Abstract
Hemodynamic assessment of patients with pulmonary embolism (PE) remains a fundamental component of early risk stratification that in turn, influences subsequent monitoring and therapeutic strategies. The current body of literature and international evidence-based clinical practice guidelines focus mainly on the use of systolic blood pressure (SBP). The accuracy of this single hemodynamic parameter, however, and its optimal values for the identification of hemodynamic instability have been recently questioned by clinicians. For example, abnormal SBP or shock index may be a late indicator of adverse outcomes, signaling a patient in whom the cascade of hemodynamic compromise is already well underway. The aim of the present article is to review the current evidence supporting the use of SBP and analyze the potential integration of other parameters to assess the hemodynamic stability, impending clinical deterioration, and guide the reperfusion treatment in patients with PE, as well as to suggest potential strategies to further investigate this issue.
Graphical Abstract

Hemodynamic assessment, and therefore risk stratification, of patients with pulmonary embolism influences subsequent monitoring and therapeutic strategies. The urgent need arises for the identification of precise hemodynamic parameters, or a set thereof, capable of non-invasively and reliably assessing the hemodynamic condition of patients with pulmonary embolism. These parameters should swiftly identify individuals at higher risk of hemodynamic instability and other immediate and long-term complications, while also facilitating the monitoring of changes in risk status over time
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Current international guidelines on the management of acute pulmonary embolism (PE) reaffirm the role of early risk stratification as a cornerstone for identification of patients with increased mortality and subsequent decision-making focused on management strategies [1,2,3]. While mortality in patients with PE has improved overall [1], PE-related mortality has increased in subpopulations, including young adults and those with hemodynamic compromise [4, 5]. As right ventricular (RV) failure represents the major cause of death in acute PE, the goal of such assessment is to use clinical surrogates to understand the state of this ventricle and its compensation to acutely increased afterload [1]. By consensus, a systolic blood pressure (SBP) < 90 mmHg is currently used as the cutoff to identify hemodynamically unstable PE patients (high-risk or massive PE) which typically has a favorable risk-to-benefit ratio for early reperfusion treatment due the higher risk of short-term mortality [6]. However, the optimal cutoff for SBP level that defines a high-risk PE may need to be re-defined through rigorous scientific study in the context of our contemporary care of patients with PE [7]. The landscape of PE management has changed considerably in recent years with widespread adoption of multidisciplinary PE response teams and rapid integration of advanced therapies for reperfusion including catheter-based intervention. Accordingly, a critical appraisal of our current risk stratification tools is warranted.
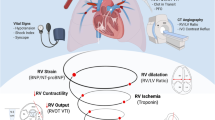
The RV is a thin-walled, compliant chamber that operates at low pressures and adapts poorly to acute increases in afterload. Such a rapid increase in pulmonary vascular resistance may trigger a series of events including RV pressure overload, changes in left ventricular morphology and filling, reduction in cardiac output, and decreases in coronary perfusion [1]. An array of factors contributes to the extent of RV compensation that may be clinically observed in PE, including its underlying function prior to PE, the presence of RV hypertrophy, and the degree of afterload increase related to the integration of clot burden, neurohumoral response, and hypoxic vasoconstriction [1, 8]. The frequently utilized SBP cut-off of < 90 mmHg has been mainly derived from historical investigations. While predictive of mortality, this threshold may miss subtle or developing RV dysfunction, particularly when used in isolation for initial risk stratification as opposed to a serial measures [6]. While an SBP reduction of ≥ 40 mmHg from baseline sustained for 15 min and related to only PE is also outlined in guideline definitions of high-risk PE, this measure suffers from similar limitations [1, 2]. Furthermore, patients with baseline systemic blood pressure abnormalities—and particularly those on anti-hypertensives—may be particularly vulnerable to misdiagnosis based on such a cut-off.
The potential for RV dysfunction is widely recognized even in PE with normal or mildly reduced blood pressure [8]. Studies in intermediate-risk PE patients have demonstrated there may be only a mild decrement in SBP with the presence of RV hypokinesis; as such, it has been suggested that an SBP between 90 and 110 mmHg may indicate systemic hypoperfusion and therefore a poor clinical prognosis [8]. Further challenging our use of SBP as a risk stratification tool is the observation that cardiogenic shock can occur in patients with PE who manage to maintain a technically normotensive blood pressure [9]. Over the latest years, different noninvasive hemodynamic parameters, such as the diastolic blood pressure (DBP), shock index (SI), Composite Pulmonary Embolism Shock (CPES) score, and mean arterial pressure (MAP) have been investigated with the aim to identify the most accurate index for the hemodynamic status in PE patients and guide their treatment [10,11,12,13,14]. The purpose of this review is to highlight the current evidence supporting the use of alternative parameters in assessing the hemodynamic status in PE patients, highlighting their strengths and limitations as well as summarizing areas of current knowledge gaps and clinical need.
Current recommendations
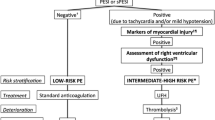
The current definitions of hemodynamic instability in acute PE patients, provided by international clinical practice guidelines [1,2,3], are shown in Table 1. As evidenced by these documents, the identification of hemodynamically unstable PE patients focuses primarily on the SBP measurement. High-risk (also known as massive) PE patients, including those with systemic arterial hypotension, should typically undergo prompt reperfusion therapy, as per guidelines [1]. In patients with advanced stages of cardiovascular decompensation such as refractory shock, salvage with mechanical circulatory support (MCS) may be considered [15]. Identification of patients with PE in the pre-shock state may permit intervention before systemic arterial hypotension with end-organ hypoperfusion leads to devastating complications including poor cerebral perfusion, acute kidney dysfunction, and less frequently hepatic injury, all of which may increase complexity of management of these already tenuous patients [16,17,18]. However, recognition of a pre-shock state has been hampered by lack of consensus on optimal tools to identify such patients.
Although not currently recommended by evidence-based guidelines, periodic reassessment of hemodynamic parameters, respiratory status, and organ hypoperfusion markers—including creatinine, urine output, temperature, liver function tests, serum lactate, and mental status—offers additional information. Firstly, the holistic assessment of global patient status ensures all potential contributors to hemodynamic derangements are recognized, a particularly salient feature in a patient population with high rates of predisposing comorbidities such as infection in malignancy. Secondly, certain values have the potential to provide incremental prognostic information. For example, baseline serum lactate correlates [19] with mortality in both septic and cardiogenic shock, and longitudinal evolution of this level carries important prognostic relevance, with an early decrease indicating a resolution of global tissue hypoxia and decreased risk of mortality [20, 21]. The use of this measure as an adjunct to risk stratification, however, requires rigorous validation in patients with PE [21].
Hemodynamic parameters
Over the years, several hemodynamic parameters, including SBP, have been proposed to assess the hemodynamic stability of PE patients and to guide subsequent treatment (Table 2).
Systolic blood pressure
SBP is currently recommended as the key parameter to identify hemodynamically unstable PE patients and is inversely associated with 30-day all-cause mortality [1]. Several large investigations, such as the International Cooperative Pulmonary Embolism Registry (ICOPER) [9], the Management Strategy and Prognosis of Pulmonary Embolism (MAPPET) Registry [22] and the Registro Informatizado de la Enfermidad Tromboembolica (RIETE) [23] have supported the use of this vital sign to identify PE patients who might benefit from reperfusion therapies. However, most of these studies analyzed prospective multicenter registries, focused on all-cause mortality rather than PE-related death, and ultimately relied upon data that predates the widespread implementation of multidisciplinary PE response teams and current reperfusion techniques [24, 25]. Therefore, the validity of SBP as a marker of hemodynamic instability in acute PE, as well as its optimal cutoff, has recently come into question. From clinical perspective, arguments highlighting the limitation of SBP focus on the observations that blood pressure (BP) measurements may be normal or relatively normal despite concomitant finding of shock [25]. Indeed, compensatory mechanisms may preserve blood pressure through vasoconstriction, while tissue perfusion and oxygenation are already significantly compromised [26]. Furthermore, the evaluation of SBP allows the assessment of only one-third of the cardiac cycle (Figs. 1 and 2) [27], ignoring the diastolic phase, and is inherently subject to potential error related to differences in in noninvasive BP cuff and invasive arterial line measures.
Different stage of pulmonary embolism – related shock. Different hemodynamic parameters could be used in according to the different stages of hemodynamic condition in acute PE. MODS: multi-organ dysfunction syndrome; SCAI: Society for Cardiovascular Angiography and Interventions; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HR: Heart rate; SI: Shock index; MAP: Mean arterial pressure, MCS: Mechanical circulatory support; ECMO: Extra-Corporeal Membrane Oxygenation; CPR: Cardiopulmonary resuscitation
Diastolic blood pressure
DBP measurements may also represent a critical variable in ensuring adequate coronary perfusion, especially in patients with acute PE [13]. Moreover, an accurate DBP assessment remains difficult using non-invasive techniques. Ischemic electrocardiographic changes, such as negative T waves in anterior leads and ST-segment elevations or depressions, are frequently observed in patients with PE [28, 29]. Underlying coronary hypoperfusion is mainly due to the lower DBP in the setting of simultaneously increased right ventricular (RV) myocardial wall tension and results in RV dilatation, myocardial ischemia, and reflex vasoconstriction [28]. To this regard, previous analyses have demonstrated that a DBP < 65 mmHg at admission was associated with a higher 30-day mortality rate in PE patients, due to a higher prevalence of myocardial ischemia and positive biomarkers of cardiac injury [11, 13]. From a pathophysiological point of view, DBP is significantly influenced by the arterial elastance which is, in turn, related to certain cardiovascular chronic processes such as long-standing hypertension and aging [30]. Therefore, its assessment and subsequent use for risk stratification could be inaccurate in a large proportion of PE patients, especially considering that the incidence is directly related with aging [31, 32]. Such features can also further alter measurement accuracy when taken by noninvasive BP cuff [25]. The evaluation of DBP informs hemodynamic status for only two-thirds of the cardiac cycle (Fig. 1).
Heart rate
HR, a simple and readily available vital sign, is widely recognized as an independent predictor of adverse outcomes in PE patients [33]. Over the years, different clinical PE prognostic scores have included HR such as the Pulmonary Embolism Severity Index (PESI) [34], its simplified version (sPESI) [35], Bova [36] and H-FABP, Syncope, and Tachycardia (FAST) [37] scores. However, an optimal cut-off defining tachycardia in patients with PE has not yet determined [38]. From a pathophysiological perspective in PE patients, increased HR may result from neurohumoral factors, such as an adrenergic response, with the purpose of maintaining end-organ perfusion [39]. However, increased HR can also be due to pain, anxiety, and dysrhythmia, all of which can be encountered in PE. For example, atrial fibrillation with a rapid ventricular response in PE may result in tachycardia that is neither compensatory nor associated with hemodynamic deterioration. Tachycardia as a compensatory mechanism depends on the baseline cardiovascular functional status, adaptation of neurohumoral systems and chronic medications. In contrast, absence of tachycardia in a patient receiving atrioventricular nodal blocking agents, such as beta-adrenergic receptor antagonists, should not routinely be interpreted as reassuring. On the opposite end of the spectrum, the presence of significant bradycardia may signal progressive RV failure [2]. Therefore, due its wide variability and susceptibility of concomitant medications, HR cannot be used in isolation as a prognostic marker. Additionally, in rare cases, reflex bradycardia could be observed due to vagal stimulation which is in turn due to RV dilation and pressure overload or intensive pleuritic pain or in patients with pre-existing left bundle branch block (LBBB) who develop right bundle branch block (RBBB) with consequent high degree atrioventricular block [40]. Serial telemetry monitoring and progressive increase in HR may serve as a better marker of PE patients at higher risk of hemodynamic decompensation, but such a hypothesis remains to be tested [39, 41].
Shock index
The SI, defined as the ratio of heart rate to SBP (bpm/mmHg), has been described as independent predictor of 30-day mortality in PE patients [22]. The normal range for this unitless measure is between 0.5 and 0.7, despite some evidence suggests that ratios up to 0.9 would be reassuring; conversely, values approaching ≥ 1.0 are indicative of worsening hemodynamic status and shock [42]. The prevalence of tachycardia increases with PE severity [43]. However, not all high-risk PE patients have tachycardia at admission, with frequencies ranging from 23 to 14% [23, 44, 45]. Notably, the SI was developed to assess the severity of hypovolemic shock. Over the years, however, it has been applied to many other medical conditions, such as cardiogenic shock [46]. A known limitation in cardiac patients, SI values may be largely influenced by antihypertensive therapy and atrioventricular nodal blocking agents (beta-blockers and calcium channel blockers, for example), thereby blunting its association with mortality. Furthermore, vital signs, such as heart rate, may depend on age and history of previous cardiac disease, in particular conduction disease [47]. Moreover, previous investigations comparing the SI with the simplified pulmonary embolism score index (sPESI), showed that the former had a lower sensitivity and negative predictive value for predicting 30-day mortality with respect to the latter [48].
Mean arterial pressure
The MAP is defined as the DBP plus one-third of the difference between SBP and DBP [11, 13]. This non-invasive hemodynamic parameter represents a more complete indicator of peripheral perfusion because it reflects the entire cardiac cycle (Fig. 1). Indeed, MAP represents the time-weighted integral of the instantaneous pressures derived from the area under the curve of the pressure–time waveform during the cardiac cycle [49]. Of particular importance is the contribution derived by the concomitant evaluation of DBP, due to its role in adequate coronary perfusion pressure (CPP) [50]. Therefore, its application in acute PE patients may be very useful in reflecting the severity of right coronary artery insufficiency [13]. Despite the wide use of MAP in the intensive care unit for the management and treatment of patients with other shock states [51, 52], its use is not endorsed by international clinical practice guidelines on acute PE. Furthermore, few observational investigations have focused on this hemodynamic parameter. Chen at al [53]. showed that patients with intermediate high-risk and high-risk PE and a MAP between 80 and 90 mmHg had fewer adverse events, such as cardiogenic shock, need for cardiopulmonary resuscitation, mechanical ventilation, and vasopressor requirement. Similarly, a recent post-hoc analysis of intermediate-high-risk PE patients enrolled in the Italian Pulmonary Embolism Registry (IPER) demonstrated that a MAP ≤ 81.5 mmHg was an independent predictor of 48-hour clinical deterioration (Hazard ratio 3.25, 95% CI: 1.89 to 5.21, p < 0.001), with a sensitivity, specificity, positive, and negative predictive values of 77.5%, 95.0%, 63.2% and 97.7%, respectively [13]. The utility of MAP as a clinically informative hemodynamic parameter in PE patients—as well as the appropriate target based on comorbidities such as systemic hypertension—requires prospective confirmation [54].
Future directions
The mortality in acute PE remains around 7% [44], and several questions regarding the optimal hemodynamic assessment as well as early indicators of clinical deterioration remain unanswered [1,2,3] (Fig. 3). The SBP has certain intrinsic limitations and lacks specific data supporting its predictive role regarding PE-specific mortality [6]. In the light of investigations performed in cardiogenic shock and the preliminary results available for PE patients, the MAP appears to be a valuable alternative, with a higher accuracy for mortality compared with SBP. This non-invasive evaluation of the entire cardiac cycle, also including an indirect assessment of coronary perfusion, may provide important insights to guide monitoring and therapeutic strategies in acute PE (Fig. 1). Furthermore, a perfusion index that can detect early hemodynamic decompensation, before systemic arterial hypotension and shock set in, is urgently needed [13]. An example of such an index, the CPES score has shown promise for identifying stable patients with PE who might be at risk for hemodynamic deterioration or death.
However, several gaps remain in the use of a measure like MAP, including its accuracy in identifying those at risk of early PE-related clinical deterioration within 48–72 h. The overall precision of MAP for in-hospital and 30-day mortality and its optimal values in various risk groups of acute PE are unclear. While MAP may currently offer the most comprehensive PE hemodynamic evaluation [55, 56], rigorous investigation of MAP and other parameters, perhaps within the construct of randomized controlled trials (RCTs), or individual patient data meta-analysis or large collaborative networks of multidisciplinary PE response team experiences, is sorely needed. MAP, other to be identified parameters, or a combination thereof may represent the key to early identification of patients with PE and increased risk of early adverse events and who may benefit from reperfusion therapy.
Importantly, it must be recognized that risk stratification systems based solely on blood pressure measures are inherently limited by the nature of this isolated measurement itself. In fact, there is likely a role for its broader interpretation within the overall clinical picture or within a risk stratification model. Progressive hypoxemia or respiratory failure in a hemodynamically stable patient, for example, may necessitate a more aggressive management strategy to avoid intubation [57]. In addition to SBP, multiple parameters of respiratory failure including oxygen saturation, tachypnea, and requirement for supplemental oxygen are incorporated in the National Early Warning Score (NEWS), a system designed to predict early clinical worsening and provide for early intervention. While devised from a general medical population, post-hoc analysis of the YEARS study recently demonstrated that the NEWS more accurately predicted short-term ICU admission and 30-day mortality in hemodynamically stable acute PE than either than either the PESI or sPESI [58]. As the development of hypotension signals that a chain of events leading to severe RV dysfunction or failure has already transpired [1], the use of such systems for early identification of clinical deterioration may be useful, particularly as reperfusion therapy may require additional time to arrange. Furthermore, delaying advanced therapies for reperfusion until this late signal may substantially attenuate the benefit of such interventions on mortality and possibly long-term complications such as post-PE syndrome and chronic thromboembolic pulmonary hypertension [56].
Currently, hypoxemia, RV strain, and hypoperfusion are among the most pathophysiologically-based and heavily-utilized markers of adverse outcomes in PE patients. Hypoxemia can be evaluated using pulse oximetry or arterial blood gas analysis. In PE patients, oxygenation may be systematically assessed as part of a serial National Early Warning Score (NEWS) score evaluation [40]. While the ideal frequency of assessment remains uncertain, many patients undergo continuous oxygen monitoring to promptly detect any decline, particularly if they already have baseline hypoxemia. RV strain is typically diagnosed using echocardiography or chest CT. However, there are limited data supporting serial assessment and informing optimal timing of such evaluations. Furthermore, patients with chronic lung or cardiac conditions may already have baseline RV dysfunction, complicating the assessment. While point-of-care ultrasound can provide some insight, there are insufficient data to advocate for its routine serial use. The 2019 ESC evidence-based clinical practice guidelines favor a more formal echocardiographic evaluation [1]. Finally, hypoperfusion can be non-invasively and continuously assessed using the MAP [11]. Lactate levels may provide further insight into perfusion although are not formally incorporated into current algorithms. Again, the optimal timing for these serial assessments remains uncertain [11].
The refinement of current PE risk classes would have significant implications for the prognosis and selection of different therapeutic strategies, including systemic fibrinolysis, catheter-based intervention, surgery, and mechanical circulatory support (Visual Abstract). Pivotal clinical trials may inform the field on the prognostic value of these other markers of medical acuity with PE with focus on the occurrence of short-term PE-related and/or cardiovascular mortality, early clinical deterioration, and long-term complications. Moreover, dedicated analyses are also needed to identify the optimal hemodynamic indications for advanced treatments such as catheter-directed therapies (CDT and mechanical circulatory support) and a longitudinal monitoring strategy that weighs hemodynamics and other factors to detect subtle but potentially progressive RV dysfunction. For such purposes, both observational studies and registry randomized clinical trials (RRCTs), which are pragmatic trials using registries as a platform for case records, data collection, randomization, and follow-up, may represent other valid sources of data on these prognostic and risk stratification parameters. Specifically, observational studies, conducted using appropriate statistical methods for balancing data and reduce the effects of covariates, such as propensity score matching, stratification, and regression adjustments may provide important findings in estimating novel prognostic strategies and relative treatment effects [59]. Similarly, RRCTs may allow the collection of ‘real-world’ data from patients in a daily clinical setting, many of whom would be excluded from RCTs. These pragmatic trials offer the further advantage of a rapid consecutive enrollment, are associated with lower cost, and may provide a more comprehensive longitudinal picture of patient treatment and outcomes compared with conventional randomized controlled trials [60, 61]. Moreover, PE responding team (PERT)-based registries and other cohort studies can aid in generating hypotheses. However, prospective data, such as that obtained from ongoing trials, like the HI-PEITHO [40] and the SONIC-PE (NCT06310018), will provide more rigorous data for applications of tools like the NEWS score, risk score derivation, and impact on clinical outcomes.
The international scientific community has expressed great interest in outcomes research focused on PE, as evidenced by the research support provided by the National Institute of Health (NIH) and industry, funding pivotal trials including the PE-TRACT (NCT05591118), HI-PEITHO (NCT04790370), PEERLESS (NCT05111613), PEERLESS 2 (NCT06055920) and STORM-PE (NCT05684796). The goal of more precise hemodynamic assessment is to guide the need and optimal pathway for reperfusion in PE patients. Such determinations based on patient symptoms, respiratory status, and hemodynamics would be aimed at reducing PE-related morbidity and mortality. Future trials focused on mechanical thrombectomy or catheter-based fibrinolysis may benefit from the identification of higher risk phenotypes.
Conclusions
The identification of more accurate hemodynamic parameters, or a collection of parameters, able to accurately and non-invasively assess the hemodynamic status of PE patients, to promptly identify subjects at higher risk of hemodynamic decompensation and other short- and long-term complications, and to monitor temporal transitions in risk status are sorely needed. With the goal of timely reperfusion to mitigate short- and long-term complications, investigation into such early measures of hemodynamic perturbation, before systemic arterial hypotension and shock have set in, will likely be critical.
References
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL, ESC Scientific Document Group (2020) 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 41:543–603
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK, American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, thrombosis and Vascular Biology (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123:1788–1830
Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores LK (2021) Executive Summary: antithrombotic therapy for VTE Disease: second update of the CHEST Guideline and Expert Panel Report. Chest 160:2247–2259
Zuin M, Bikdeli B, Armero A, Porio N, Rigatelli G, Bilato C, Piazza G (2023) Trends in Pulmonary Embolism deaths among young adults aged 25 to 44 years in the United States, 1999 to 2019. Am J Cardiol 202:169–175
Zuin M, Bikdeli B, Davies J, Krishnathasan D, Rigatelli G, Roncon L, Bilato C, Piazza G (2023) Contemporary trends in mortality related to high-risk pulmonary embolism in US from 1999 to 2019. Thromb Res 228:72–80
Jiménez D, Bikdeli B, Barrios D, Quezada A, Del Toro J, Vidal G, Mahé I, Quere I, Loring M, Yusen RD, Monreal M, RIETE investigators (2018) Epidemiology, patterns of care and mortality for patients with hemodynamically unstable acute symptomatic pulmonary embolism. Int J Cardiol 269:327–333
Secemsky E, Chang Y, Jain CC, Beckman JA, Giri J, Jaff MR, Rosenfield K, Rosovsky R, Kabrhel C, Weinberg I (2018) Contemporary Management and outcomes of patients with massive and submassive pulmonary embolism. Am J Med 131:1506–1514e0
Zhao S, Friedman O (2020) Management of right ventricular failure in pulmonary embolism. Crit Clin Care 35:505–515
Kucher N, Rossi E, De Rosa M, Goldhaber SZ (2005) Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm hg or higher. Arch Intern Med 165:1777–1781
Bangalore S, Horowitz JM, Beam D, Jaber WA, Khandhar S, Toma C, Weinberg MD, Mina B (2023) Prevalence and predictors of cardiogenic shock in Intermediate-Risk Pulmonary Embolism: insights from the FLASH Registry. JACC Cardiovasc Interv 16:958–972
Zuin M, Rigatelli G, Picariello C, Carraro M, Zonzin P, Roncon L (2017) Prognostic role of a new risk index for the prediction of 30-day cardiovascular mortality in patients with acute pulmonary embolism: the Age-Mean arterial pressure index (AMAPI). Heart Vessels 32:1478–1487
Otero R, Trujillo-Santos J, Cayuela A et al (2007) Haemodynamically unstable pulmonary embolism in the RIETE registry. Systolic blood pressure or shock index? Eur Respir J 30:1111–1116
Zuin M, Rigatelli G, Bongarzoni A, Enea I, Bilato C, Zonzin P, Casazza F, Roncon L (2023) Mean arterial pressure predicts 48 h clinical deterioration in intermediate-high risk patients with acute pulmonary embolism. Eur Heart J Acute Cardiovasc Care 12:80–86
Chen J, Lin J, Wu D, Guo X, Li X, Shi S (2020) Optimal Mean arterial pressure within 24 hours of admission for patients with Intermediate-Risk and High-Risk Pulmonary Embolism. Clin Appl Thromb Hemost 26:1076029620933944
Zuin M, Rigatelli G, Daggubati R, Nguyen T, Roncon L (2020) Impella RP in hemodynamically unstable patients with acute pulmonary embolism. J Artif Organs 23:105–112
Desai PV, Krepostman N, Collins M, De Sirkar S, Hinkleman A, Walsh K, Fareed J, Darki A (2021) Neurological complications of Pulmonary Embolism: a literature review. Curr Neurol Neurosci Rep 21:59
Murgier M, Bertoletti L, Darmon M, Zeni F, Valle R, Del Toro J, Llamas P, Mazzolai L, Villalobos A, Monreal M, RIETE Investigators (2019) Frequency and prognostic impact of acute kidney injury in patients with acute pulmonary embolism. Data from the RIETE registry. Int J Cardiol 291:121–126
Aslan S, Meral M, Akku M, Acemoglu H, Ucar EY, Gorguner M, Mirici A (2007) Liver dysfunction in patients with acute pulmonary embolism. Hepatic Res 37:205–213
Marbach JA, Di Santo P, Kapur NK, Thayer KL, Simard T, Jung RG, Parlow S, Abdel-Razek O, Fernando SM, Labinaz M, Froeschl M, Mathew R, Hibbert B (2022) Lactate Clearance as a surrogate for mortality in cardiogenic shock: insights from the DOREMI Trial. J Am Heart Assoc 11:e023322
Elder M, Blank N, Kaki A, Alraies MC, Grines CL, Kajy M, Hasan R, Mohamad T, Schreiber T (2018) Mechanical circulatory support for acute right ventricular failure in the setting of pulmonary embolism. J Interv Cardiol 31:518–524
Revelly JP, Tappy L, Martinez A, Bollmann M, Cayeux MC, Berger MM, Chiolero RL (2005) Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit Care Med 33:2235–2240
Otero R, Trujillo-Santos J, Cayuela A, Rodríguez C, Barron M, Martín JJ, Monreal M, Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators (2007) Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 30:1111–1116
Zuin M, Rigatelli G, Bilato C, Bongarzoni A, Casazza F, Zonzin P, Roncon L (2022) Prognostic role of serial electrocardiographic changes in patients with acute pulmonary embolism. Data from the Italian Pulmonary Embolism Registry. Thromb Res 217:15–21
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1795–1815
Picone DS, Schultz MG, Otahal P, Aakhus S, Al-Jumaily AM, Black AJ et al (2017) Accuracy of cuff-measured blood pressure: systematic reviews and meta-analyses. J Am Coll Cardiol 70:572–586
Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV (2005) Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 25:843–848
Nielsen TT, Lund O, Rønne K, Schifter S (1989) Changing electrocardiographic findings in pulmonary embolism in relation to vascular obstruction. Cardiology 76:274–284
Zuin M, Rigatelli G, Zonzin P, Casazza F, Roncon L (2018) Short- and long-term prognostic role of diastolic blood pressure in intermediate-high risk patients with acute pulmonary embolism. Eur J Intern Med 55:e23–e24
Sheng FQ, Xu R, Xia JD, He MR (2017) ECG patterns indicate severity of Acute Pulmonary Embolism: insights from serial ECG changes in a patient treated with Thrombolysis. J Emerg Med 52:e251–e253
Magder S (2018) The meaning of blood pressure. Crit Care 22:257
Keller K, Beule J, Coldewey M, Dippold W, Balzer JO (2015) Impact of advanced age on the severity of normotensive pulmonary embolism. Heart Vessels 30:647–656
Ramos A, Murillas J, Mascías C, Carretero B, Portero JL (2000) Influence of age on clinical presentation of acute pulmonary embolism. Arch Gerontol Geriatr 30:189–198
Jaureguízar A, Jiménez D, Bikdeli B, Ruiz-Artacho P, Muriel A, Tapson V, López-Reyes R, Valero B, Kenet G, Monreal M, Registro Informatizado De La Enfermedad TromboEmbólica investigators (2022) Heart rate and mortality in patients with Acute Symptomatic Pulmonary Embolism. Chest 161:524–534
Aujesky D, Roy PM, Le Manach CP et al (2006) Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J 27:476–481
Jiménez D, Aujesky D, Moores L, Gómez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A, Yusen RD, RIETE Investigators (2010) Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 170:1383–1389
Bova C, Vanni S, Prandoni P, Morello F, Dentali F, Bernardi E, Mumoli N, Bucherini E, Barbar S, Picariello C, Enea I, Pesavento R, Bottino F, Jiménez D, Bova Score Validation Study Investigators (2018) A prospective validation of the Bova score in normotensive patients with acute pulmonary embolism. Thromb Res 165:107–111
Hobohm L, Becattini C, Konstantinides SV, Casazza F, Lankeit M (2020) Validation of a fast prognostic score for risk stratification of normotensive patients with acute pulmonary embolism. Clin Res Cardiol 109:1008–1017
Hobohm L, Becattini C, Ebner M, Lerchbaumer MH, Casazza F, Hasenfuß G, Konstantinides SV, Lankeit M (2020) Definition of tachycardia for risk stratification of pulmonary embolism. Eur J Intern Med 82:76–82
Goldhaber SZ, Elliott CG (2003) Acute pulmonary embolism: part I: epidemiology, pathophysiology, and diagnosis. Circulation 108:2726–2729
Zuin M, Rigatelli G (2017) Complete heart block as presenting symptom of massive pulmonary embolism in an elderly patient. J Geriatr Cardiol 14:593–594
Klok FA, Piazza G, Sharp ASP, Ní Ainle F, Jaff MR, Chauhan N, Patel B, Barco S, Goldhaber SZ, Kucher N, Lang IM, Schmidtmann I, Sterling KM, Becker D, Martin N, Rosenfield K, Konstantinides SV (2022) Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: Rationale and design of the HI-PEITHO study. Am Heart J 251:43–53
Jentzer JC, Burstein B, Van Diepen S, Murphy J, Holmes DR Jr, Bell MR, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA (2021) Defining shock and preshock for Mortality Risk Stratification in Cardiac Intensive Care Unit patients. Circ Heart Fail 14:e007678
Yamashita Y, Morimoto T, Takase T, Hiramori S, Kim K, Oi M, Akao M, Kobayashi Y, Chen PM, Murata K, Tsuyuki Y, Nishimoto Y, Sakamoto J, Togi K, Mabuchi H, Takabayashi K, Kato T, Ono K, Kimura T, COMMAND VTE Registry Investigators (2023) Impact of heart rate at diagnosis on clinical outcomes in patients with Acute Pulmonary Embolism. Am J Cardiol 187:38–47
Casazza F, Becattini C, Bongarzoni A, Cuccia C, Roncon L, Favretto G, Zonzin P, Pignataro L, Agnelli G (2012) Clinical features and short term outcomes of patients with acute pulmonary embolism the Italian Pulmonary Embolism Registry (IPER). Thromb Res 130:847–852
Ebner M, Sentler C, Harjola VP, Bueno H, Lerchbaumer MH, Hasenfuß G, Eckardt KU, Konstantinides SV, Lankeit M (2021) Outcome of patients with different clinical presentations of high-risk pulmonary embolism. Eur Heart J Acute Cardiovasc Care 10:787–796
Del Castillo Gordillo C, Avila Cisternas C, Yanez Vidal F, Appiani Florit F, Ibanez Mora A, Van Grieken Garcia J, Mora Valdes R, Luque Gonzales M, Begazo Gonzales A (2010) The relationship between shock index and measures of cardiac output in cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2021. https://doi.org:10:zuab020.172. [Epub Ahead of print]
Houghton D, Jones TW, Cassidy S, Siervo M, MacGowan GA, Trenell MI, Jakovljevic DG (2016) The effect of age on the relationship between cardiac and vascular function. Mech Ageing Dev 153:1–6
Sam A, Sánchez D, Gómez V, Wagner C, Kopecna D, Zamarro C, Moores L, Aujesky D, Yusen R, Jiménez Castro D (2011) The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 37:762–766
Meaney E, Alva F, Moguel R, Meaney A, Alva J, Webe R (2000) Formula and nomogram for the sphygmomanometric calculation of the mean arterial pressure. Heart 84:64
Mathew R, Fernando S, Hu K et al (2022) Optimal perfusion targets in cardiogenic shock. JACC Adv. https://doi.org/10.1016/j.jacadv.2022.100034
Parlow S, Di Santo P, Mathew R, Jung RG, Simard T, Gillmore T, Mao B, Abdel-Razek O, Ramirez FD, Marbach JA, Dick A, Glover C, Russo JJ, Froeschl M, Labinaz M, Fernando SM, Hibbert B, CAPITAL DOREMI investigators (2021) The association between mean arterial pressure and outcomes in patients with cardiogenic shock: insights from the DOREMI trial. Eur Heart J Acute Cardiovasc Care 10:712–720
Burstein B, Tabi M, Barsness GW, Bell MR, Kashani K, Jentzer JC (2020) Association between mean arterial pressure during the first 24 hours and hospital mortality in patients with cardiogenic shock. Crit Care 24:513
Chen J, Lin J, Wu D, Guo X, Li X, Shi S (2020) Optimal Mean arterial pressure within 24 hours of admission for patients with Intermediate-Risk and High-Risk Pulmonary Embolism. Clin Appl Thromb Hemost 26:1076029620933944. https://doi.org/10.1177/1076029620933944
Russell JA (2020) Personalized blood pressure targets in shock: what if your normal blood pressure is low? Am J Respir Crit Care Med 202:10–12
Becattini C, Agnelli G, Maggioni AP, Dentali F, Fabbri A, Enea I, Pomero F, Ruggieri MP, Di Lenarda A, Gulizia M (2022) Contemporary clinical management of acute pulmonary embolism: the COPE study. Intern Emerg Med 17:715–723
Piazza G (2020) Advanced Management of Intermediate- and high-risk Pulmonary Embolism: JACC Focus Seminar. J Am Coll Cardiol 76:2117–2127
Hoeper MM, Granton J (2011) Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. AJRCCM 184:1114–1124
Bavaria R, Stals MAM, Mulder FI, Bistervels IM, Coppens M, Faber LM et al (2023) Use of the National Early warning score for predicting deterioration of patients with acute pulmonary embolism: a post-hoc analysis of the YEARS study. Emerg Med J 40:61–66
Kitsios GD, Dahabreh IJ, Callahan S, Paulus JK, Campagna AC, Dargin JM (2015) Can we trust Observational studies using propensity scores in the critical care literature? A systematic comparison with randomized clinical trials. Crit Care Med 43:1870–1879
Li G, Sajobi TT, Menon BK, Korngut L, Lowerison M, James M, Wilton SB, Williamson T, Gill S, Drogos LL, Smith EE, Vohra S, Hill MD, Thabane L (2016) Symposium on Registry-Based Randomized Controlled Trials in Calgary (2016) Registry-based randomized controlled trials- what are the advantages, challenges, and areas for future research? J Clin Epidemio l80:16–24
Doherty DA, Tong SYC, Reilly J, Shrapnel J, McDonald S, Ahern S, Harris I, Tam CS, Brennan AL, Hodgson C, Wilcox L, Balagurunathan A, Butcher BE, Reid CM (2023) Registry randomised trials: a methodological perspective. BMJ Open 13:e068057
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
M.Z. and G.P.: Conceptualization, Methodology, Data curation, Validation.: S.H., E.M.H. Writing—original draft. M.Z. Critical revision; G.P., S. H., E.M.H.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
GP received research grants from BMS/Pfizer, Janssen, Alexion, Bayer, Amgen, BSC, Esperion and 1R01HL164717-01. GP reports an advisory Role for BSC, Amgen, BCRI, PERC, NAMSA, BMS, Janssen, Regeneron. EMH reports research grants from T32-HL0007633 (Brigham and Women’s Hospital, Division of Pulmonary and Critical Care Medicine T32 grant) but has no conflicts of interest. MZ, SH report no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuin, M., Henkin, S., Harder, E.M. et al. Optimal hemodynamic parameters for risk stratification in acute pulmonary embolism patients. J Thromb Thrombolysis 57, 918–928 (2024). https://doi.org/10.1007/s11239-024-02998-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-024-02998-9