Abstract
The effects of two elicitors: jasmonic acid and methyl jasmonate on cell growth as well as on rosmarinic acid accumulation in cell suspension cultures of Mentha × piperita were investigated. The highest rosmarinic acid accumulation 117.95 mg g−1 DW (12% DW) was measured 24 h after addition of 100 μM methyl jasmonate. A similar concentration 110.12 mg g−1 DW was detected 48 h after application of 200 μM jasmonic acid. Those values were nearly 1.5 times higher compared to the control sample, without elicitation. There was no substantial influence of elicitors on rosmarinic acid secretion into the culture media. Extracellular concentrations of rosmarinic acid were similar to the values from the control variants. It was documented that suspension cultures of M. piperita treated with elicitors showed a decrease in biomass accumulation when compared to the control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants constitute basic ingredients of our everyday diet. Moreover, their nutritional and medicinal values have been known since ancient centuries. Numerous studies have demonstrated the relationship between the diet and the health status. Diet rich in bioactive phytochemicals was shown to have health enhancing as well as disease preventing properties. This belief has led to expansion of dietary supplements, phytonutrients and nutraceuticals market (Dillard and German 2000; Zhao 2007). Among them, externally supplied antioxidants effectively counteract oxidative damages in the human body. Oxidative stress is a leading factor in the development of cardiovascular diseases, hypertension, neurodegenerative disorders, cancers, arthritis and aging (Shahidi and Chandrasekara 2010). In this context, ready-to-eat snacks were established within the NUTRA-SNACK project funded by the European Commission in FP6. They were enriched with antioxidant/radical-scavenging compounds which are able to counteract the oxidative stress that daily threatens our health (Rea et al. 2011).
Rosmarinic acid (RA) is one of the most common caffeic esters found in plants. It occurs mainly within Boraginaceae and Lamiaceae families, however, it is also a constituent of other 13 plant families (Petersen and Simmonds 2003; Petersen et al. 2009). RA is an ingredient of several medicinal plants as well as common culinary herbs such as basil, lavender, lemon balm, marjoram, mint, oregano, rosemary, sage and thyme (Shetty 2007; Petersen et al. 2009). In plants, this molecule is supposed to act as a constitutively accumulated defense compound against pathogens and herbivores due to its tannin-like properties (Szabo et al. 1999).
RA reveals several biological activities, however the most important is its high antioxidant capacity (Chen and Ho 1997; Lu and Foo 2001, Soobrattee et al. 2005). This activity is mainly due to RA redox properties, which play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen or decomposing peroxides (Furtado et al. 2008). Among other factors antioxidant activity of polyphenols is attributed to their hydroxyl groups. Ferulic acid has one hydroxyl group, caffeic acid contains two, while RA posseses four of them (ortho-dihydroxybenzene/catechol structure). They are responsible for enhancement of its antioxidant efficiency. RA showed the strongest DPPH scavenging activity in different in vitro assays comparing with other hydroxycinnamic acids (Chen and Ho 1997). Furthermore, RA is also considered as an astringent agent. It shows anti-inflammatory activity, antimutagenic ability, antiviral activity, as well as antibacterial properties (Petersen and Simmonds 2003). Literature data reveal its antiviral activity against Herpes simplex virus 1 (HSV-1) (Sanchez-Medina et al. 2007) and Human immunodeficiency virus (HIV-1) as well (Mazumder et al. 1997; Tewtrakul et al. 2003). Other studies reported RA antimicrobial activity against Helicobacter pylori (Chun et al. 2005). Moreover, it was shown that RA reveals cognitive-enhancing effect (Park et al. 2010). This molecule may be used as a neuroprotective factor preventing and helping to treat Alzheimer’s disease (Iuvone et al. 2006; Hamaguchi et al. 2009). Finally, RA has also cardioprotective (Psotova et al. 2005), hepatoprotective (Osakabe et al. 2002; Lima et al. 2006) and photoprotective activity (Yamada et al. 2006; Sánchez-Campillo et al. 2009). Due to its multiple properties mentioned above, RA arouses interest of pharmaceutical and food industry, as well as cosmetics production.
Several factors like: phytohormones, microelements level, presence of precursors from biosynthetic pathway, affect secondary metabolite accumulation in various in vitro plant cultures. However, the most important factor enhancing their synthesis is elicitation process (Smetanska 2008). Among signaling molecules jasmonic acid (JA) and its methyl ester (MeJA) were applied to induce the production of various metabolites e.g. isoflavonoids (Korsangruang et al. 2010), hypericins and hyperforin (Liu et al. 2007; Coste et al. 2011), phenylpropanoids (Gadzovska et al. 2007), bakuchiol (Lystvan et al. 2010), centellosides (Bonfill et al. 2011), saponins (Kim et al. 2009) as well as coumarin derivatives (Rhee et al. 2010). Some of them are credited with therapeutic properties. Jasmonates were also applied to induce RA production in suspension cultures of several species (Mizukami et al. 1993; Szabo et al. 1999; Ogata et al. 2004; Tsuruga et al. 2006; Georgiev et al. 2007). Although M. piperita suspension cultures were the subject of several in vitro experiments, most of them were applied to investigate and stimulate menthol production (Park et al. 1997; Chang et al. 1998; Kim et al. 2002; Chakraborty and Chattopadhyay 2008). To the best of our knowledge, an impact of jasmonates on RA production in M. piperita has not been elucidated.
This study examines the effectiveness of JA and MeJA in inducing RA accumulation in M. piperita suspension cultures. Furthermore, an impact of these molecules on cell growth, as well as RA secretion into the culture media were also investigated.
Materials and methods
Cell suspension culture conditions
Cell suspension cultures of M. piperita were established from callus tissue as described previously (Krzyzanowska et al. 2011). They were maintained in LS medium (Linsmayer and Skoog 1965) supplemented with 2 mg L−1 2iP (6-γ,γ-dimethylallylaminopurine) and 0.5 mg L−1 2,4-d (2,4-dichlorophenoxyacetic acid), sucrose was added at 30 g L−1 and pH of the medium was adjusted with NaOH or HCl to 5.8 before autoclaving for 20 min at 121°C. The suspension cultures were kept in an incubator shaker Innova 44 (New Brunswick, USA) under continuous agitation (110 rpm) at 25°C and a 16-h photoperiod. Subculturing of suspension cultures to fresh media was done every week.
Preparation of elicitors
JA and MeJA were applied as elicitors. Elicitors were purchased from Duchefa Biochemie (Haarlem, Netherlands). Elicitors were dissolved in pure 96% ethanol to prepare a stock solutions. These solutions were sterilized by microfiltration through 0.20-μm Rotilabo®—syringe filters (ROTH, Germany). The elicitors were then added individually to the suspension cultures at various concentrations; the time of exposure was also diversified.
Elicitation
Cell suspension cultures of M. piperita were grown in 100-mL Erlenmeyer flasks (25 mL of working volume). To prepare homogenous cells inoculum, suspension cultures from several flasks were mixed together before inoculation. Inoculation was prepared using 5 mL of cell suspension. Elicitors were added aseptically to the individual flasks on 7th day from the beginning of cultivation, to give final concentrations of 50, 100, 200 μM. Equal volumes of ethanol were added to the control variants. The experiments were performed in triplicate. Suspension cultures were incubated on a rotary shaker in the same conditions as described above. Cell suspension cultures of M. piperita were harvested at time 0 (just after addition of the elicitor) and next 6, 12, 24, 48 and 72 h after elicitation, to monitor the influence of JA as well as MeJA on biomass growth and RA production.
Determination of dry cell weight
The growth of cell suspension was monitored by measurement of fresh and dry biomass. Suspension cultures were filtered through filter paper (Whatmann No-1). Collected biomasses and culture filtrates were analyzed for RA presence. Filtered cells were washed with deionized water and then fresh weight (FW) were determined. After that, biomass was frozen and lyophilized, then dry cell weight was calculated (expressed as DW g L−1). This material was powdered mechanically in a mortar with a pestle and finally used for chemical analysis.
Extraction and purification of RA
Liophilized and powdered suspension biomass (100 mg) was extracted as described previously (Krzyzanowska et al. 2011). Extraction was performed using a pressurized liquid extractor, ASE 200 (Dionex, Sunnyvale, CA). Extracts (25 mL) were collected in vials and then evaporated to dryness at 40°C under reduced pressure in a rotary evaporator (Laborota 4000, Heidolph, Germany). The residues were dissolved in Mili-Q water (Milipore, Billerica, MA) and purified with SPE technique. Extracts were loaded on C18 Sep-Pak cartridges (1 cm3, 360 mg; Waters Corp., Milford, MA) preconditioned with methanol and water. At first, cartridges were washed with water to remove carbohydrates, and then with 40% (v/v) methanol to elute RA. Fractions obtained in this way were evaporated and suspended in 1 mL of 40% MeOH. Until analysis all samples were stored in a freezer at −20°C.
Chromatographic analysis
RA content in methanolic extracts was determined by reversed-phase ultra-high-pressure liquid chromatography, performed on an ACQUITY UPLC® System (Waters Corporation, Milford, MA, USA), equipped with a Waters ACQUITY UPLC BEH column (50 mm × 2.1 mm, 1.7 μm; Waters Corporation, Milford, CT, USA), according to the previously described method (Krzyzanowska et al. 2011). Briefly, chromatographic analyses were carried out utilizing a gradient elution programme with a mobile phase A (MiliQ water containing 0.1% HCOOH) and a mobile phase B (MiliQ water-ACN, 60:40 v/v containing 0.1% HCOOH). The solvent A concentration was changed as follows: 0 min (80%); 5.1 min (50%); 6.0 min (0%); 6.5 min (0%); 7.0 min (80%); and finished at 7.5 min. The flow rate was 0.4 mL/min, and column temperature was 50°C. The data was acquired and processed using Empower 2.0 software (Waters Corp., Milford, MA, USA). Determination of RA was performed in triplicate, using seven-point calibration curves in the concentration range between 7.5 and 150 μg mL−1. Standard of RA was obtained from Sigma–Aldrich (St. Louis, Mo, USA).
LOD, LOQ, linearity range, R2 data
Limits of detection (LOD) and quantification (LOQ) were based on calibration curves. In case of JA treatment experiment LOD was estimated as 3.4 μg mL−1; LOQ was 10.3 μg mL−1 and the square of correlation coefficient (R2) was 0.9996. When MeJA was used as an elicitor the data was calculated as follows: LOD was 3.2 μg mL−1, LOQ was 10.6 μg mL−1 and R2 was 0.9989. In both cases linearity ranges between 7.5 and 150 μg mL−1.
Statistical analysis
All extracts were prepared in triplicate. Two-way analysis of variance (two-way ANOVA) at 0.05 level of significance was performed to check influence of both elicitors on RA accumulation and on the growth of suspension cultures. In order to find distinction between comparisons a post-hoc test—Duncan’s Multiple Range Test was performed. All experimental data were expressed as mean ± SD of three independent replications.
Results and discussion
Characteristics of M. piperita suspension culture
Callus cultures of M. piperita were obtained from leaf explants of in vitro grown plantlets. Different plant growth regulators at various concentrations were applied to induce callus cultures (data not shown). The best result was observed on LS media enriched with 2 mg L−1 2iP and 0.5 mg L−1 2,4-D. It grew as a homogeneus, friable and light green in color callus. Cell suspension cultures were initiated from this tissue. After filtration, the synchronized cells grew rapidly and the growth parameters like viability and growth curve based on packed cell volume (PCV), fresh and dry weight of cells were measured. These data gave the essential information for the management of the cultures.
Mentha piperita suspension culture presented a typical growth curve. A growth of cell population inoculated into a fresh medium ensued almost immediately, so it seems that the lag phase was brief. The cells reached the linear phase, performing the multiplication in geometric progression (2–8 days of cultivation). The maximum accumulation of biomass was achieved on the 8th day of cultivation (18.97 g L−1 DW). At the end of the culture, cell suspension reached the stationary phase.
RA can accumulate in cell cultures to amounts much higher than those in intact plants. Its biosynthesis under in vitro conditions was confirmed for several species and diverse in vitro culture systems like: callus cultures, suspension cultures, shoot cultures and hairy roots cultures (Shetty 2007; Matkowski 2008). Our previous study has shown that the extract from M. piperita cell suspension culture contained RA as a main compound. Moreover, this molecule constituted 98.05% of total phenolics (Krzyzanowska et al. 2011). However, RA accumulation varied depending on the phase of the cell growth cycle. Its concentration was enhanced during linear phase of growth (2–8 days) being especially intensive between the 6th and the 8th day, when the highest concentration of RA was found (80.28 mg g−1 DW). It was also the end of linear phase of growth, afterwards M. piperita suspension culture reached the stationary phase, where the RA concentration decreased considerably. The same schema of RA accumulation, where its content increased as cells grew, reached the maximum and thereafter decreased rapidly, was observed in many cases. Suspension cultures of Coleus blumei synthesized and accumulated RA during a few days, at the end of growth phase (Petersen 1992). The same phenomenon was also noticed in the case of Agastache rugosa suspension cultures (Kim et al. 2001). Similarly, in cell suspension cultures of Lavandula vera accumulation of RA was also correlated with phase of growth. In this case RA was synthesized during the linear phase of growth, between the 4th and 8th day of cultivation. The maximum amount of RA was determined as 68 mg L−1 DW (Ilieva and Pavlov 1997).
In the present study the effect of JA and MeJA on the RA biosynthesis in M. piperita suspension cultures was researched to answer the question to what degree RA concentration can be enhanced using elicitors. Taking into consideration essential information about M. piperita suspension cultures, which were described below, the elicitors were added to 7 day-old suspension cultures of M. piperita, at the end of linear growth phase.
RA was successfully identified in all tested samples using UPLC technique and monitored by UV detection at 329 nm. Identity of this compound in the chromatograms was verified by comparison with pure standard. Typical chromatogram of M. piperita suspension culture biomass extract, containing RA as a major peak is presented in the Fig. 1. Accumulation of biomass of M. piperita suspension cultures and the production of RA upon elicitation are presented in Figs. 2, 3, respectively. The ANOVA analysis revealed a significant change in RA accumulation levels as well as biomass production in response to elicitor treatment during cultivation period of 72 h.
Effect of elicitors on M. piperita suspension culture growth
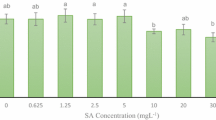
Influence of different concentrations of JA and MeJA on M. piperita suspension cultures growth were examined during 72 h. Cell growth was monitored by measuring the dry cell weight (DW). In case of elicited samples accumulated biomass was lower as compared with the control cells. Both elicitors suppressed growth of cell suspension cultures, however, this inhibition was more diversified in the case of MeJA application. The inhibition of growth was observed already 6 h after addition of JA and MeJA. Dry weights of elicited suspension cultures successively decreased during the cultivation. The addition of JA at 50 μM (Fig. 2) had almost no effect on cell growth, only slight decreases in cell growth were observed. Higher concentrations of JA (100 and 200 μM) were slightly suppressive in biomass growth. These data are in an agreement with general view that JA has been constitutively present in plant cells in a low amounts, but higher concentrations of this plant hormone cause inhibition of cell growth (Creelman and Mullet 1995). In comparison, treatment with MeJA (Fig. 2) resulted in substantial decreases in cell growth in all tested concentrations; the highest decrease was recorded in treatment with 200 μM of MeJA. In this case the biomass of cells was 10–12% lower as compared to the biomasses of the control cells. Some literature data also reveal suppressive activity of both elicitors on the growth of different in vitro cultures applied for RA synthesis. The biomass accumulation in MeJA elicited suspension cultures of Coleus blumei was slightly reduced (Szabo et al. 1999). Also in MeJA treatment the biomass of Lavandula vera MM suspension culture was generally lower comparing to the control. However, 4 h after MeJA addition, accumulation of biomass was enhanced at all of the applied concentrations of elicitor. Eight hours after MeJA addition till the end of the growth phase the elicited suspensions were inhibited comparing to the control. Moreover, a decrease in accumulated biomasses was proportional to applied concentration of MeJA. Within tested concentrations of MeJA, 100 and 150 μM doses turned out to be the most suppressing (Georgiev et al. 2007).
During the cultivation of M. piperita suspension cultures, the color changed from greenish to green–brown or even brown at the end of the growth. Browning of the cell cultures is a strong indicator for increased accumulation of phenolic compounds. This phenomenon was also noticed in the case of elicited cultures. This change was visible after 24 h of JA or MeJA addition. Observed changes depended on elicitor concentration and the time of exposure. For the lowest JA as well as MeJA concentrations, i.e. 50 μM these changes were hardly noticeable. They were visible in case of suspension cultures elicited with higher concentrations of elicitors (100 and 200 μM) especially after 72 h after treatment. This phenomenon was more intense in case of MeJA addition. It may be caused by oxidation of phenolic compounds due to polyphenol oxidase activity (Zhou et al. 2010). In case of Lavandula intermedia suspension cultures, oxidative destruction of RA caused by peroxidase-like enzymes was considered to be responsible for the formation of brown pigments during aging of the culture and resulted in the decrease of RA content (López-Arnaldos et al. 1994). Similar observation was reported in case of Agastache rugosa suspension culture (Kim et al. 2001).
Elicitors did not affect the suspension cultures aggregation. Microscopic pictures of examined samples (treated with MeJA or JA and control variants) were very much alike. In all cases we observed both rounded single cells and small aggregates reaching about one millimetre in diameter as well.
Effect of elicitors on rosmarinic acid biosynthesis
Both elicitors influenced RA synthesis. An increase in RA accumulation was noticed in all applied elicitor concentrations but the maximum accumulation was observed at various time. In the case of JA treatment (Fig. 3), the beginning of enhancement of RA synthesis was observed 6 h after its addition to the cultures. Accumulation of this molecule constantly increased until 48 h after elicitation, where maximum value of RA was achieved. At this time 200 μM of JA was found to be the most effective and RA concentration was 110.12 mg g−1 DW. A little lower content of this antioxidant 106.31 mg g−1 DW was reached when 50 μM was used. These values are nearly 1.5 times higher as compared to the untreated cells. After 72 h since JA was added, accumulation of RA decreased in all tested variants and its concentration values ranged between 82 and 88 mg g−1 of DW. MeJA also influenced RA accumulation (Fig. 3). Among the applied concentrations, the dose of 100 μM within 24 h was the most effective and RA concentration was 117.95 mg g−1 DW. The highest dose of elicitor (200 μM) also caused an enhancement of RA synthesis, however this increase was noticed 48 h after its addition. In this case RA concentration was determined as 111.93 of DW. This concentration remains quite stable, after 72 h amounts to 110. 98 mg g−1 of DW.
Several studies conducted on cell cultures derived from various plant species have shown that elicitation enhances the production of secondary metabolites. However, parameters such as elicitor specificity, its concentration and time of its exposure, as well as the culture conditions and growth stage of the cultured cells, influence the elicitation process (Vasconsuelo and Boland 2007). Jasmonates: JA and MeJA, both induced resveratrol accumulation in Vitis vinifera cell suspension cultures, but with different quantitative and qualitative composition. MeJA was highly effective in stimulating both trans- and cis-resveratrol endogenous accumulation, as well as their release into the culture medium. Cis-resveratrol was absent or detected in very low amounts. JA was less efficient than MeJA in promoting endogenous resveratrol accumulation, but it stimulated the release in the culture medium especially of cis-resveratrol (Tassoni et al. 2005).
It was shown that the activity of enzymes of biosynthetic pathway leading to RA production increased in response to MeJA elicitation. However, the time required for reaching the maximum level of their activity is different for each enzyme (Yamamura et al. 2001). MeJA elicitation was shown to up-regulate activity of phenylalanine ammonia-lyase (PAL) and 4-hydroxyphenylpyruvate reductase (HPR) crucial enzymes for phenylpropanoid pathway (Yamamura et al. 2001; Matsuno et al. 2002). Enhanced PAL level will correspond to enhanced RA production. Latest studies have revealed that MeJA increased activity of isorinic acid 3-hydroxylase (IA3′H) in parallel with increased RA accumulation. This enzyme catalyzes a final step in the biosynthetic pathway leading to RA production (Tsuruga et al. 2006).
JA and MeJA influenced RA biosynthesis in M. piperita suspension cultures. It was found that the best elicitation effect within all treatments was observed in the case of using 100 μM MeJA, 24 h after its application. At this phase, the RA concentration was 117.95 mg g−1 DW, and constituted nearly 12% of DW. This value was nearly 1.5 time higher comparing with untreated samples (control), where RA concentration reached values slightly over 8% of DW. Upon elicitation experiments with different plant species the accumulation of RA was enhanced even more. Addition of 50 μM MeJA to suspension cultures of Lavandula vera MM led to 2.4-fold (3,348 mg L−1) stimulation of RA accumulation (Georgiev et al. 2007). Another study showed that suspension cultures of Coleus blumei exposed to MeJA enhanced RA concentration by approximately three times. Elicitor increased activities of PAL and HPR (Szabo et al. 1999). When 100 μM MeJA was added to Lithospermum erythrorhizon cell suspension cultures the maximum RA concentration was ten times higher (Mizukami et al. 1993). Similar enhancement of RA accumulation in L. erythrorhizon suspension cultures after MeJA addition was observed by Ogata and co-workers. RA concentration reached 10-fold higher content compared with the control 48 h after elicitor application. Between 48 and 72 h after MeJA addition RA concentration was considerably decreased; RA might be metabolized to some other compounds (Ogata et al. 2004). Similar effects to those were recorded in our study in the case of JA treatment. The best RA enhancement was observed after 48 h since the exposure: 110.12 and 106.31 mg g−1 DW for 200 and 50 μM, respectively. Moreover, it was observed that 72 h after elicitation, RA accumulation was significantly reduced, RA concentration reached 77.81–89.94% of maximum values. When MeJA was applied as an elicitor the reduction in RA accumulation after 72 h since elicitor addition was noticed for 50 and 100 μM doses. RA accumulation in this case achieved 90.75 and 80.41% of maximum values observed 24 h after treatment with MeJA. Longer period of elicitor contact (48 and 72 h) caused enhancement in RA synthesis when 200 μM MeJA was applied. Similarly, the addition of various doses of MeJA to Lavandula vera MM suspension cultures enhanced RA accumulation, however the maximum levels were observed at different time. When 12.5 and 50 μM MeJA were used the maximum of RA accumulation was achieved 12 h after its addition. Higher doses of MeJA (100 and 150 μM) caused maximal RA accumulation 4 h after its addition. However, the RA concentration was lowered compared to the maximum content achieved when 50 μM was used (Georgiev et al. 2007). MeJA addition to the Lithospermum erythrorhizon cell suspension cultures rapidly enhanced RA production. At the beginning within 4–8 h after elicitation, small increase of RA accumulation was observed. Similarly to our results, considerable increase in RA accumulation was observed 72 h after MeJA addition. At this time the RA concentration reached 8.9 μM per gram of fresh weight, value about 16-fold higher than this detected in the control (Tsuruga et al. 2006). Another study on MeJA elicitation of Lithospermum erythrorhizon cell suspension cultures showed that RA accumulation achieved maximum 48–72 h after treatment (Mizukami et al. 1993). In case of Coleus blumei suspension cultures, enhanced level of RA was detected 16 h after MeJA addition, moreover its concentration stayed higher until 5th day after elicitation comparing with the control (Szabo et al. 1999).
Rosmarinic acid was found to reveal cytostatic activity at high extracellular concentrations, and this is the main reason which cause that RA represses the growth of plant cells. As a response to this, the plant cells secrete peroxidases, which rapidly destroy this molecule. For this reason concentration of RA in the culture media depends on two competitive processes: its secretion and degradation by cell enzymes and on the influence of the elicitor on both processes (Georgiev et al. 2007). Presence of RA in the culture media of M. piperita suspension cultures after treatment with JA and MeJA was confirmed, however in trace amounts. Concentration of this molecule increased gradually parallel to the time of cultivation. Generally, the higher concentrations of RA were noticed after 72 h of treatment with both elicitors used. This corresponds also to the browning of cell suspension cultures. Small increases in the level of RA accumulation in the culture media after 48, and especially after 72 h, following the addition of elicitors, may be also correlated with the decrease of the intracellular accumulation of this compound. The applied concentrations of elicitors had a slight effect on the release of RA into the culture medium, but a little higher concentrations of RA were recorded after application of 200 μM elicitors. The highest detected concentration of RA in the culture medium occurred 72 h after addition of 200 μM MeJA to the cell cultures, however this concentration was similar to those observed in the untreated variant (control). This phenomenon was shown in case of Lithospermum erythrorhizon suspension cultures after MeJA application. Concentration of RA in the culture media were negligible in both elicitor treated as well as control variants (Mizukami et al. 1993). On the other hand MeJA added to Lavandula vera MM suspension cultures influenced RA secretion into the media. In this case RA was also accumulated mainly intracellularly, however detected in the culture media RA concentration ranged between 5 and 19.8 mg L−1 (Georgiev et al. 2007).
Conclusion
The time courses of M. piperita growth and RA accumulation upon elicitors application were determined. The addition of JA and MeJA enhanced the RA accumulation in cell suspension culture compared with the untreated control. Elicitor concentration and the time of incubation with elicitor showed to be crucial for the elicitation process. The highest accumulation of RA 117.95 mg g−1 DW was noticed 24 h after addition of 100 μM MeJA. Similar concentration—110.12 mg g−1 DW was detected 48 h after application of 200 μM JA. Those values were nearly 1.5 times higher compared to the control variant. These results reveal that in M. piperita suspension cultures effects of JA and MeJA on RA accumulation were comparable. RA was almost exclusively detected in the cells, in the culture media throughout the culture period only traces of RA were noticed, especially 72 h after elicitor addition.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- 2iP:
-
6-γ,γ-Dimethylallylaminopurine
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- RA:
-
Rosmarinic acid
- LS:
-
Linsmayer and Skoog medium
- HCOOH:
-
Formic acid
- UPLC:
-
Ultra performance liquid chromatography
- SPE:
-
Solid phase extraction
References
Bonfill M, Mangas S, Moyano E, Cusido RM, Palazón J (2011) Production of centellosides and phytosterols in cell suspension cultures of Centella asiatica. Plant Cell Tiss Organ Cult 104:61–67
Chakraborty A, Chattopadhyay S (2008) Stimulation of menthol production in Mentha piperita cell culture. In Vitro Cell Dev Biol Plant 44:518–524
Chang JH, Shin JH, Chung IS, Lee HJ (1998) Improved menthol production from chitosan-elicited suspension culture of Mentha piperita. Biotech Lett 20:1097–1099
Chen JH, Ho CT (1997) Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agaric Food Chem 45:2374–2378
Chun SS, Vattem DA, Lin YT, Shetty K (2005) Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem 40:809–816
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tiss Organ Cult. doi: 10.1007/s11240-011-9919-5
Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA 92:4114–4119
Dillard CJ, German JB (2000) Phytochemicals: nutraceuticals and human health. J Sci Food Agric 80:1744–1756
Furtado MA, Almeida LCF, Furtado RA, Cunha WR, Tavares DC (2008) Antimutagenicity of rosmarinic acid in Swiss mice evaluated by the micronucleus assay. Mutat Res Genet Toxicol Environ Mutagen 657:150–154
Gadzovska S, Maury S, Delaunay A, Spasenoski M, Joseph C, Hagége D (2007) Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tiss OrganCult 89:1–13
Georgiev MI, Kuzeva SL, Pavlov AI, Kovacheva EG, Ilieva MP (2007) Elicitation of rosmarinic acid by Lavandula vera MM cell suspension culture with abiotic elicitors. World J Microbiol Biotechnol 23:301–304
Hamaguchi T, Ono K, Murase A, Yamada M (2009) Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am J Pathol 175:2557–2565
Ilieva M, Pavlov A (1997) Rosmarinic acid production by Lavandula vera MM cell-suspension culture. Appl Microbiol Biotechnol 47:683–688
Iuvone T, De Filippis D, Esposito G, D’Amico A, Izzo AA (2006) The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J Pharmacol Exp Ther 317:1143–1149
Kim HK, Oh SR, Lee HK, Huh H (2001) Benzothiadiazole enhances the elicitation of rosmarinic acid production in a suspension culture of Agastache rugosa O. Kuntze. Biotech Lett 23:55–60
Kim GS, Park SH, Chang YJ, Lim YH, Kim SU (2002) Transformation of menthane monoterpenes by Mentha piperita cell culture. Biotech Lett 24:1553–1556
Kim OT, Bang KH, Kim YC, Hyun DY, Kim MY, Cha SW (2009) Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tiss Organ Cult 98:25–33
Korsangruang S, Soonthornchareonnon N, Chintapakorn Y, Saralamp P, Prathanturarug S (2010) Effects of abiotic and biotic elicitors on growth and isoflavonoid accumulation in Pueraria candollei var. candollei and P. candollei var. mirifica cell suspension cultures. Plant Cell Tiss Organ Cult 103:333–342
Krzyzanowska J, Janda B, Pecio L, Stochmal A, Oleszek W, Czubacka A, Przybys M, Doroszewska T (2011) Determination of polyphenols in Mentha longifolia and M. piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J AOAC Int 94:43–50
Lima CF, Fernandes-Ferreira M, Pereira-Wilson C (2006) Phenolic compounds protect HepG2 cells from oxidative damage: Relevance of glutathione levels. Life Sci 79:2056–2068
Linsmayer EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Liu XN, Zhang XQ, Zhang SX, Sun JS (2007) Regulation of metabolite production by precursors and elicitors in liquid cultures of Hypericum perforatum. Plant Cell Tiss Organ Cult 91:1–7
López-Arnaldos T, López–Serrano M, Ros Barceló A, Calderón AA, Zapata JM (1994) Tentative evidence of rosmarinic acid peroxidase in cell cultures from Lavandin (Lavandula × intermedia) flowers. Biochem Mol Biol Int 34:809–816
Lu Y, Foo LY (2001) Antioxidants activities of polyphenols from sage (Salvia officinalis). Food Chem 75:197–202
Lystvan K, Belokurova V, Sheludko Y, Ingham JL, Prykhodko V et al (2010) Production of bakuchiol by in vitro systems of Psoralea drupacea Bge. Plant Cell Tiss Organ Cult 101:99–103
Matkowski A (2008) Plant in vitro culture for the production of antioxidants—a review. Biotechnol Adv 26:548–560
Matsuno M, Nagatsua A, Ogiharaa Y, Ellisb BE, Mizukami H (2002) CYP98A6 from Lithospermum erythrorhizon encodes 4-coumaroyl-4P-hydroxyphenyllactic acid 3-hydroxylase involved in rosmarinic acid biosynthesis. FEBS Lett 514:219–224
Mazumder A, Neamati N, Sunder S, Schulz J, Pertz H, Eich E, Pommier Y (1997) Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J Med Chem 40:3057–3063
Mizukami H, Tabira Y, Ellis BE (1993) Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep 12:706–709
Ogata A, Tsuruga A, Matsunob M, Mizukami H (2004) Elicitor-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures: Activities of rosmarinic acid synthase and the final two cytochrome P450-catalyzed hydroxylations. Plant Biotechnol 21:393–396
Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T (2002) Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in d-galactosamine (d-GalN)- sensitized mice. Free Radic Biol Med 33:798–806
Park SH, Kim KS, Suzuki Y, Kim SU (1997) Metabolism of isopiperitenones in cell suspension culture of Mentha piperita. Phytochemistry 44:623–626
Park DH, Park SJ, Kim JM, Jung WY, Ryu JH (2010) Subchronic administration of rosmarinic acid, a natural prolyl oligipeptidase inhibitor, enhances cognitive performances. Fitoterapia 81:644–648
Petersen M (1992) New aspects of rosmarinic acid biosynthesis in cell cultures of Coleus blumei. Planta Med 58:578
Petersen M, Simmonds MSJ (2003) Rosmarinic acid. Phytochemistry 62:121–125
Petersen M, Abdullah Y, Benner J, Eberle D, Gehlen K et al (2009) Evolution of rosmarinic acid biosynthesis. Phytochemistry 70:1663–1679
Psotova J, Chlopcikova S, Miketova P, Simanek V (2005) Cytoprotectivity of Prunella vulgaris on doxorubicin-treated rat cardiomyocytes. Fitoterapia 76:556–561
Rea G, Antonacci A, Lambreva M, Pastorelli S, Tibuzzi A, et al. (2011) Integrated plant biotechnologies applied to safer and healthier food production: the Nutra-Snack manufacturing chain. Trends Food Sci Technol (in press). doi:10.1016/j.tifs.2011.04.005
Rhee HS, Cho HY, Son SY, Yoon SYH, Park JM (2010) Enhanced accumulation of decursin and decursinol angelate in root cultures and intact roots of Angelica gigas Nakai following elicitation. Plant Cell Tiss Organ Cult 101:295–302
Sánchez-Campillo M, Gabaldon JA, Castillo J, Benavente-García O, Del Baño MJ et al (2009) Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol 47:386–392
Sanchez-Medina A, Etheridge CJ, Hawkes GE, Hylands PJ, Pendry BA, Hughes MJ, Corcoran O (2007) Comparison of rosmarinic acid content in commercial tinctures produced from fresh and dried lemon balm (Melissa officinalis). J Pharm Pharmaceut Sci 10:455–463
Shahidi F, Chandrasekara A (2010) Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem Rev 9:147–170
Shetty K (2007) Rosmarinic acid biosynthesis and mechanism of action. In: Shetty K, Paliyath G, Pometto AL, Levin RE (eds) Functional foods and biotechnology. CRC Taylor & Francis Group, Boca Raton, London, New York, pp 187–207
Smetanska I (2008) Production of secondary metabolites using plant cell cultures. Adv Biochem Engin Biotechnol 111:187–228
Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T (2005) Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutation Res 579:200–213
Szabo E, Thelen A, Petersen M (1999) Fungal elicitor preparation and methyl jasmonate enhance rosmarinic acid accumulation in suspension cultures of Coleus blumei. Plant Cell Rep 18:485–489
Tassoni A, Fornalè S, Franceschetti M, Musiani F, Michael AJ, Perry B, Bagni N (2005) Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol 166:895–905
Tewtrakul S, Miyashiro H, Nakamura N, Hattori M, Kawahata T, Otake T, Yoshinaga T, Fujiwara T, Supavita T, Yuenyongsawad S, Rattanasuwon P, Dej-Adisai S (2003) HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother Res 17:232–239
Tsuruga A, Terasaka K, Kamiya K, Satake T, Mizukami H (2006) Elicitor-induced activity of isorinic acid 3’-hydroxylase, an enzyme catalyzing the final step of rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Biotechnol 23:297–301
Vasconsuelo A, Boland R (2007) Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci 172:861–875
Yamada Y, Yasui H, Sakurai H (2006) Suppressive effect of caffeic acid and its derivatives on the generation of UVA-induced reactive oxygen species in the skin of hairless mice and pharmacokinetic analysis on organ distribution of caffeic acid in ddY mice. Photochem Photobiol 82:1668–1676
Yamamura Y, Ogihara Mizukami H Y, Mizukami H (2001) Cinnamic acid 4-hydroxylase from Lithospermum erythrorhizon: cDNA cloning and gene expression. Plant Cell Rep 20:655–662
Zhao J (2007) Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: a perspective on plant biotechnology application. Recent Pat Biotechnol 1:75–97
Zhou B, Wei X, Wang R, Jia J (2010) Quantification of the enzymatic browning and secondary metabolites in the callus culture system of Nigella glandulifera Freyn et Sint. Asian J Tradit Med 5:109–116
Acknowledgments
The work was performed under the 6th Framework Program of European Union NUTRA-SNACKS project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krzyzanowska, J., Czubacka, A., Pecio, L. et al. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha × piperita cell suspension cultures. Plant Cell Tiss Organ Cult 108, 73–81 (2012). https://doi.org/10.1007/s11240-011-0014-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-0014-8