fig. 4f).
Chlorophyll content and fluorescence
The acclimatization process induced an increase in the amount of chlorophylls, being statistically higher in plants acclimatized in high CO2 than in those cultured in control conditions (Fig. 5a–c). The amounts of Chl a and Chl a + b started to be significantly higher in plants under CO2 enrichment, when compared with control plants, on day 9 while the significant differences in Chl b started on day 5. In contrast, no significant differences in Fv/Fm were found between treatments, although an increment occurred from in vitro to ex vitro, acclimatized plants (Fig. 5d).
The chlorophyll a (Chl a) (a), chlorophyll b (Chl b) (b) and chlorophyll a plus chlorophyll b (Chl a + b) (c) contents and the maximum potential quantum efficiency of photosystem II (Fv/Fm) (d) in Cynara scolymus before, during and after acclimatization at 800 μmol mol−1 CO2 (800) and at the ambient concentration of CO2 (control). Values are mean ± SE (n = 10) (P < 0.005)
Proline
The highest content of proline was detected at the end of the experiment, when the plants were acclimatized (Fig. 6). The level increased gradually in both treatments during the process and no statistical differences were found between them with the exception of day 5, where proline levels were much higher in plants cultured under elevated CO2 than in control plants.
Free amino acids
The free amino acids were measured at the beginning (in vitro plants) and at the end of the experiment in plants from both treatments (800 μmol mol−1 CO2 and control). The total amount of free amino acids was higher in control plants when they were acclimatized than in plants before acclimatization (in vitro) and in plants grown under elevated CO2 (Fig. 7). No statistical differences were found in Asp, His or Met among the in vitro, control and high-CO2 plants. Higher levels of Arg, Val, Ile, Leu, Phe and Tyr were detected in in vitro plants when compared with acclimatized plants, but only Arg and Tyr showed statistically significant differences (Fig. 7). On the other hand, the levels of Gly, Glu and Ala were higher in control than in in vitro or high-CO2 plants and the contents of Ser, Thr and Lys were lower in plants cultured in high CO2 than in plants cultured in vitro and acclimatized at control CO2. The free proline measured was in accordance with the previous analysis presented in Fig. 6.
Serine (Ser), arginine (Arg) glycine (Gly), alanine (Ala), proline (Pro), lysine (Lys), tyrosine (Tyr), glutamic acid (Glu), threonine (Thr) and total aminoacids (aa) contents in Cynara scolymus before (in vitro) and after acclimatization at 800 μmol mol−1 CO2 (800) and at the ambient concentration of CO2 (control). Values are mean ± SE (n = 10) (P < 0.005). Asterisk denotes significant differences (P < 0.005)
Discussion
It is widely acknowledged that CO2 enrichment increases plant growth and production. Nevertheless, only limited information is available about the potential benefits that CO2 may confer on a very sensitive phase, namely plant acclimatization. To date, there are only a few studies in which CO2 enrichment was used to improve plant acclimatization from in vitro to ex vitro conditions. However, there is a lack of information on the physiological and biochemical changes that occur during the transition of tissue-cultured plantlets to fully-acclimatized plants. This study presents a continuous physiological analysis of the plants during the acclimatization process when supplied with high CO2, which resulted in attenuation of the damage produced by desiccation through a shortening of the acclimatization period.
After ex vitro transfer, the plantlets became wilted and dehydrated and thus seemed to be experiencing water deficit due to the change in the environmental conditions. These tissue-cultured plantlets had to adjust from nearly heterotrophic to autotrophic growth, a process that may result in poor growth and mortality of the plantlets (Xiao et al. 2011). The main cause of plantlet loss in the acclimatization phase is dehydration due to a deficient functionality of the stomata (Dias et al. 2014). Stomatal opening and closure are not regulated in the in vitro phase due to the high relative humidity. Thus, the stomata remain open during the in vitro phase and the first days of acclimatization, which causes water loss and compromises plant survival (Chaves et al. 2003; Xiao et al. 2011). In this study, the stimulatory effect of CO2 enrichment provoked earlier control of stomatal closure, hence protecting the plantlets from dehydration and eventually from death. This better control of stomatal closure was revealed by a better water status in plants treated with elevated CO2: specifically, faster decreases in the gs and higher RWC and Ψw. In contrast, Brainerd and Fuchigami (1982) applied 1,200 μmol mol−1of CO2 to in vitro plants for 1 h and the stomata closure was not observed. Thus, CO2 may need a minimum period of time to induce stomatal closure in tissue-cultured plants.
The effect of high CO2 was also manifested as greater acclimatization growth, both in weight and in LA, supporting previous reports that showed that high CO2 increases plant growth even under water stress (Wall et al. 2006; Vu and Allen 2009). It has been reported that the increased growth of plantlets is a consequence of their increased photosynthetic rate, due to the control of ambient conditions (Hahn and Paek 2001; Xiao et al. 2011; Shin et al. 2014). Here, the high CO2 increased the photosynthetic rate—which, along with the better water status, enhanced plant quality, survival and growth. By contrast, the control plants needed more time to acclimatize to the environmental conditions; gs decreased slowly from its in vitro value to reach the level of the plants treated with elevated CO2. Furthermore, the Ψw and ACO2 in control plants never reached the levels of the plants cultured with high CO2 in this experiment, due to the stress that they experienced upon the sudden changes in the environment (Shin et al. 2014). This is supported by the results obtained for WUE. The plants acclimatized with elevated CO2 were able to use water more efficiently than the control plants during the whole experiment. However, the amount of carbon gained along the experiment was the same in both treatments (Ci/Ca).
Water stress contributes to detrimental effects on the photosynthetic apparatus, such as altered chlorophyll components (Xu et al. 2013) and a decreased efficiency of PSII photochemistry (Hamerlynck et al. 2000; Xu and Zhou 2006), which indicates down-regulation of photosynthesis or photoinhibition (Maxwell and Johnson 2000). In this current study, the Fv/Fm value did not differ critically between the control plants and plants cultured with elevated CO2, which indicates that the photosynthetic efficiency and apparatus were not damaged in the control plants and that the rate of photosynthesis was affected not by a malfunction of the photosynthetic apparatus but because of the CO2. Furthermore, the levels of chlorophylls in the control plants were markedly lower than in the plants under elevated CO2. An increment in the chlorophyll content in leaves has been explained as a response to photoautotrophy (Osório et al. 2005); also, chlorophyll degradation may be induced by water stress (Baker et al. 1997). This is supported by the results obtained for the control plants, since the chlorophylls did not start to increase until day 13. The results of this experiment indicate that elevated CO2 not only protects chlorophylls from degradation in the acclimatization process, but also increases the amount of chlorophylls in leaves. Thus, the plant is better able to use the energy from incoming light, allowing better adaptation to the environmental conditions.
The proline levels increased as acclimatization was accomplished in both control and CO2-treated plants, with no differences between them. This result manifests that this stress related molecule is not affected by high CO2 during acclimatization. Proline has been described as a protector, to overcome abiotic stress (Ashraf and Foolad 2007), and it has been found in large amounts in drought resistant cultivars (Rai 2002). However, Dias et al. (2013) found high levels of proline in tissue-cultured plants and lower levels in acclimatized plants. In recent decades, many authors have questioned the role of proline in stress adaptation (Hare and Cress 1997), establishing a relationship between proline accumulation and development (Mattioli et al. 2009). According to our results, proline started to accumulate after day 9, which may mean that the plants were adapting to the environment, growing and being more resistant to dessication. Therefore, proline may act as a protector of new cells in the development process as well as conferring water loss tolerance on the plant as the acclimatization process advances.
Amino acids can modulate membrane permeability and ion uptake, which probably is the major way in which they contribute to drought mitigation (Rai 2002). Rai and Kumari (1983) found that certain amino acids (pro, arg, ala, leu, phe, thr, ser) reduce membrane permeability, by 40–100 %, while others, such as gly, glu and asp, have no effect. The lower levels of these amino acids in plants grown at high CO2, compared with control plants, could be the cause of the increase of the membrane permeability, which was more elevated in plants cultured under high CO2—hence their greater resilience to water stress. Thus, an increase in the EL may be related to a lower content of these amino acids and not only to a rupture of the membrane integrity (Lutts et al. 1996) produced by an increase in lipid peroxidation under elevated CO2. These results agree with previous studies where the plants that acclimatized better had higher EL (Dias et al. 2014).
With the above in mind, this study has demonstrated that acclimatization under CO2 enrichment reduces the plant loss through a reduction of plant acclimatization, alleviating the risk of wilting and water deficiency damage. The use of elevated CO2 in plant acclimatization enhances resistance to desiccation, increases WUE and photosynthesis and accelerates stomatal regulation, resulting in an increase of plant survival and quality.
References
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Baker JT, Allen LH Jr, Boote KJ, Pickering NB (1997) Rice responses to drought under CO2 enrichment. 1. Growth and yield. Glob Chang Biol 3:119–128
Balestrasse KB, Gallego SM, Tomaro ML (2006) Aluminium stress affects nitrogen fixation and assimilation in soybean (Glycine max L.). Plant Growth Regul 48:271–281
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Brainerd KE, Fuchigami LH (1982) Stomatal functioning of in vitro and greenhouse apple leaves in darkness, mannitol, ABA and CO2. J Exp Bot 33:388–392
Buysse J, Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44:1627–1629
Chaves MM (1994) Environmental constraints to photosynthesis in ex vitro plants. In: Lumsden PJ, Nicholas JR, Davies WJ (eds) Physiology, growth and development of plants in culture. Kluwer Academic Publishers, Dordrecht, pp 1–8
Chaves MM, Maroco JP, Pereira JS (2003) Review: understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
del Amor FM, Cuadra-Crespo P, Walker DJ, Camara JM, Madrid R (2010) Effect of foliar application of antitranspirant on photosynthesis and water relations of pepper plants under different levels of CO2 and water stress. J Plant Physiol 167:1232–1238
Dias MC, Pinto G, Guerra C, Jesus C, Amaral J, Santos C (2013) Effect of irradiance during acclimatization on content of proline and phytohormones in micropropagated Ulmus minor. Biol Plant 57(4):769–772
Dias MC, Correia C, Mountinho-Pereira J, Oliveira H, Santos C (2014) Study of the effects of foliar application of ABA during acclimatization. Plant Cell Tissue Organ Cult 117:213–224
Hahn EJ, Paek KY (2001) High photosynthetic photon flux and high CO2 concentration under increased number of air exchanges promote growth and photosynthesis of four kinds of orchid plantlets in vitro. In Vitro Cell Dev Biol Plant 37:678–682
Hamerlynck EP, Huxman TE, Loik ME, Smith SD (2000) Effects of extreme high temperature, drought and elevated CO2 on photosynthesis of Mojave Desert evergreen shrub, Larrea dridentata. Plant Ecol 148:183–193
Hare PD, Cress WA (1997) Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hazarika BM (2006) Morpho-physiological disorders in in vitro culture of plants. Sci Hortic 108:105–120
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Kozai T, Zobayed S (2000) Acclimatization. In: Spier RE (ed) Encyclopaedia of cell technology. Wiley, New York, pp 1–12
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Mattioli R, Costantino P, Trovato M (2009) Proline accumulation in plants: not only stress. Plant Signal Behav 4:1016–1018
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51(345):658–659
McKay HM (1992) Electrolyte leakage from fine roots of conifer seedlings: a rapid index of plant vitality following cold storage. Can J For Res 22:1371–1377
Mena-Petite A, Ortega-Lasuen U, Gonzalez-Moro B, Lacuesta M, Munoz-Rueda A (2001) Storage duration and temperature effect on the functional integrity of container and bare-root Pinus radiata D. Don stock-types. Trees 15:289–296
Osório ML, Gonçalves S, Osório J, Romano A (2005) Effects of CO2 concentration on acclimatization and physiological responses of two cultivars of carob tree. Biol Plant 49(2):161–167
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll a and chlorophyll b extracted with 4 different solvents —verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975:384–394
Rai VK (2002) Role of amino acids in plant responses to stresses. Biol Plant 45(4):481–487
Rai VK, Kumari A (1983) Modulation of membrane permeability by amino acids in Vinca petals. Experiencia 39:301–303
Rodriguez R, Aragon CE, Escalona M, Gonzalez-Olmedo JL, Desjardins Y (2008) Carbon metabolism in leaves of micropropagated sugarcane during acclimatization phase. In Vitro Cell Dev Biol Plant 44:533–539
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346
Shin K-S, Park S-Y, Paek K-Y (2014) Physiological and biochemical changes during acclimatization in a Doritaenopsis hybrid cultivated in different microenvironments in vitro. Environ Exp Bot 100:26–33
Toler JE, Adelberg JW, Bishop D (2003) Growth and net photosynthetic rates of Hosta ‘blue vision’ during acclimatization in bright, natural light with CO2 enrichment. In Vitro Cell Dev Biol Plant 39:338–342
Vu JCV, Allen LH Jr (2009) Growth at elevated CO2 delays the adverse effects of drought stress on leaf photosynthesis of the C4 sugarcane. J Plant Physiol 166:107–116
Walker DJ, Romero P, de Hoyos A, Correal E (2008) Seasonal changes in cold tolerance, water relations and accumulation of cations and compatible solutes in Atriplex halimus L. Environ Exp Bot 64:217–224
Wall GW, Garcia RL, Kimball BA, Hunsaker DJ, Pinter PJ, Long SP, Osborne CP, Hendrix DL, Wechsung F, Wechsung G, Leavitt SW, LaMorte RL, Idso SB (2006) Interactive effects of elevated carbon dioxide and drought on wheat. Agron J 98:354–381
Waters (2006) UPLC amino acid analysis solution. Waters Corporation, Milford
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult 105:149–158
Xu ZZ, Zhou GS (2006) Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 224:1080–1090
Xu Z, Shimizu H, Yagasaki Y, Ito S, Zheng Y, Zhou G (2013) Interactive effects of elevated CO2, drought, and warming on plants. J Plant Growth Regul 32:692–707
Acknowledgments
This work was financed by the European Social Fund (ESF) European Union-FEDER 80 %—Región de Murcia (PO07-034). MC Piñero gratefully acknowledges a doctoral contract in the INIA-CCAA system, supplied by INIA and cofinanced by the ESF. We thank Dr. David J. Walker for assistance with the correction of the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Jiménez, M., López-Pérez, A.J., Otálora-Alcón, G. et al. A regime of high CO2 concentration improves the acclimatization process and increases plant quality and survival. Plant Cell Tiss Organ Cult 121, 547–557 (2015). https://doi.org/10.1007/s11240-015-0724-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0724-4