Abstract
We did a cost-utility analysis for the new oral anticoagulants (NOACs) in the German population based on the quality-adjusted life years (QALY), total costs, and incremental cost-effectiveness ratios (ICER). The aim of our investigation was to examine cost-utility for current German drug market costs and compared to other countries. Outcome data were taken from dabigatran’s RE-LY, rivaroxaban’s ROCKET AF, and apixaban’s ARISTOTLE trials. A Markov decision model, the Monte Carlo simulation (MCS), and further sensitivity analyses were used to simulate comparisons between NOACs over a follow up period of 20 years. The main perspective used for the analyses is from a German public health care insurance perspective. The base-case analyses of a 65 years old person with a CHADS2 score >1 resulted in 7.56–7.64 QALYs gained for warfarin. NOACs added 0.04–0.19 QALYs. Total costs for warfarin ranged from 7622 to 9069€ and for NOACs from 19537 to 20048€. The sensitivity analysis indicated that current German market costs for the NOACs exceed a willingness-to-pay threshold of (hypothetical) 50000€/QALY in all treatment regimen. The MCS showed willingness-to-pay thresholds from 60500€/QALY for apixaban to 278000€/QALY for dabigatran 110 mg bid, with values for dabigatran 150 mg bid and rivaroxaban in between. In conclusion, from a German public health care insurance perspective current market costs are high in relation to the quality of life gained. These results from clinical studies (efficacy) remain to be confirmed under real life conditions (effectiveness).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demographic change with an increase of the proportion of the elderly leads to an increased incidence and prevalence of atrial fibrillation. The annual incidence of stroke in patients with atrial fibrillation (NVAF) who are not receiving antithrombotic therapy is 4.5 % [1, 2]. Patients with NVAF and two risk factors for stroke (CHADS2-score) have to undergo antithrombotic therapy which has proven beneficial and clinically relevant endpoint effects on stroke, mortality and quality of life [3]. For NVAF patients with a CHADS2-Score of two or above, major international and national clinical guidelines recommend long-term anticoagulation with vitamin K antagonists adjusted to an international normalized ratio (INR) target of 2.0–3.0 or new oral anticoagulants (NOAC) [4–8].
Disadvantages of warfarin include a genetic determination of the metabolism, narrow therapeutic window, wide inter- and intra-individual variability of the dose-effect relation, drug- and food-interactions; thus its clinical use is difficult and frequent INR monitoring and dose adjustments are mandatory [9–12]. Over- or under-anticoagulation may lead to serious bleeding or increased risk of embolic events [13, 14]. In clinical practice the time spend in therapeutic INR range is about 60 % lower than in an clinical trial setting due to the lack of protocol-driven management [15, 16].
NOACs (dabigatran etexilate, rivaroxaban and apixaban) have proven to be superior or at least equivalent for stroke prevention and occurrence of severe bleeding complications in patients with non-valvular atrial fibrillation (NVAF) compared to dose-adjusted warfarin. In the RE-LY trial 110 mg bid dabigatran was non-inferior and 150 mg bid dabigatran was superior to dose-adjusted warfarin for prevention of stroke and systemic embolism and both doses resulted in less cerebral bleeding [17]. In the double blind ROCKET AF trial [18] patients on rivaroxaban 20 mg od had reduced rates of stroke and systemic embolism and comparable major bleeding incidences compared to warfarin [19]. In the double blind ARISTOTLE trial [20] apixaban was associated with lower rates of strokes and major bleeding and reduced mortality in comparison to warfarin [21]. A total of four independent network meta-analyses demonstrated differences for the efficacy and safety of the four NOAC treatment regimens across the studies (for review see [22]). Similar differences may be identified for the pharmacoeconomic effects of the four NOAC treatment regimes using the same body of trial information.
The main restriction for prescribing NOACs is their higher daily costs compared to warfarin. However, from a health care insurance perspective the NOACs would be beneficial, if the insurance is prepared to pay defined additional costs for the benefit of the NOACs. Recent publications addressed the pharmacoeconomic aspects of dabigatran in the US [23], Canada [24], England [25, 26], Denmark [27], and Sweden [28], and of rivaroxaban in the US [29]. Dabigatran 150 mg bid, rivaroxaban and apixaban were analysed for the cost-effectiveness in US [30]. In this study we also include dabigatran 110 mg bid in addition to these three treatment options to cover a broader spectrum of the cost-effectiveness assessment in Germany. Our aim was to scientifically challenge the general assumption that retail costs for the NOACs in Germany may not be cost-effective. For this, quality-adjusted live years (QALYs), total costs (one time costs for events, rehabilitation costs for inpatient and ambulant care, inpatient medical treatment costs, daily costs for drugs) and incremental cost-effectiveness ratio (ICER) based on the data of the three large studies were analysed and compared.
Methods
Markov decision model and data sources
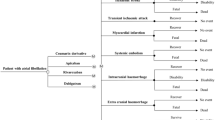
We used four structural identical, but different for input parameters, Markov decision models to compare 5 treatment options for the prevention of stroke in patients with NVAF [31, 32]. These treatments were dose-adjusted warfarin versus dabigatran 110 and 150 mg bid (RE-LY study [17]), warfarin versus rivaroxaban 20 mg od (ROCKET AF trial [19]) and warfarin versus apixaban 5 mg bid (ARISTOTLE trial [21]). The following health states and outcome events were included: healthy with NVAF, transient ischemic attack, ischemic stroke (fatal, moderate to severe, mild), hemorrhage (fatal, moderate to severe intracranial, mild intracranial, major non-cerebral, minor non-cerebral), myocardial infarction (MI), recurrent and combined events, and death (Fig. 1). We extracted or calculated the mortality rates (death from vascular cause and death from any other cause) from the clinical trials [17, 19, 21] and published mortality tables for the German population [33]. Definitions of these events were taken from the clinical studies [17, 19, 21]. Event probabilities were not included if they were not reported consistently across the studies (systemic embolism, pulmonary embolism, hemorrhagic stroke, bleeding in other location) (Tables 1, 2, 3, 4).
The base case population was a hypothetical cohort of patients with the starting age of 65 years with NVAF who were at increased risk for stroke (CHADS2-score > 1) and had no contraindications to anticoagulation, in accordance with the study data [17, 19, 21]. Our results were expressed in QALY, 2012 euro, and incremental cost-effectiveness ratios (ICER; total costs (€, NOAC)-total costs (€, warfarin)/QALY (NOAC)-QALY/warfarin)). We applied utilities and costs to each outcome yearly or event driven, and discounted costs and benefits at 5 % annually [34, 35]. A half cycle correction was done for each model, using a cycle length of 1 year. We quantified QALYs, risk for adverse events, and net cost for a time horizon of 20 years for the German population [36]. Our perspective is from a German public health care insurance.
Probability of adverse outcome events and of endpoints
The 95 % confidence intervals (CIs) for the RE-LY, ROCKET AF and ARISTOTLE studies were calculated from the published data [17, 19, 21] and were found to be identical, in case of dabigatran, as published by Freeman et al. [23].
Intention to treat values for ischemic stroke, myocardial infarction, death from cardio-vascular cause and death from non-vascular cause were taken from the RE-LY [17], ROCKET AF [19] and ARISTOTLE trials [21]. The on-treatment values (OT) were also taken for bleeding events (minor bleeding, major bleeding and ICH) [17, 19, 21]. These data were used for further calculations in the sensitivity analyses.
Severity of stroke and hemorrhage
Ischemic stroke was classified into four categories: fatal, moderate to severe, mild, and no neurologic deficit. This kind of classification was also used by Freeman et al. [23]. We assumed that a second mild ischemic stroke resulted in a moderate to severe ischemic stroke or death and that a second moderate stroke resulted in a severe ischemic stroke, reduced life quality or death. This assumption is analogous to that published by Shah and Gage [37].
Hemorrhage was classified in five categories: fatal, ICH with moderate to severe neurologic sequelae, ICH with no neurological deficit, major extracerebral hemorrhage, and minor extracerebral hemorrhage. This is categorization combines those described by Shah and Gage [37] and Freeman et al. [23], and, thereby represents a novel approach. If a moderate to severe ischemic stroke occurred in one patient together with or following an ICH a moderate to severe neurologic outcome was anticipated. Each outcome was assigned to a different decrease of quality of life and different costs based on the German health care system (in Euro [38]).
Mortality rates
We extracted or calculated the mortality rates (death from vascular cause and death from any other cause) from the clinical trials and published data for each treatment option. In detail the annual rates for death from vascular cause were 2.69 % for warfarin versus 2.43 % for dabigatran 110 mg (Table 1) and 2.28 % for dabigatran 150 mg (Table 2) [17]. Rates for warfarin versus rivaroxaban (Table 3) were 1.53 and 1.71 %, and for warfarin versus apixaban (Table 4) 2.02 and 1.80 % [19, 21].
The annual rates for death from any other cause were taken from published German mortality tables [33].
Quality of life utilities
To calculate quality-adjusted life years (QALY = survived life years adjusted for quality of live) [39]), we multiplied the time spent within a health state with the corresponding utility value. There was discounting for all utility values in our model [40]. We took the utility values for warfarin from data on patients with NVAF who underwent time trade-off and standard gamble methods to estimate their quality of life [23, 41]. A utility of “1” represents a completely healthy status and a utility of “0” represents death. The mean utility was 0.987 for warfarin, it was taken from published data [23, 41, 42]. To estimate the utilities for dabigatran, rivaroxaban and apixaban we used published estimates of utility for ximelagatran [23, 42]. This resulted in a utility of 0.994 for both doses of dabigatran; for our model it was assumed that these estimates were similar for rivaroxaban and apixaban [23, 42]. The utilities for the other diseases were taken from published data (Tables 1, 2, 3, 4) [23, 37, 41–46].
Costs for drugs and outcome events
Costs were expressed in Euro and reflected from the health care insurance perspective in Germany in 2012. The one-time costs for most events were taken from the Institute for payment regulations in German hospitals (Institut für Entgeldsystem im Krankenhaus, InEK) which included German-Diagnosis Related Groups (G-DRGs) [38]. Only the costs for bleeding events were taken from published literature because they are not included into the G-DRGs [47]. This analysis excluded indirect costs. We projected costs over a time horizon of 20 years for the German population [36]; future costs and life-years were discounted at 5 % (0 and 10 % in the sensitivity analyses) per year. Our discount rate was derived from the mean of common discount rates for better comparability between countries [34, 35, 48, 49]. Rehabilitation costs were used after ischemic strokes, ICH, and myocardial infraction, we assumed that other major bleedings were just temporally and did not need any rehabilitation.
We calculated 153€ as annual cost for warfarin therapy combining drug costs with average established patient office visits per year [50, 51] including a mean of three weeks as interval for INR measurements. The retail costs were taken from pharmacies and the “Red list” (German equivalent of “The Physicians’ Desk Reference Manual” in the US, for example) [50]. Cost ranges for the NOACs were set between less than the real-world retail costs and up to 5.00€ per day and for warfarin the costs ranges from 0.10€ per day up to 1.00€ per day. Additionally we calculated total costs for warfarin and NOAC groups, based on events from the three NOAC trials and on event costs according to the InEK. These entries were used to examine the cost-effectiveness of each NOAC, depending on the event probabilities from each trial (Tables 1, 2, 3, 4).
Sensitivity analyses
One-way sensitivity analyses of all variables were included in the decision models over their plausible ranges. Ranges for clinical events were derived from CI for event probabilities of the RE-LY, ROCKET AF, and ARISTOTLE trials [17, 19, 21]. Medication costs for warfarin/phenprocoumon ranged from 0.10 to 1.00€ per day [50, 51]. For the NOACs we evaluated a cost range from 1.00€ to a maximum of 5.00€ per day. Two-way sensitivity analyses were performed for combinations of stroke and ICH using the values of warfarin.
These results were then compared with data from other countries [23–25, 27–30, 52, 53].
Subgroup analyses with different discount rates (0 and 10 %) were done to analyse the influence of discount rates on the model [40]. These analyses are shown in detail in the technical appendix.
Probabilistic sensitivity analysis
The Monte Carlo simulation (MCS) were made using random sampling and random distribution of variables for 10,000 times to simulate outcomes in probabilistic sensitivity analyses. Beta distribution of the event probabilities was assumed for the calculation except for sub-categories of stroke using dirichlet distribution as in published data for every model to ensure a better comparability [23]. We used dirichlet distribution to show how probable our sub-classification could occur. Utilities followed a beta-distribution. We were calculating the maximum and minimum range of costs for each adverse event based on the German InEK and we used gamma- and log normal distribution.
Statistical methods
The models and analyses were created with TreeAge Pro 2012 and Microsoft Excel 2003.
Results
Base-case analysis
Dabigatran 110 mg bid versus warfarin
The quality-adjusted life expectancy was 7.64 QALYs with warfarin and 7.68 QALYs with dabigatran 110 mg bid. Total costs were 7622€ for warfarin and 20048€ for dabigatran 110 mg bid. The ICER for dabigatran 110 mg bid was 294349€ per QALY compared to warfarin (Table 5).
Dabigatran 150 mg bid versus warfarin
In the case of the higher dose of dabigatran the QALYs were 7.64 for warfarin and 7.71 for dabigatran 150 mg bid. Total costs were 7622€ for warfarin and 19537€ for dabigatran 150 mg bid. The ICER was 163184€ per QALY for dabigatran 150 mg bid compared to warfarin (Table 5).
Rivaroxaban 20 mg od versus warfarin
From the ROCKET AF trial 7.59 QALYs were calculated for patients under treatment with warfarin and 7.67 QALYs for patients on treatment with rivaroxaban, respectively. This resulted in total costs of 9069€ for warfarin and 19874€ for rivaroxaban over the whole observation period. The ICER for rivaroxaban was calculated with 133926€ per QALY compared to warfarin (Table 5).
Apixaban 5 mg bid versus warfarin
Calculations from the data of the ARISTOTLE trial resulted in quality-adjusted life years of 7.56 QALYs for warfarin and 7.75 QALYs for apixaban. Altogether the cost over of the whole observation period were 8915€ for warfarin and 19885€ for apixaban. The ICER compared apixaban with warfarin was 57245€ per QALY (Table 5).
One-way sensitivity analyses
Some variables showed greater importance for the outcome of the cost-effectiveness of the NOACs than others in the one-way sensitivity analyses. These key variables included drug costs, utilities for drugs, and risks for stroke and major bleeding for warfarin and NOACs.
Costs
The daily cost of NOACs had the greatest effect on the cost-effectiveness. At daily costs of dabigatran 110 mg bid from 1.00 to 5.00€ the ICERs varied from 97489€ per QALY to over 500000€ per QALY. For dabigatran 150 mg bid the ICERs ranged from 49904 to 372339€ per QALY at daily costs of 1.00–5.00€. Rivaroxaban had ICERs ranging from 40371 to 333132€ per QALY at 1.00–5.00€ per day, and for apixaban the ICERs were 9808–134784€ per QALY at 1.00–5.00€ per day. Increasing the costs of warfarin up to 1.00€ per day did not decrease the ICERs below 50000€ per QALY in case of all NOACs, except of apixaban bid.
Utility
The quality of life utilities for the drugs were sensitive in our models. ICERs stood between dominance for warfarin to above 290000€ per QALY for dabigatran 110 mg bid, above to 150000€ per QALY for dabigatran 150 mg bid, above to 120000€ per QALY for rivaroxaban and above to 50000€ per QALY for apixaban.
Ischemic stroke
The cost-effectiveness for the NOACs compared to warfarin was sensitive to changes in the rates of ischemic stroke. Over a range of different event probabilities, the ICERs for dabigatran 110 mg bid ranged from 200912€/QALY to over 500000€/QALY, for dabigatran 150 mg bid from 132622 to 209980€/QALY, for rivaroxaban from 105699 to 179773€/QALY and for apixaban from 52717 to 62443€/QALY compared to warfarin.
Risks for major bleeding
ICERs for the NOACs compared to warfarin changes moderately in relation to different risk-rates for major bleeding. The ranges of the ICERs for dabigatran 110 mg bid were 266979–327767€/QALY, for dabigatran 150 mg bid 153821–173693€/QALY, for rivaroxaban 127816–140591€/QALY and for apixaban 56380–58133€/QALY compared to warfarin.
Risk for intracerebral hemorrhage
The ICER differed slightly in case of ICH for all NOACs. Differences were about 1000€ (for apixaban) and 5000€ per QALY (for dabigatran 110 mg), dabigatran 150 mg and rivaroxaban were in between these values.
Two-way sensitivity analyses
The two-way sensitivity analyses of key variables for varying risk-rates for ischemic stroke and ICH showed that none of the NOACs were preferred as a therapy for combinations of moderate to high risks for ischemic stroke and any type of ICH at a set willingness to pay of 50000€ per QALY against INR dose adjusted warfarin.
Probabilistic sensitivity analyses—Monte Carlo simulation
We checked various willingness-to-pay thresholds using the probabilistic sensitivity analysis (PSA) in the MCS by varying all variables simultaneously. As results dabigatran 110 mg bid was cost-effective at willingness-to-pay threshold of 278000€ per QALY and higher (Fig. 2 in the technical appendix), dabigatran 150 mg bid at a threshold of 175500€ per QALY and higher (Fig. 3 in the technical appendix), rivaroxaban at a threshold of 136500€ per QALY and higher (Fig. 4 in the technical appendix), and apixaban at a threshold of 60500€ per QALY and higher (Fig. 5 in the technical appendix). The PSA results were similar to the base case results.
Subgroup analyses
Base-case data for a 65–85 year old cohort from the German public health care insurance perspective with 0 % discount
When discounting costs and utility values with 0 % the absolute numbers of QALYs and total costs increased and the ICER decreased (Table 7, technical appendix).
Base-case data for a 65–85 year old cohort from the German public health care insurance perspective with 10 % discount
When discounting costs and utility values with 10 % the absolute numbers of QALYs and total costs decreased, but the ICER increased (Table 8, technical appendix).
Discussion
The present study compares the cost-effectiveness of the four treatment regimens with dabigatran 110 mg bid, dabigatran 150 mg bid, rivaroxaban 20 mg od, and apixaban 5 mg bid for prevention of ischemic stroke and systemic embolic events in patients with NVAF based on the data of the RE-LY, ROCKET AF, and ARISTOTLE trial using the costs of the German health care system. Warfarin data of each study were used as comparator. The QALYs and ICERs gained versus warfarin differed between the NOACs. Dabigatran 110 mg bid was the least cost-effective and apixaban 5 mg bid the most cost-effective treatment with dabigatran 150 mg bid and rivaroxaban 20 mg od in between. From the public health care insurance view, none treatment regimen was cost-effective at a hypothetical willingness to pay threshold of EUR 50000 for patients at a moderate or higher risk of stroke (CHADS2-score > 1) compared to INR-adjusted warfarin with current German market costs.
Cost-utility analysis were reported so far for the two doses of dabigatran using health care costs and willingness to pay in US [23, 37], Canada [24], United Kingdom [25, 26], Denmark [27] and Sweden [28], for rivaroxaban in the US [29], as well as for all NOACs versus warfarin in the US [54], and as a comparative analysis for dabigatran and rivaroxaban in Canada [55]. All analyses for dabigatran used the Markov model for calculation of the QALYs and ICERs, and a one-way and two-way sensitivity analysis. In addition, we calculated these data for rivaroxaban and apixaban as well as for a certain range of daily costs for warfarin and daily costs of the NOACs for Germany. The cost data we used for the Markov model were comparable to those used in other countries [23–27, 29] (Table 6).
In the US, Freeman et al. showed 10.28 QALYs for warfarin in comparison to 10.70 QALYs for dabigatran 110 mg bid and 10.84 QALYs for dabigatran 150 mg bid [23]. Another cost-effectiveness study from the US showed 8.40 QALYs for warfarin, 8.54 QALYs for dabigatran 110 mg and 8.65 QALYs for dabigatran 150 mg [37]. Other results from the US reported 3.91 QALYs for warfarin and 4.27 QALYs for dabigatran 150 mg bid [53]. However, these and our data showed absolute differences of about 0.04 QALYs up to 0.56 QALYs gained by treatment with dabigatran over warfarin. This may be caused by the lower incidence of ischemic stroke and systemic embolism reported for the higher dose of dabigatran in the RE-LY study. For rivaroxaban about 0.10 higher QALYs were reported in the US [29] as in our study were 0.08 QALYs compared to warfarin using the data of the ROCKET AF trial. In the publication of Harrington et al. the QALYs were 8.41 for dabigatran 150 mg, 8.26 for rivaroxaban and 8.47 for apixaban [54]. For the present study apixaban shows improvement by about 0.19 QALYs as compared to warfarin based on the data of the ARISTOTLE trial. The detailed description of these data show that the absolute values for QALYs yield higher variations between studies than the relative QALYs gained with one treatment over another and across studies. The differences of QALYs between rivaroxaban and apixaban need confirmation across countries before drawing specific conclusions.
These data from the literature support our decision to use the warfarin control of every study individually because age, gender, CHADS2 score, and time in therapeutic range (TTR) of the INR differed between the studies. The individual control groups of every study are also used for indirect comparisons of the efficacy and safety of the 4 treatment regimens of the RE-LY, ROCKET AF, and ARISTOTLE trials [56]. Therefore we decided to perform our cost effectiveness analysis strictly only using the results of these studies and German mortality tables. Harrington et al. performed the analysis for each NOAC versus warfarin by pooling the data of the RE-LY, ROCKET AF, and ARISTOTLE studies. We used the data of the warfarin control group of the individual studies. For the cost effectiveness analysis we included the actual market prices from Germany which are available in the US only for dabigatran.
When comparing the ICERs for different countries results are related to the comparison of the QALYs in these countries. Data from the US showed that at a cost over 9.36$ per day for low-dose dabigatran, the ICER compared with warfarin exceeded 50000$ per QALY. At a cost over 13.70$ per day for high-dose dabigatran, the ICER compared with warfarin exceeded 50000$ per QALY. These results were robust over a wide range of model assumptions but were sensitive to dabigatran costs [23]. Other cost-utility analyses showed that the ICER were less than 25000$ per QALY at daily costs for warfarin of 1.14$ versus dabigatran 150 mg bid of 6.75$ [53]. Shah and Gage showed that 9$ for dabigatran 110 mg bid and 150 mg bid both exceeding 50000$ per QALY [37]. Others reported lower daily costs for both doses of dabigatran and only 12286$ per QALY using other input variables such as age below and above 80 years [52]. The cost-effectiveness model illustrated that costs of ICER were not exceeding 30000$ per QALY, when the costs for warfarin were 1$ per day and for rivaroxaban 6.80$ per day [29].
In our model daily costs of 3.38€ for dabigatran 110 mg bid and for dabigatran 150 mg bid and 3.20€ for rivaroxaban and for apixaban at daily costs of 3.54€ exceeded the (theoretical) willingness to pay threshold of 50000€ per QALY compared to daily costs of warfarin of 0.20€.
The comparisons show the high variation of results when comparing different daily costs for NOACs and warfarin depending on the input variables into the Markov model and the Monte-Carlo simulation. The results of our model using input variables for a >65 year old person with NVAF and a CHADS2 score > 1 and the costs for outcome events according to reimbursement by public health insurance demonstrate values for QALYs, total costs and ICERs in Germany within the range of other European and north-American countries [23–30, 37, 52, 53]. The difference to the other countries is that a willingness to pay threshold is not defined by authorities in Germany [35] and we therefore used a theoretical willingness to pay threshold.
Some limitations of our study have to be addressed. RE-LY trial was open for warfarin therapy and blinded for the two doses of dabigatran [17], whereas the ROCKET-AF trial [19] and ARISTOTLE trial [21] were double blind and double dummy. The three studies differed regarding the risk factor profile of patients included into the trial and the time in therapeutic range of the INR and some other biographic data. Only one study was available for each treatment option, treatment could not be changed in the Markov model, and a return to a complete health status following an event was not included because this was not reported in the publications [17, 19, 21]. The minor and non-major bleeding complications are reported differently in the studies and were included as reported. The investigation included a large set of variables which may increase the variance of the results: 4 NOACs, 7 endpoints, and sub-classifications of the outcome of events were included into the comparison. Quality of life utilities were taken from the literature [23, 42, 44, 45, 57] because they were not reported in the studies. In case of warfarin the quality of life value was taken from published data [23, 41, 42] and is slightly lower than for the NOACs. Probably one reason for a lower quality of life value for warfarin in comparison to the NOACs is the INR-determination to adjust the correct warfarin dose and therefore the visit of the doctor’s office. In none other CEA we found other warfarin values for quality of life different from the used in this model. The QALYs of the NOACs were taken from published data using ximelagatran [42]. However, the same approach was used in the literature to analyse the cost-effectiveness for one [23–28, 37] or more NOACs in NVAF [29] and following knee and hip replacement surgery [58]. The model utility values are mostly derived from published data, as you can see in the Tables 1, 2, 3, and 4. We had to made some assumptions, for myocardial infraction, major and minor bleeding events because the published data seemed to be different from our clinical practice experience. We lower the utility value after a MI and increased the utility value for major and minor bleeding events to correlate them with our clinical practice experience. It still can be questioned how far this influenced our model results; because of similar model results in other countries we think our approach is appropriate.
In conclusion, at current market costs of the NOACs none therapeutic regimens seem to be cost-effective from a German public health care insurance perspective. The larger reduction in medical cost by apixaban was mainly driven by reductions in the risks for ischemic stroke and major bleeding events as compared to the two doses of dabigatran and rivaroxaban. Real life use of NOACs for prevention of embolic events in patients with NVAF should be generated to identify cost effectiveness analyses in clinical practice for Germany and across countries.
References
Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22(8):983–988
Hart RG (1999) Warfarin in atrial fibrillation: underused in the elderly, often inappropriately used in the young. Heart 82(5):539–540
You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GY, Physicians ACoC (2012) Antithrombotic therapy for atrial fibrillation: antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e531S–e575S. doi:10.1378/chest.11-2304
Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Agladze V, Aliot E, Balabanski T, Blomstrom-Lundqvist C, Capucci A, Crijns H, Dahlof B, Folliguet T, Glikson M, Goethals M, Gulba DC, Ho SY, Klautz RJM, Kose S, McMurray J, Perrone Filardi P, Raatikainen P, Salvador MJ, Schalij MJ, Shpektor A, Sousa J, Stepinska J, Uuetoa H, Zamorano JL, Zupan I (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31(19):2369–2429. doi:10.1093/eurheartj/ehq278
Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M (2011) Canadian Cardiovascular Society Atrial Fibrillation Guidelines 2010: prevention of Stroke and Systemic Thromboembolism in Atrial Fibrillation and Flutter. Can J Cardiol 27(1):74–90. doi:10.1016/j.cjca.2010.11.007
Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr., Priori SG, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Tarkington LG, Yancy CW (2011) 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. In: Circulation, vol 123. vol 10. United States, pp e269-367. doi:10.1161/CIR.0b013e318214876d
Wann LS, Curtis AB, Ellenbogen KA, Estes Iii NAM, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ (2011) 2011 ACCF/AHA/HRS Focused update on the management of patients with atrial fibrillation (update on dabigatran)a report of the American college of cardiology foundation foundation/American heart association task force on practice guidelines. J Am Coll Cardiol 57(11):1330–1337. doi:10.1016/j.jacc.2011.01.010
Wann LS, Curtis AB, Ellenbogen KA, Estes NAM, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK (2011) 2011 ACCF/AHA/HRS Focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation 123(10):1144–1150. doi:10.1161/CIR.0b013e31820f14c0
Hylek EM, Skates SJ, Sheehan MA, Singer DE (1996) An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 335(8):540–546. doi:10.1056/nejm199608223350802
Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, Singer DE (2004) Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 141(10):745–752
Harenberg J (2009) New anticoagulants in atrial fibrillation. Semin Thromb Hemost 35(06):574–585. doi:10.1055/s-0029-1240018
Harenberg J (2008) Development of new anticoagulants: present and future. Semin Thromb Hemost 34(08):779–793. doi:10.1055/s-0029-1145260
Levine MN, Raskob G, Landefeld S, Kearon C (2001) Hemorrhagic complications of anticoagulant treatment. Chest 119(1 Suppl):108S–121S
Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, Singer DE (2003) Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 349(11):1019–1026. doi:10.1056/NEJMoa022913
van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ (2006) Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest 129(5):1155–1166. doi:10.1378/chest.129.5.1155
Dolan G, Smith LA, Collins S, Plumb JM (2008) Effect of setting, monitoring intensity and patient experience on anticoagulation control: a systematic review and meta-analysis of the literature. Curr Med Res Opin 24(5):1459–1472. doi:10.1185/030079908x297349
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361(12):1139–1151. doi:10.1056/NEJMoa0905561
Rationale and Design of the ROCKET AF study (2010) Rivaroxaban—Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation. American Heart Journal 159(3):340–347. doi:10.1016/j.ahj.2009.11.025
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM (2011) Rivaroxaban versus Warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):883–891. doi:10.1056/NEJMoa1009638
Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J (2010) Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J 159(3):331–339. doi:10.1016/j.ahj.2009.07.035
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L (2011) Apixaban versus Warfarin in patients with atrial fibrillation. N Engl J Med 365(11):981–992. doi:10.1056/NEJMoa1107039
Harenberg J, Marx S, Wehling M (2012) Head-to-head or indirect comparisons of the novel oral anticoagulants in atrial fibrillation: what’s next? Thromb Haemost. doi:10.1160/TH12-07-0463
Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, Wang PJ, Turakhia MP (2011) Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 154(1):1–11. doi:10.1059/0003-4819-154-1-201101040-00289
Sorensen SV, Kansal AR, Connolly S, Peng S, Linnehan J, Bradley-Kennedy C, Plumb JM (2011) Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation:a Canadian payer perspective. Thromb Haemost 105(5):908–919. doi:10.1160/th11-02-0089
Pink J, Lane S, Pirmohamed M, Hughes DA (2011) Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ (Clinical research ed) 343:d6333
Kansal AR, Sorensen SV, Gani R, Robinson P, Pan F, Plumb JM, Cowie MR (2012) Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 98(7):573–578. doi:10.1136/heartjnl-2011-300646
Langkilde L, Asmussen M, Overgaard M (2012) Cost-effectiveness of dabigatran etexilate for stroke prevention in non-valvular atrial fibrillation. Applying RE-LY to clinical practice in Denmark. J Med Econ 15(4):695–709. doi:10.3111/13696998.2012.673525.
Davidson T, Husberg M, Janzon M, Oldgren J, Levin LA (2012) Cost-effectiveness of dabigatran compared with warfarin for patients with atrial fibrillation in Sweden. Eur Heart J 34(3):177–183. doi:10.1093/eurheartj/ehs157
Lee S, Anglade MW, Pham D, Pisacane R, Kluger J, Coleman CI (2012) Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol 5(4):472–479. doi:10.1016/j.amjcard.2012.05.011
Deitelzweig S, Amin A, Jing Y, Makenbaeva D, Wiederkehr D, Lin J, Graham J (2012) Medical cost reductions associated with the usage of novel oral anticoagulants vs warfarin among atrial fibrillation patients, based on the RE-LY, ROCKET-AF, and ARISTOTLE trials. J Med Econ 15(4):776–785. doi:10.3111/13696998.2012.680555
Sonnenberg FA, Beck JR (1993) Markov models in medical decision making: a practical guide. Med Decis Mak 13(4):322–338
Beck JR, Pauker SG (1983) The Markov process in medical prognosis. Med Decis Mak 3(4):419–458
Amtliche Sterbetafeln und Entwicklung der Sterblichkeit (https://www.destatis.de/DE/Publikationen/WirtschaftStatistik/Bevoelkerung/AmtlicheSterbetafeln_32011.pdf?__blob=publicationFile,abgerufen am 27.01.2013)
Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB (1996) Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA, J Am Med Assoc 276(15):1253–1258
Adam H, Ahlert M, Breyer F, Cassel D, Daumann F, Eisen R, Ernst C, Felder S, Firnkorn H-J, v. d. Schulenburg J-M, Greiner W, Henke K-D, Jacobs K, Karmann A, Kifmann M, Kuhn M, Leidl R, Neubauer G, Nuscheler R, Oberender P, Prinz A, Ried W, Schellhorn M, Schneider M, Ulrich V, Wambach A, Wasem J, Wille E (2009) Dokumentation der Stellungnahmen zum „Entwurf einer Methodik für die Bewertung von Verhältnissen zwischen Nutzen und Kosten im System der deutschen gesetzlichen Krankenversicherung Version 2.0“. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Retrieved September 1, 2012 from https://www.iqwig.de/download/Wuerdigung_der_Stellungnahmen_KNB-Methodenentwurf_2.0.pdf
Statistisches Bundesamt Deutschland, Hrsg. Periodensterbetafeln für Deutschland 2007/2009 (http://www.destatis.de/jetspeed/portal/cms/Sites/destatis/Internet/DE/Content/Statistiken/Bevoelkerung/GeburtenSterbefaelle/AktuellLebenserwartung,templateId=renderPrint.psml,abgerufen am 05.07.2011)
Shah SV, Gage BF (2011) Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 123(22):2562–2570. doi:10.1161/circulationaha.110.985655
InEK gGmbH, Hrsg. Vereinbarung zum Fallpauschalensystem für Krankenhäuser für das Jahr 2011 (2010) (http://www.g-drg.de/cms/G-DRG-System_2011/Fallpauschalen-Katalog/Fallpauschalen-Katalog_2011; abgerufen am 10.07.2011). http://www.g-drg.de/cms/G-DRG-System_2011/Fallpauschalen-Katalog/Fallpauschalen-Katalog_2011
Drummond MF, Sculpher MJ, Torrance GW, O′Brien BJ, Stoddart GL (eds) (2005) Methods for the economic evaluation of health care programmes, 3rd edn. Oxford Univ. Press, NY
Hay JW, Smeeding J, Carroll NV, Drummond M, Garrison LP, Mansley EC, Mullins CD, Mycka JM, Seal B, Shi L (2010) Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report–Part I. Value Health 13(1):3–7. doi:10.1111/j.1524-4733.2009.00663.x
Gage BF, Cardinalli AB, Owens DK (1996) The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med 156(16):1829–1836
O’Brien CL, Gage BF (2005) Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA, J Am Med Assoc 293(6):699–706. doi:10.1001/jama.293.6.699
Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, Martin PA (1993) The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Mak 13(2):89–102
Sullivan PW, Ghushchyan V (2006) Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Mak 26(4):410–420. doi:10.1177/0272989x06290495
Tengs TO, Lin TH (2003) A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics Vols. 21,3:191–200
Thomson R, Parkin D, Eccles M, Sudlow M, Robinson A (2000) Decision analysis and guidelines for anticoagulant therapy to prevent stroke in patients with atrial fibrillation. Lancet 355(9208):956–962. doi:10.1016/s0140-6736(00)90012-6
Bufe A, Frey S, Briswalter S (2009) Durch Blutungen verursachte Kosten bei der Therapie des akuten Koronarsyndroms in Deutschland. Herz 34(6):479–484
Guidelines for the economic evaluation of health technologies: Canada [3rd Edition] (2006). Canadian Agency for Drugs and Technologies in Health, Ottawa
Von der Schulenburg JM, Greiner W, Jost F, Klusen N, Kubin M, Leidl R, Mittendorf T, Rebscher H, Schöffski O, Vauth C, Volmer T, Wahler S, Wasem J, Weber C, Konsens uMdH (2007) German recommendations on health economic evaluation—third and updated version of the Hanover Consensus. Gesundheitsökon Qualitätsmanage 12:285–290
Rote Liste Online. http://www.rote-liste.de. Accessed 28 April 2013
Hein L (2011) Arzneimittelverordnungs-Report 2011 - Antithrombotika und Antihämorrhagika. Springer-Verlag Berlin Heidelberg NewYork. doi:10.1007/978-3-642-21992-4_15
Adcock AK, Lee-Iannotti JK, Aguilar MI, Hoffman-Snyder CR, Wingerchuk DM, Wellik KE, Demaerschalk BM (2012) Is dabigatran cost effective compared with warfarin for stroke prevention in atrial fibrillation?: a critically appraised topic. Neurologist 18(2):102–107. doi:10.1097/NRL.0b013e318247bcb6
Kamel H, Johnston SC, Easton JD, Kim AS (2012) Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke 43(3):881–883. doi:10.1161/STROKEAHA.111.641027
Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC (2013) Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 44(6):1676–1681. doi:10.1161/STROKEAHA.111.000402
Kansal AR, Sharma M, Bradley-Kennedy C, Clemens A, Monz BU, Peng S, Roskell N, Sorensen SV (2012) Dabigatran versus rivaroxaban for the prevention of stroke and systemic embolism in atrial fibrillation in Canada. Comparative efficacy and cost-effectiveness. Thromb Haemost 108(4):672–682. doi:10.1160/TH12-06-0388
Harenberg J, Marx S, Diener HC, Lip GY, Marder VJ, Wehling M, Weiss C (2012) Comparison of efficacy and safety of dabigatran, rivaroxaban and apixaban in patients with atrial fibrillation using network metaanalysis. Int Angiol 31(4):330–339
Carr-Hill RA (1992) Health related quality of life measurement–Euro style. Health policy 20(3):321–328 Discussion 329–332
Lereun C, Wells P, Diamantopoulos A, Rasul F, Lees M, Sengupta N (2011) An indirect comparison, via enoxaparin, of rivaroxaban with dabigatran in the prevention of venous thromboembolism after hip or knee replacement. J Med Econ 14(2):238–244. doi:10.3111/13696998.2011.564699
Claes C, Mittendorf T, Grond M, Graf von der Schulenburg J-M (2009) Inkrementelle Kosteneffektivität von Dipyridamol + Acetylsalicylsäure in der Sekundärprävention bei ischämischem nichtkardioembolischem Schlaganfall. Med Klin 103(11):778–787. doi:10.1007/s00063-008-1122-z
Lee S, Anglade MW, Meng J, Hagstrom K, Kluger J, Coleman CI (2012) Cost-effectiveness of apixaban compared with aspirin for stroke prevention in atrial fibrillation among patients unsuitable for warfarin. Circ Cardiovasc Qual Outcomes 5(4):472–479. doi:10.1161/CIRCOUTCOMES.112.965251
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krejczy, M., Harenberg, J., Marx, S. et al. Comparison of cost-effectiveness of anticoagulation with dabigatran, rivaroxaban and apixaban in patients with non-valvular atrial fibrillation across countries. J Thromb Thrombolysis 37, 507–523 (2014). https://doi.org/10.1007/s11239-013-0989-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-013-0989-6