Abstract
The activity of the main enzymes related to the sucrose metabolism, photosynthesis, and sucrose concentration were studied in sugarcane (Saccharum spp hybrid) plantlets. Acclimatization was developed in two steps. (1) Light intensity of 1,000 μmol m−2 s−1 and 90% relative humidity during the first 21 d; followed by 2,000 μmol m−2 s−1 and approximately 80% of relative humidity. All measurements were carried out at the end of rooting phase concomitant with day 0 of acclimatization and at 7-d intervals thereafter (0, 7, 14, 21, 28, 35, 42 d). As the in vitro plantlets were transferred to the acclimatization phase, photosynthesis increased significantly during the first 7 d. After this period, the increase was constant with only a small but nonsignificant decline after being transferred to the uncontrolled external conditions. The activity of the sucrose synthase began to show a decrease, starting from day 7, and was related to the changes that began to happen in these plants from its adaptation to new ex vitro conditions. Due to the increase of fresh weight favored by the high light intensity and lower relative humidity, an increase of the sucrose phosphate synthase activity was observed. The maximum activity of the acid and neutral invertases was reached at 14 and 21 d, respectively, after 21 d of acclimatization period. There was a marked tendency for the activity of both enzymes to decrease. The sucrose content was decreased only in the first 7 d. The metabolism of sugarcane plantlets seemed to be susceptible to the environmental changes during the acclimatization phase but did not contribute to inhibitory factors for normal development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Limited information is available on the physiological changes that occur during the transition of micropropagated plantlets from in vitro to the acclimatization phase. This acclimatization phase is critical within micropropagation. In this phase, the plants can be classified in two fundamental groups: (1) those that are completely incompetent and unable to adapt to the new ex vitro conditions and require replacing all leaves and (2) those whose leaves need gradual changes in order to adapt themselves to photoautotrophic conditions. This classification, however, is rather arbitrary and dependent on the culture conditions, as shown by Yue et al. (1993) for strawberry.
The use of photomixotrophic conditions (increase of light intensity and CO2 atmosphere inside of culture flask) improves plant quality and can lead to a greater efficiency during the acclimatization phase (Hymus et al. 2001; Kadlecek et al. 2001; Nguyen and Kozai 2001; Vanle et al. 2001). The main indicators that characterize the gradual change of heterotrophic to autotrophic metabolism in the plantlets are based on the structural and functional development during this stage (Van Huylenbroeck and De Riek 1995; Van Huylenbroeck and Debergh 1995; Van Huylenbroeck et al. 1998; Pospisilova et al. 1999; Ulman et al. 2000).
The effects of both light and relative humidity on photoinhibition, sucrose metabolism, oxidative metabolism, physiological change, and photosynthetic activity have been described for Spathiphyllum and Calathea (Van Huylenbroeck 1994; Van Huylenbroeck and De Riek 1995; Van Huylenbroeck et al. 1998, 2000) and grapevine and chestnut (Carvalho and Amancio 2002).
Sucrose is the major transport sugar in higher plants and the principal form that carbon fixed in photosynthesis is exported from mature source leaves. Two enzymes may cleave imported sucrose: Invertases (EC 3.2.1.26) which catalyzes the cleavage of sucrose to glucose + fructose and sucrose synthase (EC 2.4.1.13) which catalyzes the reversible interconvention of sucrose and uridine diphosphate (UDP) to UDP-glucose + fructose. The sucrose phosphate synthase (SPS) enzyme (EC 2.4.1.14) catalyzes the production of sucrose phosphate from the monosaccharide UDP-glucose and fructose 6-phosphate (Stitt and Quick 1989). In this study, the sucrose metabolism and photosynthetic activity of micropropagated sugarcane plantlets were examined during acclimatization process.
Material and Methods
Plant material and growth conditions. Sugarcane plantlets (Saccharum spp. hybrid) were propagated in temporary immersion bioreactors (TIB) using the protocol of Lorenzo et al. (1998). The TIB is constituted of two transparent glass containers: one for growing plants and one as a reservoir for liquid medium. The two containers were connected by silicone tubes. In each case, the airflow was sterilized by passage through 0.2-μm hydrophobic filters. Positive pressure from an air compressor pushed the medium from one container to the other to immerse the plants completely. The airflow was reversed to withdraw the medium from the culture container. Electronic timers controlled the frequency and length of the immersion period. Three-way solenoid valves provided on–off operation. In brief, five axillary shoots were cultured in a 1-L bioreactor, containing 250-mL MS medium (Murashige and Skoog 1962) with 1.33 μM benzyladenine (Sigma, St. Louis, Mo) +3.40 μM paclobutrazol (Sigma) for 30 d. The plantlets were then placed in an MS medium with 2.88 μM gibberellic acid (Sigma) for an additional 15 d. The photoperiod was 16 h light at a photosynthetic photon flux (PPF) of 75 μmol m−2 s−1. The plantlets produced in the bioreactor were transferred to 144-cell multitray containers for acclimatization (52.5 × 29.5 × 4 (H) cm) with a sterilized mixture of substrate composed of filter cake and sugarcane ashes (1:1, v:v). The filter cake was derived from sugarcane plants at the end of the industrial process. Uniform micropropagated plantlets of approximately 5.34-cm height, 0.31–0.50-g fresh weight, and three to four true leaves were selected from the glass culture vessels and were transferred to a greenhouse to 90% relative humidity (RH), a PPF of 1,000 μmol m−2 s−1, and 27 ± 2°C for the first 21 d of acclimatization, followed by removal from the environmentally controlled condition to a natural environment in field of 80% RH and PPF of 2,000 μmol m−2 s−1; in both conditions, the photoperiod was 16 h light. The climatic variables under both conditions were measured at noon during experiment. The obtained data were averaged. The climatic variables of growing of the sugarcane plant during natural environment are shown in Table 1.
Photosynthesisand physiological measurements. Photosynthesis measurements were carried out during the in vitro elongation phase, at the end of the rooting phase concomitant with day 0 of acclimatization phase and at 7-d intervals thereafter (0, 7, 14, 21, 28, 35, 42 d).
Fully expanded sugarcane leaves (leaf 3 and 4 from the top) were collected early in the morning (10:00–11:00 am) and used for all photosynthesis and physiological measurements. A portable photosynthesis system (LI-COR 6400) equipped with a LED Light Source attachment (6400-02) was used. The photosynthesis measurements were conducted at ambient relative humidity and air temperature. The Infra-Red Gas Analyzer was automatically matched before every measurement. The light was fixed at 600 μmol m−2 s−1 for the determination of the A/Ci. (net photosynthesis/internal CO2 concentration).
At the time of the photosynthesis measurement, separate plant samples were collected and growth parameters were taken, specifically fresh (g) and dry weight (g; fw and dw). For dry weight determination, plants were dried for 48 h at 90°C.
Sucrose determinations. Leaf samples were taken before 10:00 am and immediately frozen in liquid N2 and stored at −80°C until use. For sucrose extraction, 200 mg of frozen tissue was ground to a very fine powder with a mortar and pestle in liquid N2. Sucrose was extracted by the addition of 1 mL of ethanol 70% (v/v); then, the solution was placed at 90°C for 60 min. We took 1 mL of the supernatant was taken and concentrated it at 0°C in a Speed-vac concentrator and redissolved in 100-mL distilled sterile H2O. The solution then was refiltered through a Sep-Pak C 18 cartridge and 0.45-μm membrane filters, using a syringe. The sucrose was measured by high-resolution liquid chromatography. Pharmacia LKB equipment with an IR detector and an HPX-87 H (BIORAD, Hercules, CA 300 × 7.8 mm) column was used for the determination of the sucrose. Sulfuric acid (0.005 M) was used in proportion with 0.3 mL min−1, as the mobile phase. The volume injected was 20 μL. As is standard, sucrose was used at 1 mg mL. The results were expressed in milligram of sucrose per gram fresh weight.
Enzyme extraction and assays. Leaf samples were taken before 10:00 am and immediately frozen in liquid N2 and stored at −80°C until use. A 200-mg frozen tissue was ground to a very fine powder with a mortar and pestle in liquid N2. The enzyme was extracted by the method described by Geigenberger and Stitt (1991).
The Vmax of SPS (EC 2.4.1.14) was determined in 70 μL of an assay medium containing 50 μM Hepes–KOH pH 7.5, 15 μM MgCl2, 10 μM fructose-6-phosphate (Sigma), 40 μM glucose-6-phosphste (Sigma), 10 μM UDP-glucose (Sigma), and 50 μL of extract. The mixture was incubated for 20 min at 30°C. The incubation was terminated with the addition of 70 μL of 30% KOH. The enzyme blanks were immediately terminated with 30% KOH at 0 min. Sucrose synthase (SS; EC 2.4.1.13) was assayed as described above but with 10 μM fructose instead of 10 μM fructose-6-phosphate (Sigma) and in the absence of Glc-6-phosphate, according to the method of Miron and Schàffer (1991). The Vmax of soluble invertase (EC 3.2.1.26) was assayed in a reaction mixture (500 μL total volume) containing desalted enzyme extract, 50 μM sucrose, and a citrate–phosphate buffer with a pH of 5.0 for the acid invertase and 50 μM Hepes–NaOH pH 7.0 for the neutral invertase. The reactions were initiated by the addition of enzyme extract. The solution was then incubated for 20 min at 30°C. The resulting hexose was determined enzymatically, according to the method of Miron and Schàffer (1991).
Statistical analysis. At each sampling date, six plantlets were randomly selected to measure photosynthesis. For sucrose and enzymatic determination, randomized plantlets were used. Three extraction in each evaluation moments and three repetitions for each extraction were performed. Twenty plantlets were used for measuring fresh and dry weight.
Analysis of variance was conducted using SPSS Program. Duncan’s multiple range tests were used for mean separation at the p < 0.05 level.
Results and Discussion
The sugarcane plantlets had a positive net photosynthesis (0.65 μmol CO2 m−2 s−1) at the end of in vitro period (0 d in acclimatization). During the first week of acclimatization, the photosynthesis activity significantly increased (1.90 μmol CO2 m-2s-1) due to change in climatic conditions. At 14 d, a maximum and stable value was measured during the acclimatization process (Fig. 1).
Only on the 28th day, an insignificant decrease was observed. This reduction was related to the change of the new environmental conditions (from 1,000 μmol m−2 s−1 and 90% RH to 2,000 μmol m−2 s−1 and 80% RH) where the plantlets were transferred at 21 d. A decline in carbon exchange rate was observed in sugarcane leaf growth in the greenhouse, which was caused by CO2 deficit, that is, stomatal closure, not by biochemical reactions (Du et al. 1998). The results show that in vitro leaves of sugarcane are photosynthetically competent and show photosynthetic activity during acclimatization phase. As is typical in C4 species, photosynthesis in sugarcane was tolerant to relatively high environmental stress (Crafts-Brandner and Salvucci 2002). Nevertheless, the photosynthetic activity was low compared with the values obtained in plantain plantlets (C3 species) cultivated under similar in vitro conditions (Aragón et al. 2005). Maybe the dual way to fix carbon dioxide in the sugarcane plants as C4 metabolism are the reason for the not-so-high photosynthetic activity in this young plants. Other authors refer that the in vitro photosynthesis may have the problem of the leaf conductance to CO2 diffusion (Fila et al. 2006). In this case, this could be the problem for the low in vitro photosynthesis activity, even when the micropropagation systems were completely different (TIB).
During the first 3 d of Calathea leaf acclimatization, a reduction in photosynthetic activity was measured, which later increased towards the end of the process. However, Spathiphyllum plantlets showed a photosynthetic (1.15 μmol CO2 m−2 s−1) light response immediately after being transferred to ex vitro conditions (Van Huylenbroeck et al. 1996). The results suggest that during the first 14 d of acclimatization, plantlets use the reserves obtained during the in vitro culture. During the ex vitro stage, the enzymes related to the degradation of sucrose displayed maximum activity. At 14 d, conditions began to be observed in the plantlets. The increase in the photosynthesis levels was in accordance with development and evolution of the plants. Figure 2 shows the fresh and dry weight dynamics evaluated during 42 d in acclimatization.
In the 7- to 14-d interval, plantlet dry weight quadrupled (0.04 to 0.15 g), while fresh weight doubled (0.52 to 1.04 g), although statistical differences were not observed. At the end of the acclimatization phase, both variables reach the maximum values. Interesting behavior was observed in the increment of the fresh and dry weight in only 7 d (from the 14th day to the 21st day). It was related to the new rooting emission (data not shown). This behavior is associated with new and autotrophic tendencies in the plantlets. After 21 d, plantlets were changed to a new acclimatization atmosphere (2,000 μmol m−2 s−1 and 80% RH). Consequently, a slight but nonsignificant decrease in the fresh and dry weight was observed. Sugarcane plantlets propagated in the temporary immersion bioreactor seemed to have sufficient reserves during the first week of the acclimatization, which helped in the constant increase of both variables.
During the early months of sugarcane crop growth and development, the majority of the daily produced photoassimilates were used by the plant in order to increase body mass; moreover, the number and size of leaves, stalks, and roots were all rapidly multiplying (Moore and Maretzki 1999). Similar behavior was observed in the fresh and dry weight after 14 d in sugarcane plantlets during acclimatization phase. When the plantlets were transferred to the acclimatization phase, the metabolism began a readjustment process and reconstruction until reaching the total photoautotrophic development. An increase in the foliar area and dry mass of red pepper plants (Capsicum annuum L. cv. San Luis) after 6 d in acclimatization was observed by Estrada-Luna et al. (2001); this increase was higher at 12 d when the plantlets began their autotrophic behavior. In grapevine (Vitis vinifera L.) plantlets under high-intensity light (300 μmol m−2 s−1), the biomass increased sevenfold when compared with the threefold in plantlets grown under low-intensity light (150 μmol m−2 s−1). However, in chestnut (Castanea sativa × Castanea crenata), no significant differences between the light treatments were detected, but both ended up with a twofold increase in biomass when compared to in vitro plantlets (Carvalho et al. 2001).
As photosynthesis is responsible for the plant’s energy and carbon incorporation into the plant, the acclimatization of the photosynthetic apparatus is crucial for its survival, and also for acclimatization of other metabolic processes in the shoot and root system. The sucrose levels found in sugarcane leaves during acclimatization are presented in Fig. 3.
The high sucrose concentration (0.033 mg g−1 fw) in sugarcane leaves at the end of in vitro treatment (0 d in acclimatization) is explained by the presence of an external carbon source in the culture medium. In only 7 d after transplant, the sucrose concentration was significantly reduced to 0.016 mg g−1 fresh weight; after that, a significant increase was observed at 14 d (0.032 mg g−1 fw). When the plantlets were changed to new environmental conditions (21 d), the sucrose level remained stable, and no significant differences were observed. At the end of the acclimatization period (42 d), significant higher sucrose concentrations were measured in sugarcane leaves (0.056 mg g−1 fw).
The leaves became autotrophic, and their carbon requirement was fulfilled by photosynthesis and possibly by transient sucrose storage. The sucrose produced by photosynthesis in the leaves is loaded into the phloem by an aproton-sucrose symporter. The rapid turnover of sucrose between the apoplastic space, cytosol, and the vacuole characterized in sugarcane mature cells (Wedler et al. 1990) is proposed to enable the plant to respond rapidly to environmental changes (Moore 1995). In sugarcane leaves under mild water stress (−0.9 MPa), Du et al. (1998) found that the sucrose contents was not significantly affected.
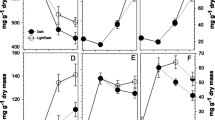
In Spathiphyllum “petite” plantlets during acclimatization, Van Huylenbroeck and De Riek (1995) found high increases in all carbohydrate pools at the end of acclimatization; these increases were related to enzymatic sucrose metabolism. These authors found that by 20 d in acclimatization, all measured carbohydrates were significantly lower in the newly formed leaves compared to those formed in vitro. The changes in sucrose concentration are governed by the activities of the different key enzymes of sucrose metabolism (Fig. 4).
Changes in sucrose synthase (A), sucrose phosphate synthase (B), and acid and neutral invertase (C, D) activities during the acclimatization of micropropagated sugarcane plantlets. Means followed by different letters within panels are significantly different using ANOVA, Duncan’s test, p < 0.05, n = 9.
SS (Fig. 4 A) in leaves showed high enzymatic activity at 7 d of being exposed to the acclimatization condition, followed by continuous reduction of this activity at the end of the process. During the first 7 d in acclimatization, the sugarcane plantlets used the reserves accumulated in vitro; this is in agreement with the high activity displayed by the enzyme. This specialized enzyme in the sucrose transformation to glucose and fructose was not important in the plants when they began to acquire an autotrophic capacity. It is probable that the high degree of heterotrophic conditions developed by the plantlets in vitro was the cause by which the activity of this enzyme was still increased during the first moments of being transferred to ex vitro conditions.
SS from sycamore cells (Acer pseudoplatanus L.) has a much lower k m for sucrose compared with the neutral invertase. Consequently, the SS pathway may be relatively more important when sucrose availability is limited. This pathway is also more energetically efficient, as the energy is preserved (Huber and Akazawa 1986).
Nguyen-Quoc et al. (1990) found that activity in the extracts of young leaves suggested that either invertase or SS may have a major role in sucrose cleavage, depending on the plant selected. Both SS and acid invertase activity decline during leaf expansion. The researchers found that SS activity was high in heterotrophic maize leaf tissue but was almost undetectable in mature green tissue.
In maize, two isoenzymes of SS (SS1 and SS2) have been identified, encoded by two different genes. In maize leaves, the young cells are full sucrose importers and SS activity is maintained at a very high level, as long as these leaves are not exposed to light. The SS is rapidly degraded at a time when the photosynthesis machinery is built up (Nguyen-Quoc et al. 1990).
Maximum activity in SPS was obtained at 21 d in acclimatization (Fig. 4 B). The activity of the enzyme significantly diminished at the moment when the plantlets were transferred to the acclimatization conditions (2,000 μmol m−2 s−1 and 80% RH). Starting from 35 d, an increase was already observed; this is perhaps due to the adaptation of the photosynthetic apparatus of the sugarcane plantlets in the new acclimatization conditions. The development obtained by the leaves at 14 d was in agreement with the high activity of SPS. Possibly, the formation of new leaves entails the development of a new and photoautotrophic photosynthetic apparatus, which produces enough sucrose content necessary for normal growth in sugarcane plantlets. This enzyme was evaluated in plantain plantlets previously propagated in TIB and the results were different. The enzymes only showed an increase in the first week of the acclimatization phase, due to the development of this enzyme during the in vitro conditions and that ex vitro is unnecessary (Aragón et al. 2005). In plantain plantlets, the starch was described as the main carbon energetic source, so the SPS is not necessary during the ex vitro phase for the sucrose synthesis (Aragón et al. 2006). At the end of the acclimatization phase, a decrease of the SPS (28–35 d) was observed, while the sucrose content kept increasing at this week. There are few possible reasons for this, one of them could be that the sugarcane leaves accepts sucrose from another part of the plant to contribute to the development of this organ. The results in the fresh and dry weight (Fig. 2) demonstrate that the plant leaves continue the anabolic process, even when the SPS synthase activity decrease.
The increase in the activity of SPS in leaves was related to the chlorophyll content and the photosynthetic activity in maize leaves (Nguyen-Quoc et al. 1990). In sugarcane leaves, the chlorophyll content had decreased during the first 7 d in acclimatization (data not shown); however, the photosynthesis activity had increased (Fig. 1).
The sugarcane plantlets seemed to be susceptible to environmental changes during the acclimatization process. Changes in the photosynthetic activities and the fresh weight occurred as the activity of SPS showed substantial changes at high-intensity light (2,000 μmol m−2 s−1) and low relative humidity (80%) in ex vitro conditions. After this period, a favorable development was observed, which was related to an increase in an unknown variable evaluated during the acclimatization process. In vivo, illumination of the leaves resulted in dephosphorylation (activation) of SPS catalyzed by a protein phosphatase (Huber and Huber 1990). However, in tomato (Lycopersicon esculentum Mill.) leaves, the SPS activity depended on the growth conditions. Under normal in vitro growth conditions, an increase of 64% was found in these tomatoes, while a decrease of 47% was observed under enriched condition of more light and CO2 (Vanle et al. 2001).
In mature leaves of Lolium temulentum, the activity of sucrose phosphate synthase was at least tenfold higher than that of sucrose synthase but almost tenfold lower than that of soluble acid invertase (Pollock and Lloyd 1977). Maximum activities in acid and neutral invertases were reached at 14 and 21 d, respectively (Fig. 4 C, D). At that moment, a significant decrease of the activity in both enzymes was observed. It has been suggested that the activity of these enzymes are associated with the degradation of sucrose fundamentally in the storage organs (Moore and Maretzki 1999). The acid invertases are more related with the mobilization of sucrose inside the plantlets because of the presence of them in the apoplastic part of the cell (Nguyen-Quoc et al. 1990). In plantain plantlets, this enzyme shows the higher activities at the beginning of the in vitro phase in TIB and at the end of the acclimatization, while in sugarcane it was at the beginning of the acclimatization phase (Aragón et al. 2005). The differences could be due to the pattern of response of both species, where possibly because of the more rapid reaction of sugarcane during the first part of the acclimatization. One of the most important aspects in the adaptation of the plant to the acclimatization conditions is the capacity of the mobilization of substances of reserve (Van Huylenbroeck and De Riek 1995).
Changes in invertase activity can occur under a range of environmental conditions and tend to provide evidence for a significant role for invertases in leaves, in terms of the regulation of sucrose metabolism. The suppression of growth in barley by a nitrogen deficiency led to a decline in the invertase activity in mature leaves, accompanied by a switch to the storage of fructose (Wang and Tillberg 1996).
In conclusion, during the acclimatization of sugarcane plantlets, it was observed that, during the first 7 d after transplanting the plantlets, there was a reduction of sucrose content and invertase activity; after the 7-d period, the leaves changed from heterotrophic to autotrophic metabolism, and the carbon requirement was supplied by photosynthesis activity. During this change, the photosynthetic apparatus provides sucrose for normal plant development and biomass production.
References
Aragón C.; Escalona M.; Capote I.; Pina P.; Cejas I.; Rodríguez R. et al. Photosynthesis and carbon metabolism in plantain (Musa AAB) growing in temporary immersion bioreactor (TIB) and ex vitro acclimatization. In Vitro Cell Dev Biol-Plant 414: 550–554; 2005 doi:10.1079/IVP2005640.
Aragón C.; Escalona M.; Capote I.; Pina P.; Cejas I.; Rodríguez R. et al. Aclimatización de plantas de plátano “CEMSA 3/4 (AAB)” provenientes de Biorreactores de Inmersión Temporal. Importancia metabólica del almidón INFOMUSA 152: 32–35; 2006.
Carvalho L. C.; Amancio S. Antioxidant defence system in plantlets transferred from in vitro to ex vitro: Effects of increasing light and CO2 concentration. Plant Sci 1621: 33–40; 2002 doi:10.1016/S0168-9452(01)00524-6.
Carvalho L. C.; Osorio M. L.; Chaves M. M.; Amancio S. Chlorophyll fluorescence as an indicator of photosynthetic functioning of in vitro grapevine and plantlets under ex vitro acclimatization. Plant Cell Tiss Org Cult 67: 271–280; 2001.
Crafts-Brandner S. J.; Salvucci M. E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129: 1773–1780; 2002 doi:10.1104/pp.002170.
Du Y. C.; Nose A.; Wasano K.; Uchida Y. Response to water stress of enzyme activities and metabolite levels in relation to sucrose and starch synthesis, the Calvin cycle and C4 pathway in sugarcane (Saccharum sp). Aust J Plant Physiol 25: 253–260; 1998.
Estrada-Luna A. A.; Davies F. T.; Egilla J. N. Physiological change and growth of micropropagated chile ancho pepper plantlets during acclimatization and post-acclimatization. Plant Cell Tiss Org Cult 66: 17–24; 2001 doi:10.1023/A:1010606430060.
Geigenberger P.; Stitt M. A futile cycle of sucrose synthesis and degradation is involved in regulating partitioning between sucrose starch and respiration in cotyledons of germinating Ricinus comunis L. seedlings when phloem transport is inhibited. Planta 185: 81–90; 1991.
Fila G.; Badeck F.; Meyer S.; Ceroviv Z.; Ghashghaie J. Relationships between leaf conductance to CO2 diffusion and photosynthesis in micropropagated grapevine plants, before and after ex vitro acclimatization. J Exp Bot 5711: 2687–2695; 2006 doi:10.1093/jxb/erl040.
Huber S. C.; Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol 81: 1008–1013; 1986.
Huber S. C.; Huber J. L. Activation of sucrose-phosphate synthase from darkened spinach leaves by an endogenous protein phosphatase. Arch Biochem Biophys 282: 421–426; 1990 doi:10.1016/0003-9861(90)90138-O.
Hymus G. J.; Baker N. R.; Long S. P. Growth in elevated CO2 can both increase and decrease photochemistry and photoinhibition of photosynthesis in a predictable manner. Dactylis glomerata grown in two levels of nitrogen nutrition. Plant Physiol 127: 1204–1211; 2001 doi:10.1104/pp.127.3.1204.
Kadlecek P.; Ticha I.; Haisel D.; Capkova V.; Schafer C. Importance of in vitro pretreatment for ex vitro acclimatization and growth. Plant Sci 161: 695–701; 2001 doi:10.1016/S0168-9452(01)00456-3.
Lorenzo J. C.; Gonzalez B. L.; Escalona M.; Teisson C.; Espinosa P.; Borroto C. Sugarcane shoots formation in an improved temporary immersion system. Plant Cell Tiss Org Cult 54: 197–200; 1998 doi:10.1023/A:1006168700556.
Miron D.; Schaffer A. A. Sucrose phosphate synthase, sucrose synthase and invertase activities in developing fruit of Lycopersicon hirsutum Humb. Plant Physiol 95: 623–627; 1991.
Moore P. H. Temporal and spatial regulation of sucrose accumulation in the sugarcane stem. Aust J Plant Physiol 22: 661–679; 1995.
Moore P. H.; Maretzki A. Sugarcane. In: ZamskiE.; SchafferA. A. (eds) Photoassimilate distribution in plants and crops, vol. 27. Marcel Dekker, New York, pp 643–665; 1999.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15: 473–479; 1962 doi:10.1111/j.1399-3054.1962.tb08052.x.
Nguyen-Quoc B.; Krivitzki M. S.; Huber C.; Lecharny A. Sucrose synthesis in developing maize leaves. Plant Physiol 94: 516–523; 1990.
Nguyen Q. T.; Kozai T. Growth of in vitro banana (Musa spp.) shoots under photomixotrophic and photoautotrophic conditions. In Vitro Cell Dev Biol-Plant 37: 824–829; 2001 doi:10.1007/s11627-001-0137-4.
Pollock C. J.; Lloyd E. J. The distribution of acid invertase in developing leaves of Lolium temulentum L. Planta 133: 197–200; 1977 doi:10.1007/BF00391919.
Pospísilová J.; Synkova H.; Haisel D.; Catsky J.; Wilhelmova N.; Sramek F. Effect of elevated CO2 concentration on acclimatization of tobacco plantlets to ex vitro conditions. J Exp Bot 50330: 119–126; 1999 doi:10.1093/jexbot/50.330.119.
Stitt M.; Quick W. P. Photosynthetic carbon partitioning: its regulation and possibilities for manipulation. Physiol Plant 77: 633–641; 1989 doi:10.1111/j.1399-3054.1989.tb05402.x.
Ulman P.; Catsky J.; Pospísilová J. Photosynthetic traits in wheat grown under decreased and increased CO2 concentration, and after transfer to natural CO2 concentration. Biol Plant 432: 227–237; 2000 doi:10.1023/A:1002752210237.
Vanle L.; Samson G.; Desjardins Y. Opposite effects of exogenous sucrose on growth, photosynthesis and carbon metabolism of in vitro plantlets of tomato (Lycopesicon esculentum Mill.) grown under two levels of irradiance and CO2 concentration. J Plant Physiol 158: 599–605; 2001 doi:10.1078/0176-1617-00315.
Van Huylenbroeck J. M. Influence of light stress during the acclimatization of in vitro plantlets. In: StruikP. C. (ed) Plant production on the threshold of a new century. Kluwer Academic, Dordrecht, pp 451–453; 1994.
Van Huylenbroeck J. M.; De Riek J. Sugar and starch metabolism during ex vitro rooting and acclimatization of micropropagated Spathiphyllum “petite” plantlets. Plant Sci 111: 19–25; 1995 doi:10.1016/0168-9452(95)04223-H.
Van Huylenbroeck J. M.; Debergh P. C. Impact of sugar concentration in vitro on photosynthesis and carbon metabolism during ex vitro acclimatization of Spathiphyllum plantlets. Physiol Plant 96: 298–304; 1996 doi:10.1111/j.1399–3054.1996.tb00217.x.
Van Huylenbroeck J. M.; Huygens H.; Deberg P. C. Photoinhibition during acclimatization of micropropagated Spathiphyllum ‘petite’ plantlets. In Vitro Cell Dev Biol-Plant 31: 160–164; 1995 doi:10.1007/BF02632013.
Van Huylenbroeck J. M.; Piqueras A.; Debergh P. C. Photosynthesis and carbon metabolism in leaves formed prior and during ex vitro acclimatization of micropropagated plants. Plant Sci 134: 21–30; 1998 doi:10.1016/S0168-9452(98)00043-0.
Van Huylenbroeck J. M.; Piqueras A.; Debergh P. C. The evolution of photosynthesis capacity and the antioxidant enzymatic system during acclimatization of micro propagated Calathea plants. Plant Sci 155: 59–66; 2000 doi:10.1016/S0168-9452(00)00201-6.
Wang C. W.; Tillberg J. E. Effects of nitrogen deficiency on accumulation of fructan and fructan metabolizing enzyme activities in sink and leaves of barley (Hordeum vulgare). Physiol Plant 97: 339–345; 1996 doi:10.1034/j.1399-3054.1996.970218.x.
Wedler R.; Veinth R.; Dancer J.; Stitt M.; Komor E. Sucrose storage in cell suspension culture of Saccharum sp. (sugarcane) is regulated by a cycle of synthesis and degradation. Planta 183: 31–39; 1990.
Yue D.; Gosselin A.; Desjardins Y. Re-examination of the photosynthetic capacity of in vitro-culture strawberry plantlets. J Am Soc Hort Sci 118: 419–424; 1993.
Acknowledgement
We thank The International Foundation for Science (IFS) for their financial support of this work (Project C/3570).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Michael Edward Kane
Rights and permissions
About this article
Cite this article
Rodriguez, R., Aragon, C.E., Escalona, M. et al. Carbon metabolism in leaves of micropropagated sugarcane during acclimatization phase. In Vitro Cell.Dev.Biol.-Plant 44, 533–539 (2008). https://doi.org/10.1007/s11627-008-9142-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-008-9142-1