Abstract
A protocol has been developed for somatic embryogenesis and subsequent plant regeneration in Allium schoenoprasum L. Calli were induced from root sections isolated from axenic seedlings and cultivated on media containing either Murashige and Skoog’s (MS) or Dunstan and Short’s mineral solution supplemented with 5 μM 2,4-dichlorophenoxyacetic acid (2,4-D) in combination with 6-benzylaminopurine (BA), 6-furfurylaminopurine (Kin) or thidiazuron (TDZ) at 1, 5 or 10 μM. The highest frequencies of callus induction were achieved on media with 5 μM 2,4-D in combination with 5 μM TDZ or 10 μM BA (78.9% and 78.4%, respectively). Calli were then transferred to 1 μM 2,4-D, where compact yellow callus turned to segmented yellowish callus with transparent globular somatic embryos at the surface. Calli that were previously grown on media with 5 μM 2,4-D in combination with 10 μM BA or 10 μM TDZ showed the highest frequencies of embryogenic callus formation (45% and 42%) as well as mean number of somatic embryos per regenerating callus. The choice of mineral solution formulation did not significantly affect callus induction or embryogenic callus formation. The embryos could complete development into whole plants on plant growth regulator (PGR)-free medium, but inclusion of Kin (0.5, 2.5 and 5 μM) in this phase improved somatic embryo development and multiplication. Subsequently transferred to 1/2 MS PGR-free medium, all embryos rooted and the survival rate of the plants in a greenhouse was 96%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chive (Allium schoenoprasum L.) is a small bulbous perennial species belonging to the genus Allium and the family Alliaceae. This plant is widely used as a culinary herb as a replacement for garlic or onion because of its mild and tasty flavour, but also as an ornamental plant in herb gardens and decorative dried plant arrangements because of its beautiful ball-like lavender umbels.
However, members of the genus Allium, both wild-growing and cultivated, also contain compounds with potent antioxidant capacity (Štajner et al. 2008; Štajner and Popović 2009). This includes all chive organs, particularly leaves (Štajner et al. 2004, 2008), contributing to a high nutrient value of the plant. The high superoxide dismutase (SOD) activity and high amounts of free thiol and carotenoid found in chive leaves (Štajner and Popović 2009) are beneficial in preventing tumour promotion, cardiovascular disease and aging, all processes associated with free radicals.
Modern biotechnology is a powerful tool for facilitating the breeding of species. For this purpose, an efficient in vitro propagation system is required. However, in vitro regeneration and genetic transformation have not been achieved in chive. By contrast, many members of the genus Allium have been successfully cultivated and propagated in vitro, either by caulogenesis or somatic embryogenesis. The most well-studied species of the genus Allium are the commercially important A. cepa (Dunstan and Short 1978; Zheng et al. 1998, 1999; Eady et al. 1998; Luthar and Bohanec 1999; Zhang et al. 2004) and A. sativum (Myers and Simon 1998, 1999; Ayabe and Sumi 1998, 2001; Barandiaran et al. 1999a, b; Fereol et al. 2002, 2005; Martín-Urdíroz et al. 2004; Luciani et al. 2006). However, protocols for other Allium species are also available: A. ampeloprasum (Buiteveld et al. 1993; Buiteveld and Creemers-Molenaar 1994; Yasseen et al. 1995), A. chinense (Xu et al. 2008), A. porrum (Debergh and Standaert-De Metsenaere 1976; Van der Valk et al. 1992; Hong and Debergh 1995), A. fistulosum (Van der Valk et al. 1992; Song and Peffley 1994; Kim and Soh 1996), A. fistulosum × A. cepa (Van der Valk et al. 1992; Song and Peffley 1994) and A. tuberosum (Matsuda and Adachi 1996).
Because root tips obtained from micro propagated plants have shown to be a convenient explant type used for both shoot initiation (Haque et al. 1997; Myers and Simon 1998; Barandiaran et al. 1999a, b; Martín-Urdíroz et al. 2004) and somatic embryo induction (Fereol et al. 2002; Luciani et al. 2006) in garlic, we decided to use this material to start A. schoenoprasum tissue culture.
The purpose of this study was to establish a protocol for in vitro regeneration of chive, and we believe that the outcome will provide the basis for further biotechnology research of this species. To the best of our knowledge, this is the first report on in vitro regeneration in chive.
Materials and methods
Plant material
Plants of A. schoenoprasum were cultivated in the botanical garden of the Faculty of Agriculture, University of Novi Sad, Serbia (voucher specimen: 1753 No 2–1917). The seeds were washed with plenty of running water and a few drops of detergent (Fairy, Procter & Gamble) and then immersed in 20% commercial bleach (4% NaClO) for 30 min, rinsed with sterile distilled water and planted in 90-mm Petri dishes (20 seeds per dish) containing 25 ml MS PGR-free medium solidified with 0.7% (w/v) agar (Torlak, Belgrade, Serbia) for germination. The Petri dishes were sealed with Parafilm® M (Pechiney Plastic Packing, Chicago, IL, USA). Within the next 1–3 weeks, non-contaminated seedlings were picked out and collected on new Petri dishes (three seedlings per dish) containing the same medium and grown for another 4 weeks until the seedlings reached approximately 5 cm and the root system was well developed. The roots were then isolated and 1-cm sections were cut off and placed on basal media with a different PGR content for callus induction.
Basal medium
The media contained either MS (Murashige and Skoog 1962) or BDS mineral solution (Dunstan and Short 1978) and 20 g/l sucrose, 100 mg/l myo-inositol, 2 mg/l thiamine, 2 mg/l pyridoxine, 5 mg/l nicotinic acid and 2 mg/l adenine, all purchased from Sigma–Aldrich (St. Louis, MO, USA). The media were gelled with 0.7% (w/v) agar and pH was adjusted to 5.8 before sterilisation by autoclaving at 114°C for 25 min.
Callus induction, plant regeneration and acclimatisation
For callus induction, root sections were cultivated on either MS- or BDS-based media supplemented with 5 μM 2,4-dichlorophenoxyacetic acid (2,4-D) in combination with 6-furfurylaminopurine (kinetin, Kin), 6-benzylaminopurine (BA) or thidiazuron (TDZ) at 1, 5 or 10 μM and grown for 8 weeks. Thereafter, all obtained calli were transferred to media with the same mineral formulation (MS or BDS) supplemented with 1 μM 2,4-D as the sole plant growth regulator (PGR) and grown for another 8 weeks to induce somatic embryogenesis. The clumps of embryogenic calli with globular embryos were divided into equal parts (5 × 5 mm) and transferred to either MS PGR-free medium or MS media with 0.5, 2.5 or 5 μM Kin for 1 month to improve somatic embryo development and multiplication. Somatic embryos grown on Kin-supplemented media were transferred to 1/2 MS PGR-free medium solidified with 0.7% agar (w/v) for 1 month to improve root growth. Rooted plants were planted in pots containing a mixture of compost and sand (2:1) and kept protected in a high-moisture environment for 1 week before being gradually exposed to normal greenhouse conditions.
Culture conditions
The cultures were maintained under cool, white fluorescent tubes with photon flux density of 45 μmol m−2 s−1 and 16 h day length at 25 ± 2°C.
Recordings and statistical analysis
All cultures were placed in a completely randomised design. For callus induction, 18 treatments were tested: three cytokinin types (BA, Kin or TDZ), three cytokinin concentrations (1, 5 and 10 μM) and two mineral solutions (MS or BDS). Each treatment consisted of five replications with 20 subsamples (root sections) (n = 100). The callus formation rate was recorded after 8 weeks of culture. The effect of all 18 callus induction treatments on subsequent induction of somatic embryogenesis was evaluated after another 8 weeks on either MS or BDS medium with 1 μM 2,4-D. Ten calli were cultured per Petri dish, with 2–8 replications per treatment (n = 20–78), depending on the number of calli acquired in the previous phase. The frequency of embryo-forming calli and number of somatic embryos per regenerating callus were recorded with the aid of a stereomicroscope.
For embryo development and multiplication, four treatments were tested (0, 0.5, 2.5 and 5 μM Kin). Each treatment consisted of two replicates with four samples (Petri dishes) and five subsamples (callus clumps) (n = 40). The number of somatic embryos and embryos with well-developed shoot and root apex were recorded with the aid of a stereomicroscope after 1 month of culture. We also evaluated the effect of Kin-supplemented media (0.5, 2.5 and 5 μM Kin) on subsequent shoot and root development after 1 month on 1/2 MS PGR-free medium. The root and shoot lengths were measured, and the number of roots was counted. At least 30 plants were used per treatment. For acclimatisation, the number of plants was recorded at 4-weekly intervals.
All percentage data were subjected to angular transformation before analysis. The data were subjected to standard analysis of variance (ANOVA), and the means were separated using Duncan’s test at P ≤ 0.05. For presentation, percentage data were inverse-transformed.
To take into account both the mean number and frequency of somatic embryo formation, we used an index of somatic embryo-forming capacity (EFC), calculated as follows:
Results
Callus induction
In a preliminary study, using 2,4-D as the sole PGR at 1, 5, 10, 20 and 40 μM, calli were induced in 36.7%, 73.3%, 70.0%, 23.3% and 20% of root explants, respectively (not shown). However, the subsequent lowering of 2,4-D concentration to 1 or 5 μM or its exclusion did not bring about regeneration of shoots or somatic embryos, so we decided to combine a cytokinin (BA, Kin or TDZ) with 5 μM 2,4-D in the callus induction media.
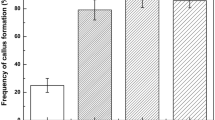
At the beginning of the experiment, seeds were germinated (at the rate of 43%; approximately 20% of seeds were contaminated) on MS PGR-free medium. Root sections were isolated and subjected to 18 treatments for callus induction. Calli formed from root explants of chive were visible after 10–15 days in culture but grew very slowly, so the frequency of callus formation was recorded after 8 weeks of culture. Analysis of variance showed that cytokinin type and its concentration significantly affected callus formation rate (Table 1), whereas the effect of mineral solution formulation (BDS or MS) was insignificant. The highest frequencies of callus induction were achieved on media containing 5 μM 2,4-D in combination with 5 μM TDZ or 10 μM BA, on both BDS and MS media. Compact yellow calli (Fig. 1a) were predominantly formed, although pale friable calli were also observed (Fig. 1b). During callus initiation and growth, a mucilaginous substance was produced by the explants (Fig. 1a, b), being more abundantly secreted by calli grown on BDS-based media (not shown).
a–n Sequential phases of somatic embryogenesis and subsequent plant regeneration from root sections of Allium schoenoprasum. a Compact yellow callus induced from root sections after 2 months on MS medium with 5 μM 2,4-D + 10 μM BA (bar = 1 mm). b Pale friable callus induced from root sections on BDS medium with 5 μM 2,4-D + 10 μM BA for 2 months and then subcultured on BDS medium with 1 μM 2,4-D for 7 weeks (bar = 1 mm). c Yellowish embryogenic callus (originating from callus presented in Fig. 1a) with globular somatic embryos at the surface after 7 weeks on MS medium with 1 μM 2,4-D (bar = 1 mm). d Somatic embryos at different stages of development after 8 weeks on MS medium with 1 μM 2,4-D (bar = 1 mm). e Formation of shoot apex on somatic embryos regenerated by direct somatic embryogenesis from root sections cultivated on MS medium with 1 μM 2,4-D (bar = 1 mm). f Multiplication of somatic embryos on MS medium with 2.5 μM Kin for 1 month (bar = 1 cm). g Multiplication and development of somatic embryos on MS medium with 5 μM Kin for 1 month (bar = 1 cm). h Germinated somatic embryos from embryogenic clumps cultivated on PGR-free MS medium for 1 month (bar = 1 cm). i Individual somatic embryos that germinated on PGR-free MS medium (bar = 1 cm). j Rooting of somatic embryos on 1/2 MS PGR-free medium (bar = 1 cm). k A plant rooted on 1/2 MS PGR-free medium, previously grown on MS medium with 5 μM Kin (bar = 10 cm). l The same plant acclimatised to greenhouse conditions, multiplied and flowered (bar = 10 cm). m The umbel, a detail from image k (bar = 1 cm). n Bulblet formation in acclimatised plants (bar = 1 cm)
Induction of somatic embryogenesis
Upon transfer of calli to MS or BDS media with 1 μM 2,4-D as the sole PGR, they started to change, becoming more segmented and yellowish until, 7–8 weeks later, globular transparent somatic embryos were visible (Fig. 1c). The friable calli never regenerated somatic embryos or buds (Fig. 1b). After an 8-week period, somatic embryos originating from the yellow callus at different stages of development were visible (Fig. 1d). Direct somatic embryo formation from root sections with no visible calli present was seldom observed (Fig. 1e). ANOVA showed that only cytokinin concentration in callus induction media significantly influenced the frequency of embryogenic callus formation. Neither cytokinin type nor mineral solution formulation influenced this process (Table 1). The highest rate of embryogenic callus formation was achieved when calli were induced on MS medium with 5 μM 2,4-D + 10 μM BA or on BDS medium with 5 μM 2,4-D + 10 μM TDZ. The highest mean number of somatic embryos per regenerating callus was obtained when calli were induced on BDS medium with 5 μM 2,4-D + 10 μM TDZ (Table 1). The EFC index showed the highest values for BDS with 5 μM 2,4-D + 10 μM TDZ (8.69) and for MS with 5 μM 2,4-D + 10 μM BA (6.84) (Table 1), and we found these media the most suitable for callus induction from root sections in chive.
Differentiation and multiplication of somatic embryos
The clumps of embryogenic tissue subcultured on MS medium with 1 μM 2,4-D showed no significant changes in tissue proliferation and somatic embryo formation after 1 month of culture (not shown), whereas those subcultured on PGR-free medium produced plantlets with well-formed shoots and roots, although younger embryo forms were arrested at the globular stage. Finally, on media with cytokinins, somatic embryos completed development and multiplied. BA and TDZ were inferior to Kin because they provoked a degree of hyperhydration, so we decided to use only Kin, with the aim of finding the optimal concentration for embryo development. As shown in Table 2, the concentration of Kin significantly affected the number of embryos regenerated from embryogenic calli, as well as the number of embryos with both a well-developed shoot and root apex. The highest embryo number was obtained with 2.5 μM Kin (Fig. 1f), whereas the best shoot apex formation was achieved with 5 μM Kin (Fig. 1g). Somatic embryos grown on Kin-supplemented media were able to root (Fig. 1g), but PGR-free medium was much more effective (Fig. 1h), although the overall number of somatic embryos obtained from PGR-free MS medium was significantly lower (Table 2).
Rooting and acclimatisation
Somatic embryos grown on MS PGR-free medium developed shoots and roots (Fig. 1i) and were ready for acclimatisation, whereas the embryos grown on media with Kin usually needed a rooting treatment on 1/2 MS PGR-free medium (Fig. 1j). Kin level from a previous treatment did not influence the mean number of roots (Table 3) or shoot length, but significantly influenced root length. Somatic embryos previously grown on 5 μM Kin formed much longer roots (up to 33 cm, average 21 cm) than others (Fig. 1k). The plantlets were green and healthy with no visible abnormalities (Fig. 1j, k).
After 1 month of acclimatisation the plant survival rate was 96.07 ± 0.42%. The plants even multiplied, and the number of plants increased by 38.4% over the initial plantlets planted after 2 months of acclimatisation, and further increased by 57.1% after the third month of acclimatisation. The plant presented in Fig. 1k acclimatised and multiplied; the same plant is presented in Fig. 1l after 3 months of acclimatisation. The plants flowered after 9 months of potting (Fig. 1l, m) and formed bulblets (Fig. 1n).
Discussion
Calli formed from root explants of chive were visible after 2 weeks but grew slowly, as was also described in onion (Dunstan and Short 1977), garlic (Myers and Simon 1998, 1999; Robledo-Paz et al. 2000; Fereol et al. 2002) and Chinese chive (Matsuda and Adachi 1996). At least two types of calli were distinguishable in chive: yellow nodular compact calli (Fig. 1a) and pale friable calli (Fig. 1b), but only opaque, yellow and compact calli showed embryogenic capacity (Fig. 1c). The same phenomenon was observed in other Allium species: onion (Eady et al. 1998; Zheng et al. 1998), garlic (Fereol et al. 2002), garden leek (Hong and Debergh 1995) and Chinese chive (Matsuda and Adachi 1996).
Since Dunstan and Short (1977) introduced their new mineral formulation to improve callus growth in onion, the majority of research has used BDS for tissue culture in different Allium species. In this study, we observed no significant differences in the efficiency of BDS in callus formation frequency compared with MS (Table 1), as was also reported for onion by Zheng et al. (1998).
According to our experience and the data presented in this work, 2,4-D seems to be indispensable for induction of somatic embryogenesis, although it was not efficient as sole PGR for subsequent regeneration. The type and concentration of auxin is considered the most important factor for callus induction in Allium species, and 2,4-D has often been used for this purpose, although some authors limited its concentration and treatment duration (Myers and Simon 1999) to avoid somaclonal variation. Fereol et al. (2002) and Barandiaran et al. (1999b) achieved the highest frequency of callus formation in garlic by using low levels (0.3–0.5 mg/l) of 2,4-D. Zheng et al. (1998) and Hong and Debergh (1995) found higher levels of 2,4-D (1 and 2 mg/l) to be optimal for callus induction in onion and garden leek, respectively. Picloram was much more effective than 2,4-D for induction of somatic embryogenesis in onion in the study of Eady et al. (1998), but Luciani et al. (2006) and Luthar and Bohanec (1999) found 2,4-D to be superior to picloram for both callus induction and plant regeneration in garlic and onion, respectively.
The efficiency of embryogenic callus induction from root segments in chive ranged from 4.3% to 45% (Table 1) and was slightly better than obtained in studies on garlic, where only up to 20% of calli were embryogenic (Myers and Simon 1998; Fereol et al. 2002; Luciani et al. 2006). Although Haque et al. (1997) induced direct shoot regeneration from garlic root tips with frequency of 75%, Luciani et al. (2006) found root tips and immature umbels of garlic to be less responsive than meristems and basal plates. In spite of this, the year-round availability of roots from in vitro grown plants and no loss through contamination of primary explants might still justify using this explant type. The regeneration process in our study was slow. It took 8 weeks for induction of somatic embryogenesis. The same was reported for A. tuberosum (Matsuda and Adachi 1996) and A. sativum (Fereol et al. 2002).
We found no significant differences in either the frequency of embryogenic callus formation or the mean number of somatic embryos per regenerating callus with regard to mineral solution used. Zheng et al. (1998) reported that MS-based medium was more effective than BDS for shoot regeneration in onion, but in garlic Luciani et al. (2001) found BDS to be superior to MS, and a mineral solution termed BLM, which was based on BDS with an increased NO −3 level, was even more effective.
In our study, calli induced on media with 2,4-D as sole PGR failed to regenerate, and the presence of a cytokinin was indispensable. By contrast, 2,4-D was sufficient for induction of somatic embryogenesis in garlic (Luciani et al. 2006) and garden leek (Hong and Debergh 1995). Matsuda and Adachi (1996) reported that the concentration of 2,4-D in callus induction medium was decisive for the type and efficiency of morphogenesis in Chinese chive. By using low 2,4-D concentration (0.2–0.5 mg/l) they induced shoot regeneration, whereas higher 2,4-D concentration (5 mg/l) provoked somatic embryogenesis. Some authors also found cytokinins to be essential for callus induction (Yasseen et al. 1995; Hong and Debergh 1995; Kim and Soh 1996; Luthar and Bohanec 1999). Luthar and Bohanec (1999) reported that presence of 2 mg/l BA in the induction medium was beneficial for subsequent regeneration, and omission or substitution of BA with 2 mg/l 2-isopentenyladenine (2iP) decreased regeneration, whereas TDZ was equally effective at 1 mg/l. Yasseen et al. (1995) and Zhang et al. (2004) also used BA to induce regeneration in A. ampeloprasum and A. cepa, respectively.
Once initiated, somatic embryogenesis in chive can precede without the presence of PGRs, but the process is much more effective in the presence of cytokinins (Table 2). This is in accordance with the results of Fereol et al. (2002), who reported that garlic somatic embryos, although capable of regeneration from embryogenic calli without PGRs, differentiated more efficiently in a 2,4-D/Kin combination at 0.1/0.3 or 0.1/0.5 mg/l. Other authors also found cytokinins useful in this phase. Buiteveld et al. (1993) used 1 mg/l Kin to induce somatic embryogenesis in leek, and Hong and Debergh (1995) used 9.8 μM 2iP for complete development of somatic embryos in garden leek. Matsuda and Adachi (1996) supplemented 1 mg/l Kin and 1 mg/l gibberellic acid (GA3) in the regeneration medium to increase the frequency of somatic embryo germination and multiplication in A. tuberosum.
Regenerating complete plants with well-developed roots required final transfer of somatic embryos from Kin-supplemented media to 1/2 MS PGR-free medium (Table 3). This is in accordance with the results of Hong and Debergh (1995) and Zheng et al. (1999). Allium species easily acclimatised to greenhouse conditions, with survival rates of nearly 100% (this study; Yasseen et al. 1995; Hong and Debergh 1995; Zheng et al. 1998; Xu et al. 2008).
To conclude, a system for in vitro regeneration of chive through indirect somatic embryogenesis has been developed for the first time. Calli were initiated from root segments of in vitro grown axenic seedlings. Somatic embryos were induced from embryogenic calli and successfully developed into healthy whole plants, nearly all of which acclimatised. The regeneration process, including an acclimation phase, took 7 months. This protocol needs further optimisation of growth regulator content and explant type used, with the aim of increasing efficiency and speeding up callus induction and subsequent somatic embryogenesis.
Abbreviations
- ANOVA:
-
Analysis of variance
- BA:
-
6-Benzylaminopurine
- BDS:
-
Dunstan and Short’s mineral solution
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- Kin:
-
6-Furfurylaminopurine
- MS:
-
Murashige and Skoog’s mineral solution
- PGR:
-
Plant growth regulator
- TDZ:
-
Thidiazuron
References
Ayabe M, Sumi S (1998) Establishment of a novel tissue culture method, stem-disc culture, and its practical application to micropropagation of garlic (Allium sativum L.). Plant Cell Rep 17:773–779
Ayabe M, Sumi S (2001) A novel and efficient tissue culture method—“stem-disk dome culture”—for producing virus-free garlic (Allium sativum L.). Plant Cell Rep 20:503–507
Barandiaran X, Martín N, Rodríguez-Conde MF, Di Pietro A, Martín J (1999a) Genetic variability in callus formation and regeneration of garlic (Allium sativum L.). Plant Cell Rep 18:434–437
Barandiaran X, Martín N, Rodríguez-Conde MF, Di Pietro A, Martín J (1999b) An efficient method for callus culture and shoot regeneration of garlic (Allium sativum L.). Hort Sci 34:348–349
Buiteveld J, Creemers-Molenaar J (1994) Plant regeneration from protoplasts isolated from suspension cultures of leek (Allium ampeloprasum L.). Plant Sci 100:203–210
Buiteveld J, Van der Valk P, Jansen J, Creemers-Molenaar J, Colijn-Hooymans CM (1993) Callus induction and plant regeneration from explants of commercial cultivars of leek (Allium ampeloprasum var. porrum L.). Plant Cell Rep 12:431–434
Debergh P, Standaert-De Metsenaere R (1976) Neoformation of bulbils in Allium porrum L. cultured in vitro. Sci Hort 5:11–12
Dunstan DI, Short KC (1977) Improved growth of tissue cultures of the onion, Allium cepa. Physiol Plant 41:70–72
Dunstan DI, Short KC (1978) Shoot production from onion callus tissue culture. Sci Hortic 9:99–110
Eady CC, Butler RC, Suo Y (1998) Somatic embryogenesis and plant regeneration from immature embryo cultures of onion (Allium cepa). Plant Cell Rep 18:111–116
Fereol L, Chovelon V, Causse S, Michaux-Ferriere N, Kahane R (2002) Evidence of a somatic embryogenesis process for plant regeneration in garlic (Allium sativum L.). Plant Cell Rep 21:197–203
Fereol L, Chovelon V, Causse S, Triarie D, Arnault I, Auger J, Kahane R (2005) Establishment of embryogenic cell suspension cultures of garlic (Allium sativum L.), plant regeneration and biochemical analyses. Plant Cell Rep 24:319–325
Haque MS, Wada T, Hattori K (1997) High frequency shoot regeneration and plantlet formation from root tip of garlic. Plant Cell Tiss Org Cult 50:83–89
Hong W, Debergh P (1995) Somatic embryogenesis and plant regeneration in garden leek. Plant Cell Tiss Org Cult 43:21–28
Kim JW, Soh WY (1996) Plant regeneration through somatic embryogenesis from suspension cultures of Allium fistulosum L. Plant Sci 114:215–220
Luciani G, Marinangeli PA, Curvetto NR (2001) Increasing nitrate/ammonium ratio for improvement of garlic micropropagation. Sci Hort 87:11–20
Luciani GF, Mary AK, Pellegrini C, Curvetto NR (2006) Effects of explants and growth regulators in garlic callus formation and plant regeneration. Plant Cell Tiss Organ Cult 87:139–143
Luthar Z, Bohanec B (1999) Induction of direct somatic organogenesis in onion (Allium cepa L.) using a two-step flower or ovary culture. Plant Cell Rep 18:797–802
Martín-Urdíroz N, Garrido-Gala J, Martín J, Barandiaran X (2004) Effect of light on the organogenic ability of garlic using a one-step in vitro system. Plant Cell Rep 22:721–724
Matsuda Y, Adachi T (1996) Plant regeneration via embryogenesis in commercial cultivars of Chinese chive (Allium tuberosum Rottl.). Plant Sci 119:149–156
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Myers JM, Simon PW (1998) Continuous callus production and regeneration of garlic (Allium sativum L.) using root segments from shoot tip-derived plantlets. Plant Cell Rep 17:726–730
Myers JM, Simon PW (1999) Regeneration of garlic callus as affected by clonal variation, plant growth regulators and culture conditions over time. Plant Cell Rep 19:32–36
Robledo-Paz A, Vilalobos-Arambula VM, Jofre-Garfias AE (2000) Efficient plant regeneration of garlic (Allium sativum L.) by root-tip culture. In Vireo Cell Dev Plant 36:416–419
Song P, Peffley EB (1994) Plant regeneration from suspension cultures of Allium fistulosum and an A. fistulosum × A. cepa interspecific hybrid. Plant Sci 98:63–68
Štajner D, Popović BM (2009) Comparative study of antioxidant capacity in organs of different Allium species. Cent Eur J Biol 4:224–228
Štajner D, Čanadanović-Brunet J, Pavlović A (2004) Allium schoenoprasum L., as a natural antioxidant. Phytother Res 18:522–524
Štajner D, Igić R, Popović BM, Dj Malenčić (2008) Comparative study of antioxidant properties of wild growing and cultivated Allium species. Phytother Res 22:113–117
Van der Valk P, Scholten O, Verstappen F, Jansen R, Dons J (1992) High frequency somatic embryogenesis and plant regeneration from zygotic embryo-derived callus cultures of three Allium species. Plant Cell Tiss Org Cult 30:181–192
Xu Z, Um YC, Kim CH, Lu G, Guo DP, Liu HL, Bah AA, Mao A (2008) Effect of plant growth regulators, temperature and sucrose on shoot proliferation from the stem disc of Chinese jiaotou (Allium chinense) and in vitro bulblet formation. Acta Physiol Plant 30:521–528
Yasseen MY, Barringer SA, Splittstoesser WE (1995) In vitro shoot proliferation and plant regeneration from kurrat (Allium ampeloprasum var. kurrat) seedlings. Plant Cell Tiss Org Cult 40:195–196
Zhang W, Lin X, Takano H, Takio S, Ono K (2004) Efficient plant regeneration from suspension cells of Allium cepa. Plant Cell Rep 23:371–376
Zheng S, Henken B, Sofiari E, Jacobsen E, Krens FA, Kik C (1998) Factors influencing induction, propagation and regeneration of mature zygotic embryo-derived callus from Allium cepa. Plant Cell Tiss Org Cult 53:99–105
Zheng S, Henken B, Sofiari E, Keizer P, Jacobsen E, Kik C, Krens FA (1999) Effect of cytokinins and lines on plant regeneration from long-term callus and suspension cultures of Allium cepa L. Euphytica 108:83–90
Acknowledgments
This work was supported by the Ministry of Science and Technological Development of the Republic of Serbia, Contract No. 143026B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zdravković-Korać, S., Milojević, J., Tubić, L. et al. Somatic embryogenesis and plant regeneration from root sections of Allium schoenoprasum L.. Plant Cell Tiss Organ Cult 101, 237–244 (2010). https://doi.org/10.1007/s11240-010-9682-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9682-z