Abstract
Three species of beloniform fishes were found infected with species of Monogenoidea Bychowsky, 1937 during a survey of the diversity of parasites infecting the fishes of Moreton Bay, Queensland, Australia. The diagnoses of Hareocephalus Young, 1969 and Hemirhamphiculus Bychowsky & Nagibina, 1969 (both Polyonchoinea Bychowsky, 1937: Dactylogyridae Bychowsky, 1933) were emended. Hareocephalus thiasae Young, 1969, type-species of the genus, was recorded and redescribed from the gill lamellae of the stout longtom Tylosurus gavialoides (Castelnau) (Belonidae), and six new species of Hemirhamphiculus were described from the gill lamellae of three host species: Hemirhamphiculus exserocephalus n. sp. from T. gavialoides; Hemirhamphiculus choanophallus n. sp. and Hemirhamphiculus flagrum n. sp. from the river garfish Hyporhamphus regularis (Günther) (Hemiramphidae); and Hemirhamphiculus perexiguus n. sp., Hemirhamphiculus krabsi n. sp., and Hemirhamphiculus imcomptus n. sp. from the southeastern snub-nose garfish Arrhamphus sclerolepis krefftii (Steindachner) (Hemiramphidae). An unidentified species of the Suborder Microcotylinea Lebedev, 1972 (Heteronchoinea Boeger & Kritsky, 2001) was found infecting single specimens of each of H. regularis and A. sclerolepis krefftii. Parahemirhamphiculus Bychowsky & Nagibina, 1969 is placed in junior subjective synonymy with Hemirhamphiculus, and its three species, Parahemirhamphiculus pinguis Bychowsky & Nagibina, 1969, Parahemirhamphiculus brevilamellatus Zhang, 2001, and Parahemirhamphiculus longilamellatus An & Zhang, 1988 are transferred to Hemirhamphiculus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Beloniformes includes about 270 species of primarily marine fishes known as needlefishes (Belonidae, ~ 40 species), garfishes and halfbeaks (Hemiramphidae, ~ 60 species and Zenarchopteridae, ~ 60 species), flyingfishes (Exocoetidae, ~ 70 species), saures (Scomberesocidae, 5 species), and ricefishes (Adrianichthyidae, 37 species) (Froese & Pauly, 2017; Eschmeyer & Fong, 2017). Considering that many of these fishes are relatively common and have relatively wide geographic distributions within the world’s oceans, the diversity of the monogenoidean fauna infecting them is comparatively poorly known. Prior research on their monogenoidean parasites has focused primarily on those assigned to the Suborder Microcotylinea Lebedev, 1972 (Subclass Heteronchoinea Boeger & Kritsky, 2001) and infecting flyingfishes, whereas only 16 named species of Dactylogyridae Bychowsky, 1933 (Subclass Polyonchoinea Bychowsky, 1937: Dactylogyrinea Bychowsky, 1937) from 15 species of beloniforms have been previously recorded worldwide (Table 1).
During January, 2016, a survey to determine the diversity of the parasites infecting the fishes of Moreton Bay, Queensland, Australia, was initiated by Dr Thomas Cribb, University of Queensland, Australia. The fish fauna of Moreton Bay and the adjacent continental-shelf waters comprises a minimum of 1,190 species (Johnson, 2010). During the first collection effort held over ten days during January, 2016, approximately 100 species of marine fishes were necropsied for parasites infecting their various organs, including the gills, skin, muscles, gastrointestinal tract, and olfactory sacs. Of these potential hosts, three species of beloniforms were examined, including the stout longtom Tylosurus gavialoides (Castelnau) (Belonidae), the river garfish Hyporhamphus regularis (Günther) (Hemiramphidae); and the southeastern snub-nose garfish Arrhamphus sclerolepis krefftii (Steindachner) (Hemiramphidae). Among the group of investigators responsible for the various parasite taxa, the present author had responsibility for tabulating the monogenoidean fauna.
The present paper represents the first installment recording the monogenoids infecting the fishes of Moreton Bay and includes one previously described and six undescribed dactylogyrid species. An unidentified microcotylinean from the gills of these hosts was also encountered. The respective generic diagnoses of Hareocephalus Young, 1969 and Hemirhamphiculus Bychowsky & Nagibina, 1969 (both Dactylogyridae) are emended and the six new species of the latter genus are described.
Materials and methods
Beloniform fishes were collected by various methods from Moreton Bay, Queensland, Australia, and examined for monogenoids during January, 2016. Scientific and common names of hosts were those presented by Johnson (2010), and each was verified in Froese & Pauly (2017) and Eschmeyer et al. (2017). Hosts were transported alive to the Moreton Bay Research Station located in Dunwich, North Stradbroke Island, Queensland, where they were euthanised and their gill baskets immediately removed and placed in hot (60°C) sea water to relax and kill the attached helminths. A volume of 10% formalin, equal to that of the sea water of each container, was then added for fixation in 5% formalin. When necessary, excess fluid was decanted, and the fixed gills and sediments from each fish were placed in separate vials (except for gill baskets of the river garfish, which were pooled in a single vial), labeled, and shipped to Idaho State University for study. Dactylogyrids were subsequently removed from the gills or sediment using a small probe and dissecting microscope. Some specimens were mounted unstained in Gray & Wess medium for study of sclerotised structures; other specimens were stained with Gomori’s trichrome or VanCleaves’s hematoxylin (Kritsky et al., 1978; Pritchard & Kruse, 1982) and mounted in Canada balsam for observing internal anatomy. Illustrations were prepared with the aid of a camera lucida or microprojector. Measurements, all in micrometers, represented straight-line distances between extreme points and were expressed as the range followed by the mean and number (n) of structures measured in parentheses. Body length included that of the haptor. Direction of the coil of the male copulatory organ (MCO), clockwise vs counterclockwise, was determined using the method of Kritsky et al. (1985). Type- and voucher specimens of helminths collected for the present study were deposited in the Queensland Museum, Brisbane, Australia (QM); the Invertebrate Zoology Collection, National Museum of Natural History, Smithsonian Institution, Washington, D. C. (USNM); and the University of Nebraska State Museum, Harold W. Manter Laboratory, Lincoln, Nebraska (HWML) as indicated in the following species accounts. For comparative purposes, the following museum specimens were examined: holotype, 2 paratypes, Tylosuricola amatoi Iannacone & Luque, 1990 (USNM 1376667, 1376668); 8 types, Ancyrocephalus parvus Linton, 1940 (USNM 1320891); hypotype, A. parvus (USNM 1367816); 2 paratypes, Ancyrocephalus cornutus Williams & Rogers, 1972 (USNM 1367815); paratype, Ancyrocephalus trullae Williams, 1980 (USNM 1371672); 17 syntypes, Ancyrocephalus tylosuri (MacCallum, 1917) Johnston & Tiegs, 1922 (USNM 1371672); voucher specimen, Ancyrocephalus sp. (USNM 1375570)
Results
Specimens of three species of beloniform fishes were examined for species of Monogenoidea. These included 14 stout longtoms T. gavialoides, an unrecorded number of river garfish H. regularis, and two southeastern snub-nose garfish A. sclerolepis krefftii. The gill lamellae of all three host species were infected with species of Dactylogyridae: T. gavialoides with a species of each of Hareocephalus and Hemirhamphiculus; H. regularis with two species of Hemirhamphiculus; and A. sclerolepis krefftii with three species of Hemirhamphiculus. An unidentified member of the Suborder Microcotylinea was found infecting single specimens of H. regularis (2 parasites) and A. sclerolepis krefftii (1 parasite) (USNM 1459061, 1459060, respectively).
Subclass Polyonchoinea Bychowsky, 1937
Order Dactylogyridea Bychowsky, 1937
Dactylogyridae Bychowsky, 1933
Hareocephalus Young, 1969
Emended diagnosis
Body comprising body proper (cephalic region, trunk, and peduncle) and haptor; peduncle with non-muscular, inverted saucer-shaped organ from which haptor descends. Tegument smooth. Cephalic region broad, with terminal and two pairs of subterminal bilateral cephalic lobes; three bilateral pairs of large exaggerated head organs; paired groups of cephalic-gland cells anterior and posterolateral to pharynx. Eyespots present. Mouth midventral at level of anterior margin of pharynx; buccal tube apparently absent; pharynx a muscular glandular bulb; oesophagus, two intestinal caeca with lateral diverticula; caeca non-confluent posteriorly. Common genital pore midventral, immediately posterior to intestinal bifurcation. Gonads intercaecal, tandem (germarium pre-testicular). Testis lobulate or several in number; vas deferens not observed (apparently intercaecal); seminal vesicle a simple dilation of vas deferens; prostatic reservoir present. Copulatory complex comprising tubular MCO and accessory piece. Oviduct short; oötype receives ducts of vitellarium and seminal receptacle; Mehlis’ gland not observed; uterus extending anteriorly along body midline to common genital pore. Vaginal pore dextroventral in anterior trunk; vaginal canal extending to seminal receptacle. Vitelline follicles distributed throughout trunk, except absent in regions of other reproductive organs. Haptor with dorsal and ventral anchor/bar complexes, seven pairs of similar hooks with normal dactylogyrid distribution (Mizelle 1936; Mizelle & Price 1963); each hook with undilated shank of one subunit, protruding and terminally depressed thumb. Parasitic on gills of beloniform fishes (currently restricted to members of the Belonidae). Type- and only species: Hareocephalus thaisae Young, 1969 from the stout longtom Tylosurus gavialoides (Castelnau).
Remarks
Hareocephalus is unique among the Dactylogyridae by its species having a non-muscular saucer-shaped organ originating from the peduncle and from which the haptor descends. However, hook morphology, a putative indicator of ancient relationships among some members of the Dactylogyridae, is suggestive of a phylogenetic relationship of Hareocephalus with other dactylogyrid genera having species infecting beloniform fishes, including Hemirhamphiculus, Parahemirhamphiculus Bychowsky & Nagibina, 1969, Tribaculocauda Tripathi, 1959, Tylosuricola Unnithan, 1964, and some species considered incertae sedis and assigned to Ancyrocephalus Creplin, 1839 (sensu lato) and Pseudohaliotrematoides Yamaguti, 1953 (Pseudohaliotrematoides is currently considered a junior subjective synonym of Tetrancistrum Goto & Kikuchi, 1917; see Young, 1967 and Kritsky et al., 2007). Based on published accounts and on the examination of museum specimens (see Materials and methods), the hooks of these dactylogyrids all have protruding, terminally flattened thumbs that are generally uncharacteristic of species assigned to other dactylogyrid genera.
Hareocephalus is monotypic, and the type-species has been recorded only from stout longtoms from Moreton Bay, Australia. The emended generic diagnosis was provided to include the following new information. Young (1969) indicates that 12 hooks (6 pairs), rather than the usual 14 (7 pairs) present in dactylogyrids, were present in specimens comprising the type-series of H. thaisae. Young (1969) stated that all of the hooks lie anterior to the anchor/bar complexes, suggesting that he missed the pair associated with the shafts of the ventral anchors. Further, the accessory piece of the copulatory complex was not mentioned or figured in the original description of the type-species, but was clearly present in specimens collected for this study. The presence of perforations in the germarium and the diverticula and terminations of the intestinal caeca, depicted by Young (1969), were not confirmed in present specimens. Finally, it was uncertain whether the testicular field represents multiple testes or lobes of a single testis. Young (1969) did not mention vasa efferentia, which would be expected if multiple testes were present; vasa efferentia were not observed in the present specimens.
Hareocephalus thaisae Young, 1969
Type-host: Stout longtom Tylosurus macleayanus (Ogilby) [now Tylosurus gavialoides (Castelnau)] (Belonidae).
Type-locality: Moreton Bay, Queensland, Australia.
Other localities (present study): Moreton Bay off Peel Island (27°30′S, 153°20′E) (13 January 2016) and south of the Port of Brisbane (27°23′S, 153°11′28″E) (19–22 January 2016), Queensland, Australia.
Voucher material: 20 specimens, QM G236400–G236403, USNM 1459050, 1459051, HWML 139350, 139351.
Site in host: Gill lamellae.
Prevalence: 86% (12 of 14 stout longtoms infected).
Previous record: There have been no other records except that of the original description by Young (1969).
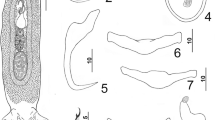
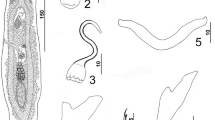
Redescription (Figs. 1–7)
Body dorsoventrally flattened. Cephalic region broad, with rounded terminal and poorly developed bilateral cephalic lobes. Head organs draining paired bilateral groups of post-pharyngeal cephalic-gland cells; paired pre-pharyngeal gland cells drained by separate ducts opening at cephalic margin between anterior-most pair of head organs. Two pairs of subequal eyespots dorsal to pharynx; members of posterior pair with lenses, slightly farther apart than those of anterior pair; chromatic granules minute, elongate ovate; free granules scattered throughout cephalic region. Mouth opening directly into pharynx; pharynx ovate, with distinct anterior and posterior regions, the latter with 6–8 short internal papillae extending into cavity of anterior portion of pharynx. Oesophagus moderately long, usually with small bilateral diverticula, bifurcating to form intestinal caeca; intestinal caeca obscured by dense vitellarium. Peduncle broad, tapered posteriorly, giving rise to non-muscular saucer-shaped organ from which the lappet-like haptor descends. Ventral anchor robust, with broad superficial root, knob-like deep root, evenly curved shaft and point; point extending slightly past level of tip of superficial root. Dorsal anchor delicate, with subequal roots, slightly curved to straight shaft, point extending to near level of tip of superficial root. Haptoral bars similar, each a slightly bowed rod having ventral groove; ventral bar elongate, with slightly inflated midregion; dorsal bar with ends directed anterolaterally. Each hook with delicate point, protruding and depressed thumb, uniform shank comprised of one subunit; filamentous hook (FH) loop about ¾ shank length. Common genital pore non-muscular. Gonads intercaecal. Testicular field in posterior half of trunk, comprised of six lobes or testes (anterior two juxtaposed, posterior four tandem). Seminal vesicle lying to left of body midline posterior to MCO. Horseshoe-shaped row of cells (prostates?) anterior to copulatory complex; small subspherical prostatic reservoir near base of MCO. Accessory piece basally articulated to MCO by elongate articulation process. MCO with inverted vase-shaped base, delicate tubular shaft forming a loose counterclockwise coil of slightly more than one ring; accessory piece variable, lightly sclerotised (often difficult to visualize), with distal complex of branches. Germarium forming cap over anterior end of testicular field, giving rise to delicate inflated (usually empty) uterus. Vaginal pore dextroventral at level of oötype; vaginal canal delicate, with small distal flare; seminal receptacle subspherical. Vitellarium dense, coextensive with intestinal caeca and often extending into anterior portion of peduncle, absent in saucer-shaped organ of peduncle; vitelline ducts empty into oötype immediately anterior to germarium. Mature egg not observed.
Measurements
Body 1,430–1,960 (1,680; n = 11); width at level of germarium 400–547 (455; n = 11). Haptor 140–187 (164; n = 11) wide; peduncular cup 417–586 (498; n = 9) wide. Ventral anchor 55–65 (59; n = 7) long; dorsal anchor 35–46 (39; n = 5) long. Ventral bar 69–84 (74; n = 6) long; dorsal bar 33–37 (36; n = 5) long. Hook 15–18 (17; n = 15) long. Pharynx 125–158 (141; n = 11) long, 88–116 (99; n = 11) wide. Ring diameter of MCO 31–42 (37; n = 5). Testicular field 377–603 (493; n = 11) long, 146–219 (188; n = 11) wide. Germarium 93–140 (118; n = 11) long, 117–179 (144; n = 11) wide.
Remarks
Hareocephalus thaisae is the type- and only species assigned to the genus.
Hemirhamphiculus Bychowsky & Nagibina, 1969
Emended diagnosis
Body comprising body proper (cephalic region, trunk and peduncle) and haptor. Tegument smooth. Terminal and two bilateral subterminal cephalic lobes; three bilateral pairs of head organs; cephalic-gland cells lateral or posterolateral to pharynx. One or two pairs of eyespots. Mouth midventral, subterminal at level of head organs, opens into buccal tube; buccal tube extends posteriorly along midline of cephalic region to pharynx to form buccal cavity; pharynx muscular, glandular; oesophagus present; intestinal caeca two, lacking diverticula, confluent posterior to gonads. Common genital pore midventral at or just posterior to intestinal bifurcation; genital atrium unarmed or armed with spines. Gonads intercaecal, overlapping. Testis entire or lobulate; vas deferens apparently looping left intestinal caecum; one or two seminal vesicles simple dilations of vas deferens; prostatic reservoir present. Copulatory complex comprising tubular MCO basally articulated to accessory piece. Germarium lying ventral or dextroventral to testis. Oviduct short; oötype receives ducts of vitellarium and seminal receptacle; Mehlis’ gland inconspicuous or absent; seminal receptacle anterior to gonads; uterus extending anteriorly along body midline to common genital pore. Vaginal pore dextroventral in anterior trunk; vaginal canal usually unsclerotised, extending to seminal receptacle. Vitellarium coextensive with intestinal caeca. Haptor with dorsal and ventral anchor/bar complexes, seven pairs of similar hooks with normal dactylogyrid distribution (Mizelle 1936; Mizelle & Price 1963). Anchor/bar complexes similar; bars rod shaped. Hook with undilated shank of one or two subunits, with protruding and terminally flattened thumb. Parasitic on gills of beloniform fishes.
Type-species: Hemirhamphiculus armatus Bychowsky & Nagibina, 1969 from the black-barred halfbeak Hemiramphus far (Forsskål).
Other species: Hemirhamphiculus brevilamellatus (Zhang, 2001) n. comb. from the Asian pencil halfbeak Hemiramphus intermedius Cantor [now Hyporhamphus intermedius (Cantor)]; Hemirhamphiculus choanophallus n. sp. and Hemirhamphiculus flagrum n. sp. both from the river garfish Hyporhamphus regularis (Günther); Hemirhamphiculus exserocephalus n. sp. from the stout longtom Tylosurus gavialoides (Castelnau); Hemirhamphiculus incomptus n. sp., Hemirhamphiculus krabsi n. sp., and Hemirhamphiculus perexiguus n. sp. all from the southeastern snub-nose garfish Arrhamphus sclerolepis krefftii (Steindachner); Hemirhamphiculus longilamellatus (An & Zhang, 1988) n. comb. from Ablennes anastomella (Valenciennes) [now Strongylura anastomella (Valenciennes)]; Hemirhamphiculus pinguis (Bychowsky & Nagibina, 1969) n. comb. from Hemiramphus far (Forsskål); Hemirhamphiculus similis Bychowsky & Nagibina, 1969 from the long billed half beak Hemiramphus georgii Valenciennes [now Rhynchorhamphus georgii (Valenciennes)] and Dussumier’s halfbeak Hemiramphus dussumieri Valenciennes [now Hyporhamphus dussumieri (Valenciennes)].
Remarks
Hemirhamphiculus was proposed by Bychowsky & Nagibina (1969) for two dactylogyrid species, H. armatus and H. similis, parasitizing the gills of halfbeaks from the western Pacific Ocean. The genus was characterised by its members having a haptor poorly separated from the body proper, a pair of eyespots, a dextral vaginal pore and weakly sclerotised vaginal canal, an elongate germarium overlapping the testis, an armed genital atrium, a vas deferens looping the left intestinal caecum, and by lacking an accessory piece in the copulatory complex. Other characters mentioned in the generic diagnosis by Bychowsky & Nagibina (1969) were those generally characterising species of most other dactylogyrid genera.
In the same paper, Bychowsky & Nagibina (1969) proposed the monotypic Parahemirhamphiculus for Parahemirhamphiculus pinguis Bychowsky & Nagibina, 1969. Parahemirhamphiculus was primarily differentiated from Hemirhamphiculus by its species having an unarmed genital atrium, an intercaecal vas deferens, and an accessory piece in the copulatory complex. The discovery of new dactylogyrid species from Australia, that clearly belong to the Hemirhamphiculus/Parahemirhamphiculus complex, suggested that a single genus should be recognised to accommodate the six new species described herein and the three species described by Bychowsky & Nagibina (1969). Whereas morphologies of the reproductive systems and the haptoral armament of all nine species were similar, the main features that Bychowsky & Nagibina (1969) used to differentiate the two taxa do not appear to justify separate genera. In the species described herein, the genital atrium may or may not be armed, the route of the vas deferens could not be verified in any of the new species because of the dense vitellarium, and the copulatory complexes were lightly sclerotised such that the MCOs and accessory pieces were observed in individual specimens with difficulty or not observed at all. As such, the three defining features of Parahemirhamphiculus could not reliably discriminate the nine species into two taxonomic entities. Thus, Parahemirhamphiculus was herein considered a subjective synonym of Hemirhamphiculus, and P. pinguis was transferred to the latter genus as Hemirhamphiculus pinguis (Bychowsky & Nagibina, 1969) n. comb. Two subsequently described species of Parahemirhamphiculus, Parahemirhamphiculus brevilamellatus Zhang, 2001 and Parahemirhamphiculus longilamellaltus An & Zhang, 1988, were also transferred as Hemirhamphiculus brevilamellatus (Zhang, 2001) n. comb. and Hemirhamphiculus longilamellaltus (An & Zhang, 1988) n. comb., respectively. Hemirhamphiculus was chosen as the valid genus for all of these species because its diagnosis was presented first in the paper by Bychowsky & Nagibina (1969).
Hemirhamphiculus exserocephalus n. sp.
Type-host: Stout longtom Tylosurus gavialoides (Castelnau) (Belonidae).
Type-locality: Moreton Bay, south of the Port of Brisbane (27°23′S, 153°11′28″E) (19–22 January 2016), Queensland, Australia.
Other localities: Moreton Bay off Peel Island (27°30′S, 153°20′E) (13 January 2016); off Green Island (27°26′S, 153°20′E) (12 January 2016); and off Dunwich (27°50′S, 153°40′E) (12 January 2016), Queensland, Australia.
Type-material: Holotype, QM G236404; 41 paratypes, QM 236405–236417, USNM 1459052, 1459053, HWML 139352.
Site in host: Gill lamellae.
Prevalence: 100% (14 stout longtoms examined).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Hemirhamphiculus exserocephalus n. sp. is urn:lsid:zoobank.org:act:FEBA8A8D-D7BD-4A7B-B555-E6ECD84CE5CE.
Etymology: The specific name (a noun) is from Latin (exser/o = to thrust forth + cephal/o = head) and refers to the enlarged cephalic region projecting from the trunk of the worm.
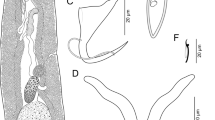
Description (Figs. 8–16)
Body flattened dorsoventrally, with elongate conspicuous cephalic region, robust trunk, short peduncle. Cephalic region with rounded terminal and moderately developed bilateral cephalic lobes; head organs well developed; cephalic-gland cells lateral to pharynx. Two pairs of eyespots; posterior pair always present, each member with lens; anterior pair of eyespots usually absent or infrequently represented by single eyespot, lacking lenses; chromatic granules minute, ovate; free granules absent or few in cephalic region. Pharynx elongate ovate; oesophagus short to moderately long. Haptor with well-developed bilateral lobes containing hook pairs 2–4, 6, 7. Ventral and dorsal anchors similar, each with moderately long roots (superficial root longest), slightly arcing shaft, elongate point extending just past level of tip of superficial root; dorsal anchor slightly smaller than ventral anchor. Ventral bar rod shaped, narrow, with enlarged ends directed laterally; dorsal bar a U- or V-shaped rod with slightly enlarged ends. Each hook with delicate point, robust depressed thumb, short shank composed of two subunits; proximal subunit of shank often difficult to observe; FH loop approaching shank length. Genital atrium unarmed, with weakly developed wall. Testis bacilliform, composed of several to many closely appressed lobes, lying in posterior half of trunk; vas deferens not observed. Two fusiform seminal vesicles posterior to MCO; proximal vesicle overlying left intestinal caecum; distal vesicle lying to right of body midline. Prostatic reservoir subovate, lying dorsal to right seminal vesicle, containing light reticulate or granular material. MCO comprising tapered tubular shaft arising from expanded proximal base, terminating in strong recurve and with diagonal distal aperture. Accessory piece variable, lightly sclerotised (often difficult to observe), distally expanded, articulated to base of MCO by articulation process. Germarium elongate, pyriform, narrow, lying ventral to testis along body midline, giving rise to delicate slightly inflated uterus; oötype apparently immediately anterior to germarium on body midline; Mehlis’ gland not observed. Vaginal pore in dextral region of anterior trunk lacking vitelline follicles; vaginal canal not observed. Seminal receptacle a small, delicate, spherical vesicle overlying oötype. Vitellarium dense, absent in regions of other reproductive organs; vitelline ducts entering oötype immediately anterior to gonads. Egg not observed.
Measurements
Body 366–648 (495; n = 29); greatest width of trunk 113–210 (160; n = 33). Haptor 137–188 (159; n = 29) wide. Ventral anchor 25–33 (30; n = 10) long; dorsal anchor 24–30 (28; n = 10) long. Ventral bar 53–66 (60; n = 10) long; dorsal bar 44–58 (50; n = 9) long. Hook 14–19 (16; n = 26) long. Pharynx 40–59 (52; n = 27) long, 25–33 (29; n = 27) wide. MCO 21–26 (24; n = 9) long. Testis 89–147 (120; n = 22) long, 42–71 (56; n = 22) wide. Germarium 64–117 (89; n = 22) long, 16–31 (23; n = 22) wide.
Remarks
Hemirhamphiculus exserocephalus n. sp. differs from all other congeners by having the germarium lying ventral to the midventral surface of the testes. In all other species of Hemirhamphiculus, the germarium lies along the dextral or dextroventral margins of the testis.
Based on the comparative morphology of the haptoral armament, H. exserocephalus is most similar to H. incomptus. It differs from H. incomptus by having a small, poorly developed and unarmed genital atrium (atrium comparatively large and with ten or 11 internal papillae with sclerotised tips in H. incomptus), ventral anchors slightly larger than the dorsal anchors (dorsal anchor larger in H. incomptus), an MCO with a strong distal recurve of the shaft (MCO a straight inverted funnel lacking a distal recurve in H. incomptus), and a longer dorsal bar with slightly expanded ends (dorsal bar comparatively short, with narrowed ends in H. incomptus).
Hemirhamphiculus choanophallus n. sp.
Type-host: River garfish Hyporhamphus regularis (Günther) (Hemiramphidae).
Type-locality: Moreton Bay off Peel Island (27°30′S, 153°20′E), Queensland, Australia (13 January 2016).
Type-material: Holotype, QM G236432; 38 paratypes, QM G236433–G236446, USNM 1459057, HWML 139355.
Site in host: Gill lamellae.
Prevalence: Not available (gills of several hosts were combined during field collection).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Hemirhamphiculus choanophallus n. sp. is urn:lsid:zoobank.org:act:842F10B9-FA28-41FA-BD15-7ED3FB51FE9C.
Etymology: The specific name (a noun) was derived from Greek (choane = a funnel + phallos = the penis) and refers to the funnel-shaped MCO.
Description (Figs. 17–23)
Body proper flattened dorsoventrally, comprising elongate conspicuous cephalic region, robust pyriform trunk, short to nonexistent peduncle. Cephalic region with poorly developed cephalic lobes, well-developed head organs, paired bilateral groups of cephalic-gland cells posterolateral to pharynx. One pair of eyespots, representing posterior pair (one paratype with unpaired anterior eyespot lacking lens); each member of posterior pair with lens often obscured by chromatic granules; chromatic granules small, ovate; free granules absent or few in cephalic region. Pharynx ovate; oesophagus short to moderately long. Haptor with well-developed bilateral lobes containing hook pairs 3, 4, 6, 7. Ventral and dorsal anchors similar, each with moderately long roots (superficial root longest), arcing shaft, elongate point extending just past level of tip of superficial root; dorsal anchor slightly smaller than ventral anchor. Bars similar, rodshaped; ventral bar narrow with enlarged ends directed anterolaterally; dorsal bar somewhat shorter than ventral bar, broadly U shaped, with slightly enlarged ends directed anterolaterally. Each hook with delicate point, robust depressed thumb, short shank composed of two subunits; proximal subunit of shank often difficult to observe; FH loop approaching shank length. Genital atrium muscular, with two zones of six to eight spines. Testis ovate, entire, lying in posterior half of trunk; vas deferens, seminal vesicle not observed; prostatic reservoir containing fine granular material and lying to right of base of MCO. MCO an elongate inverted funnel with minimally developed base, diagonal distal aperture; accessory piece weekly sclerotised (most often not visible), articulated to base of MCO by articulation process. Germarium elongate, narrow, lying along dextroventral margin of testis, giving rise to delicate slightly inflated (usually empty) uterus; oötype apparently immediately anterior to germarium on body midline; Mehlis’ gland not observed. Vaginal pore unarmed; vaginal canal inflated, weakly sclerotised. Seminal receptacle a small, delicate, spherical vesicle overlying oötype. Vitellarium dense, absent in regions of other reproductive organs; vitelline ducts entering oötype immediately anterior to gonads. Single egg occasionally present in utero, usually collapsed, lacking filaments; overall shape of egg undetermined.
Measurements
Body 243–417 (332; n = 27); width at level of testis 102–183 (148; n = 27). Haptor 123–172 (147; n = 26) wide. Ventral anchor 24–29 (27; n = 12) long; dorsal anchor 24–29 (27; n = 12) long. Ventral bar 62–75 (68; n = 12) long; dorsal bar 50–66 (60; n = 12) long. Hook 13–16 (15; n = 31) long. Pharynx 28–39 (34; n = 23) long, 21–29 (25; n = 16) wide. MCO 28–40 (36; n = 11) long. Testis 62–98 (74; n = 25) long, 39–63 (48; n = 25) wide. Germarium 42–72 (63); n = 22) long, 15–23 (19; n = 22) wide.
Remarks
Hemirhamphiculus choanophallus n. sp. most closely resembles H. similis in the morphology of the anchor/bar complexes of the haptor. It is distinguished from H. similis by lacking an armed vaginal pore (present in H. similis) and by having longer ventral and dorsal haptoral bars and a copulatory complex comprised of an MCO and accessory piece [accessory piece reported to be absent in H. similis by Bychowsky & Nagibina (1969)]. However, the presence/absence of an accessory piece may not represent a suitable differentiating character between the two species, because it is often weakly sclerotised and difficult or impossible to discern in some species of Hemirhamphiculus. Indeed, the complete structure could not be determined in H. choanophallus during the present study because of its delicate nature, and in some specimens of the species, it was not observed at all.
Hemirhamphiculus flagrum n. sp.
Type-host: River garfish Hyporhamphus regularis (Günther) (Hemiramphidae).
Type-locality: Moreton Bay off Peel Island (27°30′S, 153°20′E), Queensland, Australia (13 January 2016).
Type-material: Holotype, QM G236424; 22 paratypes, QM G236425–G236431, USNM 1459056, HWML 139354.
Site in host: Gill lamellae.
Prevalence: Not available (gills of several hosts combined during field collection).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Hemirhamphiculus flagrum n. sp. is urn:lsid:zoobank.org:act:72AC1CA1-D202-4E5B-AB35-227414401C6A.
Etymology: The specific name (a noun) is from Latin (flagrum = a whip) and refers to the accessory piece of the copulatory complex.
Description (Figs. 24–30)
Body proper flattened dorsoventrally, comprising elongate conspicuous cephalic region, robust pyriform trunk, short to nonexistent peduncle. Cephalic region with poorly developed terminal and bilateral cephalic lobes, well-developed head organs, paired bilateral groups of cephalic-gland cells lateral to pharynx. One pair of eyespots representing posterior pair; each eyespot with lens often obscured by chromatic granules; chromatic granules small, ovate; free granules absent or few in cephalic region. Pharynx ovate; oesophagus short to moderately long. Haptor with well-developed bilateral lobes containing hook pairs 3, 4, 6, 7. Ventral anchor with moderately long roots (superficial root longest), arcing shaft, elongate point extending to near level of tip of superficial root. Dorsal anchor with superficial root approximately perpendicular to anchor base, arcing anchor shaft; elongate anchor point extending to near level of tip of superficial root. Ventral bar rod shaped, narrow, with slightly enlarged ends directed anterolaterally; rod-like dorsal bar slightly shorter than ventral bar, broadly U-shaped, with slightly enlarged anterolaterally directed ends. Each hook with delicate point, robust depressed thumb, short shank composed of two subunits; proximal subunit of shank small, often difficult to observe; FH loop approaching shank length. Genital atrium muscular, unarmed. Testis entire, ovate, lying in posterior half of trunk; vas deferens, seminal vesicle, prostatic reservoir not observed. MCO an elongate tube with slightly expanded base, diagonal distal aperture, and distally tapering to fine elongate filament. Accessory piece elongate, whip like, meandering. Germarium elongate, narrow, lying along dextroventral margin of testis, giving rise to delicate slightly inflated uterus; oötype apparently immediately anterior to germarium on body midline; Mehlis’ gland not observed. Vaginal pore unarmed; vaginal canal weakly sclerotised, delicate extending to small spherical seminal receptacle lying near anterior end of germarium. Vitellarium dense, absent in regions of other reproductive organs; vitelline ducts entering oötype immediately anterior to gonads. Egg not observed.
Measurements
Body 269–340 (308; n = 11); width at level of testis 83–124 (104; n = 12). Haptor 97–119 (108; n = 8) wide. Ventral anchor 23–27 (25; n = 11) long; dorsal anchor 23–26 (25; n = 11) long. Ventral bar 32–47 (40; n = 11) long; dorsal bar 35–44 (40; n = 10) long. Hook 13–16 (14; n = 30) long. Pharynx 23–31 (27; n = 7) long, 21–23 (22; n = 7) wide. MCO 32–36 (34; n = 3) long (length of MCO does not include distal filament). Testis 50–72 (59; n = 10) long, 34–50 (40; n = 10) wide. Germarium 56–69 (62; n= 5) long, 17–21 (18; n = 5) wide.
Remarks
Hemirhamphiculus flagrum n. sp. is the only named species assigned to the genus having a whip-like accessory piece of the copulatory complex. It most closely resembles H. similis in the morphology of the haptoral sclerites and MCO. In addition to the comparative morphology of the accessory piece, H. flagrum is further distinguished from H. similus by having an unarmed genital atrium (atrium spined in H. similis), a longer MCO tapering to a fine distal filament (tapered distal end of the MCO in H. similis comparatively short), the superficial root of the dorsal anchor about perpendicular to the anchor base (superficial root less than 90° from anchor base in H. similis), and by lacking a spinous sclerite within the vaginal vestibule (present in H. similis).
Four specimens of a putative undescribed species of Hemirhamphiculus collected from the river garfish during the present study, were initially included in the type-series of H. flagrum. These specimens closely resembled H. flagrum as described above by having a similar copulatory complex, haptoral anchors, bars, and hooks (see Figs. 52–57). However, during the development of the description of H. flagrum, some subtle differences were observed in these four specimens that suggested that they may belong to distinct species of Hemirhamphiculus. These features included noticeably longer dorsal and ventral bars and smaller dorsal and ventral anchors. Whereas the superficial roots of the dorsal anchors of the four specimens approaches perpendicular to the anchor shaft as it does in H. flagrum, the roots were more robust and stubby than those of H. flagrum (compare Figs. 28, 57). In addition, the whip-like accessory pieces in these specimens appeared to meander to a lesser extent than that observed in H. flagrum, the haptoral bars were more delicate than those of H. flagrum, and the superficial and deep roots of the ventral anchors were shorter and more robust than those of H. flagrum. The four specimens were insufficient for a complete description or for determination of whether or not they represented intraspecific variation within H. flagrum. As a result, they were identified below as Hemirhamphiculus sp.
Hemirhamphiculus perexiguus n. sp.
Type-host: Southeastern snub-nose garfish Arrhamphus sclerolepis krefftii (Steindachner) (Hemiramphidae).
Type-locality: Moreton Bay, north Shore off Wynnum, Queensland, Australia (27°25’S, 153°11’E) (18 January 2016).
Type-material: Holotype, QM G236418; 15 paratypes, QM G236419–G236423, USNM 1459054, HWML 139353.
Site in host: Gill lamellae.
Prevalence: 100% (2 southeastern snub-nose garfish examined).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Hemirhamphiculus perexiguus n. sp. is urn:lsid:zoobank.org:act:FE57A1B5-B472-4CB3-AC29-333A8F6568A9.
Etymology: The specific name (an adjective) is from Latin (per- = very + exiguus = small) and refers to the comparatively small size of the helminth relative to that of most other dactylogyrids.
Description (Figs. 31–37)
Body proper flattened dorsoventrally, comprising broad elongate cephalic region, robust pyriform trunk, short to nonexistent peduncle. Cephalic region with poorly developed terminal and two moderately developed bilateral cephalic lobes, well-developed head organs, paired bilateral groups of cephalic-gland cells lying on each side of pharynx. One pair of eyespots always present, representing posterior pair (one immature specimen lacking eyespots); each eyespot with lens often obscured by chromatic granules; chromatic granules small, ovate; free chromatic granules absent or few in cephalic region. Pharynx subspherical; oesophagus short. Haptor with well-developed bilateral lobes containing hook pairs 2, 3, 4, 6. Ventral and dorsal anchors similar, each with moderately long roots (superficial root longest), arcing shaft, elongate point extending to near or slightly past level of tip of superficial root; dorsal anchor slightly smaller than ventral anchor. Haptoral bars similar; each rod shaped, with slightly enlarged ends directed anterolaterally. Each hook with delicate point, terminally flattened thumb, shank composed of two subunits; proximal subunit of shank small, often difficult to observe; FH loop approaching shank length. Genital atrium unarmed, with thick muscular wall having sclerotised anterior internal ridge. Testis ovate, lying in posterior half of trunk; vas deferens, seminal vesicle not observed; prostatic reservoir twisted, containing fine granular material, lying to right and slightly posterior to base of MCO. MCO a tapered tube with minimally developed base, reflexed expanded distal end; accessory piece not observed or absent. Germarium elongate, narrow, lying along dextroventral margin of testis, giving rise to delicate slightly inflated uterus; oötype apparently immediately anterior to germarium on body midline; Mehlis’ gland not observed. Vaginal pore inconspicuous; vaginal canal weakly sclerotised, with proximal portion obscured by vitellarium. Seminal receptacle not observed. Vitellarium dense, absent in regions of other reproductive organs; vitelline ducts entering oötype immediately anterior to gonads. Egg not observed.
Measurements
Body 192–255 (227; n = 8); width at level of testis 68–114 (94; n = 8). Haptor 76–128 (109; n = 8) wide. Ventral anchor 23–26 (25; n = 8) long; dorsal anchor 22–26 (23; n = 8) long. Ventral bar 37–44 (40; n = 6) long; dorsal bar 33–43 (38; n = 8) long. Hook 11–13 (12; n = 18) long. Pharynx 28–26 (23; n = 8) long, 17–22 (19; n = 8) wide. MCO 11–16 (13; n = 6) long. Testis 40–55 (49; n = 5) long, 27–34 (30; n = 5) wide. Germarium 33–46 (40; n = 5) long, 17–21 (18; n = 5) wide.
Remarks
Hemirhamphiculus perexiguus n. sp. is the smallest of the named species assigned to the genus. It most closely resembles H. krabsi in the general morphology of the anchor/bar complexes of the haptor. It differs from H. krabsi by lacking sclerotised caps on the papillae within the genital atrium, and by having shorter haptoral bars and an MCO with a recurved tip (MCO sigmoid and without distal recurve in H. krabsi).
Hemirhamphiculus krabsi n. sp.
Type-host: Southeastern snub-nose garfish Arrhamphus sclerolepis krefftii (Steindachner) (Hemiramphidae).
Type-locality: Moreton Bay, north Shore off Wynnum, Queensland, Australia (27°25’S, 153°11’E) (18 January 2016).
Type-material: Holotype, QM G236455; 9 paratypes, QM G236456–G236458, USNM 1459059, HWML 139357.
Site in host: Gill lamellae.
Prevalence: 100% (2 southeastern snub-nose garfish examined).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Hemirhamphiculus krabsi n. sp. is urn:lsid:zoobank.org:act:2DB3155C-66BC-4500-874A-D8A1FEC84456.
Etymology: The specific name (krabsi) was chosen because of the similar body shape of the species to that of Eugene H. Krabs (Mr. Krabs), a cartoon character in the children’s animated television series SpongeBob SquarePants.
Description (Figs. 38–44)
Body proper flattened dorsoventrally, comprising elongate conspicuous cephalic region, robust pyriform trunk, short to nonexistent peduncle. Cephalic region with well-developed terminal, bilateral cephalic lobes, well-developed head organs, paired bilateral groups of cephalic-gland cells lateral to pharynx. One pair of eyespots representing posterior pair; each eyespot with lens; chromatic granules small, ovate, absent or few in cephalic region. Pharynx elongate ovate; oesophagus moderately long. Haptor with well-developed bilateral lobes containing hook pairs 2–4, 6. Ventral and dorsal anchors similar, each with moderately long roots (superficial root longest), arcing shaft, elongate point extending just past level of tip of superficial root; dorsal anchor slightly longer than ventral anchor. Haptoral bars similar, rod shaped; each with slightly enlarged ends directed anterolaterally. Each hook with delicate point, robust depressed thumb, short shank composed of two subunits; proximal subunit of shank often difficult to observe; hooks of pair 3 slightly longer than remaining hooks; FH loop approaching shank length. Genital atrium muscular, armed with about ten internal papillae; each papilla terminating in small sclerotised spine. Testis ovate, lying in posterior half of trunk; vas deferens, seminal vesicle not observed; prostatic reservoir containing fine granular material and lying to right of base of MCO. MCO a sigmoid inverted funnel with minimally developed base; accessory piece not visible or absent. Germarium elongate, narrow, lying along dextroventral margin of testis, giving rise to delicate slightly inflated uterus; oötype apparently immediately anterior to germarium on body midline; Mehlis’ gland not observed. Vaginal pore dextroventral in anterior portion of trunk; vaginal canal a thin, weakly sclerotised duct extending diagonally toward seminal receptacle. Seminal receptacle a small spherical vesicle near oötype. Vitellarium dense, absent in regions of other reproductive organs; vitelline ducts entering oötype immediately anterior to gonads. Egg not observed.
Measurements
Body 294–387 (325; n = 5); width at level of testis 140–166 (155; n = 5). Haptor 148–168 (155; n = 5) wide. Ventral anchor 26–28 (27; n = 5) long; dorsal anchor 28–30 (29; n = 5) long. Ventral bar 63–70 (65; n = 5) long; dorsal bar 57–64 (61; n = 5) long. Hook pairs 1, 2, 4–7 13–15 (14; n = 10) long; hook pair 3 15–17 (16; n = 5). Pharynx 25–35 (31; n = 5) long, 17–23 (20; n = 5) wide. MCO 19–22 (21; n = 3) long. Testis 52–74 (61; n = 5) long, 37–48 (42; n = 5) wide. Germarium 40–71 (59; n = 5) long, 16–21 (19; n = 5) wide.
Remarks
Comparative morphology of the haptoral sclerites and MCO indicates that H. krabsi n. sp. is most similar to H. choanophallus. The two species are easily differentiated by H. krabsi having a shorter more robust MCO apparently lacking a diagonal distal opening, the dorsal anchor slightly longer than the ventral anchor (ventral anchor somewhat longer than dorsal anchor in H. choanophallus), and the genital atrium armed with ten or more papillae with sclerotised spinous caps (genital atrium with two zones of spine-like structures and lacking papillae in H. choanophallus).
Hemirhamphiculus incomptus n. sp.
Type-host: Southeastern snub-nose garfish Arrhamphus sclerolepis krefftii (Steindachner) (Hemiramphidae).
Type-locality: Moreton Bay, north shore off Wynnum, Queensland, Australia (27°25′S, 153°11′E) (18 January 2016).
Type-material: Holotype, QM G236447; 18 paratypes, QM G236448–G236454, USNM 1459058, HWML 139356.
Site in host: Gill lamellae.
Prevalence: 100% (2 southeastern snub-nose garfish examined).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Hemirhamphiculus incomptus n. sp. is urn:lsid:zoobank.org:act:C57E8307-3E6A-4A02-9AD6-CA31EDC148B4.
Etymology: The specific name (an adjective) is from Latin (incomptus = unadorned).
Description (Figs. 45–51)
Body proper fusiform, comprising elongate cephalic region, robust trunk, short posteriorly tapered peduncle. Cephalic region with moderately developed terminal and bilateral cephalic lobes, well-developed head organs, paired groups of cephalic-gland cells lateral to pharynx. One pair of eyespots representing posterior pair; each eyespot with lens often obscured by chromatic granules; chromatic granules small, ovate; free granules few in cephalic region. Pharynx elongate ovate; oesophagus short. Haptor with well-developed bilateral lobes containing hook pairs 2–4, 6. Ventral and dorsal anchors similar, each with moderately long roots (superficial root longest), slightly arcing shaft, elongate point extending just short of level of tip of superficial root; dorsal anchor slightly longer than ventral anchor. Rod-shaped ventral bar broadly U shaped, with slightly enlarged ends directed anterolaterally. Dorsal bar robust, bent into U, with ends directed laterally. Each hook with delicate point, robust flattened thumb, short shank composed of two subunits; proximal subunit of shank small, often difficult to observe; FH loop approaching shank length. Genital atrium with about ten papillae distributed around the internal wall of the atrium; each papilla terminally rounded, with distal sclerotization. Testis ovate, lying in posterior half of trunk; vas deferens, seminal vesicle, prostatic reservoir not observed. MCO a delicate inverted funnel with minimally developed base; accessory piece not visible or absent. Germarium elongate, narrow, lying along dextroventral margin of testis, giving rise to delicate slightly inflated uterus; oötype apparently immediately anterior to germarium on body midline; Mehlis’ gland not observed. Vaginal pore dextroventral in anterior portion of trunk; vaginal canal unsclerotised, extending diagonally toward seminal receptacle. Seminal receptacle a small, delicate, spherical vesicle overlying oötype. Vitellarium dense, absent in regions of other reproductive organs; vitelline ducts entering oötype immediately anterior to germarium. Egg not observed.
Measurements
Body 278–463 (380; n = 9); width at level of testis 80–132 (115; n = 9). Haptor 86–110 (101; n = 9) wide. Ventral anchor 25–28 (26; n = 9) long; dorsal anchor 28–32 (30; n = 7) long. Ventral bar 34–43 (39; n = 9) long; dorsal bar 31–36 (33; n = 9) long. Hook 12–14 (13; n = 18) long. Pharynx 24–33 (31; n = 10) long, 18–24 (21; n = 10) wide. MCO 16–21 (19; n = 3) long. Testis 56–87 (71; n = 5) long, 34–43 (38; n = 4) wide. Germarium 60–94 (76; n = 5) long, 15–23 (19; n = 5) wide.
Remarks
Hemirhamphiculus incomptus n. sp. most closely resembles H. armatus in general body shape and morphology of the haptoral anchors and bars. It differs from H. armatus by having a poorly defined funnel-shaped MCO with a minimally developed base (MCO with flange like base in H. armatus) and a genital atrium with about ten papillae having sclerotised tips (genital atrium of H. armatus lacking papillae). It differs from H. perexiguus and H. krabsi, both parasitizing the gill lamellae of the southeastern snub-nose garfish, by its body shape, its comparatively short dorsal bar, and the morphology of the sclerotised papillae of the genital atrium.
Hemirhamphiculus sp.
Host: River garfish Hyporhamphus regularis (Günther) (Hemiramphidae).
Locality: Off Peel Island (27°30′S; 153°20′E), Moreton Bay, Queensland, Australia (13 January 2016).
Site in host: Gill lamellae.
Prevalence: Not available (gills of several hosts combined during field collection).
Specimens studied: 4 voucher specimens, USNM 1459055.
Measurements (Figs. 52–57)
Body 246–286 (266; n = 2); width at level of testis 107–108 (n = 2). Haptor 109–134 (121; n = 2) wide. Ventral anchor 20–22 (21; n = 2) long; dorsal anchor 21–25 (23; n = 2) long. Ventral bar 54–57 (56; n = 2) long; dorsal bar 48–58 (53; n = 2) long. Hook 13–16 (14; n = 6) long. Pharynx 22–23 (n = 1) long, 20–21 (n = 1) wide. MCO 29–31 (30; n = 2) long (length of MCO does not include distal filament).
Remarks
The four specimens identified as Hemirhamphiculus sp. were initially included in the type-series of H. flagrum, primarily on the basis of the copulatory complex having a whip-like accessory piece and an MCO tapered to an elongate terminal filament. However, comparison of the morphometrics of the haptoral bars and anchors and accessory piece of the copulatory complex with those of H. flagrum suggested that the four specimens may represent a distinct species. The following features were determined sufficient to temporarily exclude the four specimens from H. flagrum: dorsal and ventral anchors having more robust anchor bases with shorter and heavier superficial roots; longer and more delicate dorsal and ventral haptoral bars; and an accessory piece lacking distal loops. However, the total extent of intraspecific variation of H. flagrum is unknown, and as a result, proposal of a new species for the four specimens appears premature. Determination of the taxonomic status of the four specimens was, therefore, deferred until larger samples of the two forms become available for study.
References
An, D., & Zhang, J.-Y. (1988). [Monogenea of South China freshwater fishes. V. Four new species of monogenetic trematodes from fishes of Hanjian River]. Annual Bulletin of the Society of Parasitology of Guangdong Province, 10, 121–123 (In Chinese).
Bychowsky, B. E., & Nagibina, L. F. (1969). [New genera of monogeneans of the subfamily Ancyrocephalinae (Dactylogyridae)]. Parazitologiya, 3, 518–527 (In Russian).
Collette, B. B. (1976). Indo-west Pacific halfbeaks (Hemiramphidae) of the genus Rhynchorhamphus with descriptions of two new species. Bulletin of Marine Science, 26, 72–98.
Eschmeyer, W. N., & Fong, J. D. (2017). Species by family/subfamily in the Catalog of Fishes. Accessed 16 June 2017 from http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp.
Eschmeyer, W. N., Fricke, R., & van der Laan, R. (Eds). (2017). Catalog of fishes: Genera, species, references. Accessed 16 June 2017 from http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
Froese, R., & Pauly, D. (Eds). (2017). FishBase. Accessed 16 June 2017 from www.fishbase.org (version (02/2017).
Iannacone, J., & Luque, J. L. (1990). Contribucióal conocimiento de los monogeneos parásitos de peces marinos del Perú: descripción de Tylosuricola amatoi n. sp. (Monogenea, Tetraonchidae) y lista de especies conocidas. Revista Ibérica de Parasitología., 50, 213–220.
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Johnson, J. W. (2010). Fishes of the Moreton Bay Marine Park and adjacent continental shelf waters, Queensland, Australia. Memoirs of the Queensland Museum - Nature, 54, 299–353.
Kritsky, D. C., Boeger, W. A., & Thatcher, V. E. (1985). Neotropical Monogenea. 7. Parasites of the pirarucu, Arapaima gigas (Cuvier), with descriptions of two new species and redescription of Dawestrema cycloancistrium Price and Nowlin, 1967 (Dactylogyridae, Ancyrocephalinae). Proceedings of the Biological Society of Washington, 98, 321–331.
Kritsky, D. C., Galli, P., & Yang, T. (2007). Dactylogyrids (Monogenoidea) parasitizing the gills of spinefoots (Teleostei: Siganidae): revision of Tetrancistrum Goto and Kikuchi, 1917, with descriptions of two new species from Siganus spp. of the Red Sea and Celebes. Journal of Natural History, 41, 1513–1551.
Kritsky, D. C., Leiby, P. D., & Kayton, R. J. (1978). A rapid stain technique for the haptoral bars of Gyrodactylus species (Monogenea). Journal of Parasitology, 64, 172–174.
Lester, R. J. G., & Sewell, K. B. (1989). Checklist of parasites from Heron Island, Great Barrier Reef. Australian Journal of Zoology, 37, 101–128.
Linton, E. (1940). Trematodes from fishes mainly from the Woods Hole region Massachusetts. Proceedings of the U. S. National Museum, 88, 1–172.
MacCallum, G. A. (1917). Some new forms of parasitic worms. Zoopathologica, 1, 43–75.
Mizelle, J. D. (1936). New species of trematodes from the gills of Illinois fishes. American Midland Naturalist, 17, 785–806.
Mizelle, J. D., & Price, C. E. (1963). Additional haptoral hooks in the genus Dactylogyrus. Journal of Parasitology, 49, 1028–1029.
Paperna, I., & Lahav, M. (1975). Parasites of fish of the hypersaline Bardawil Lagoon, North Sinai. A preliminary communication. Rapport de la Commission Internationale pour la Mer Méditerranée, 23, 127–128.
Price, E. W. (1937). North American monogenetic trematodes. I. The superfamily Gyrodactyloidea. Journal of the Washington Academy of Sciences, 27, 114–130.
Pritchard, M. H., & Kruse, G. O. W. 1982. The collection and preservation of animal parasites. Lincoln, Nebraska: University of Nebraska Press, 141 p.
Tripathi, R. (1959). Monogenetic trematodes from fishes of India. Indian Journal of Helminthology, 9, 1–149.
Unnithan, R. V. (1964). Four new polyonchoineans (Monogenoidea) parasitic on gills of marine fishes from the Indian Seas. Journal of Parasitology, 50, 241–247.
Williams, E. H., Jr. (1980). Two new species of Ancyrocephalus (Monogenea: Dactylogyridae) from marine fishes of the northern Gulf of Mexico. Proceedings of the Biological Society of Washington, 93, 383–387.
Williams, E. H., Jr., & Rogers, W. A. (1972). Ancyrocephalus cornutus sp. n. (Trematoda: Monogenea) and a redescription of A. parvus Linton, 1940, from the Atlantic needlefish, Strongylura marina (Walbaum). Journal of Parasitology, 58, 876–878.
Yamaguti, S. (1968). Monogenetic trematodes of Hawaiian fishes. Honolulu: University of Hawaii Press, 287 pp.
Young, P. C. (1967). Some species of the genus Tetrancistrum Goto and Kikuchi, 1917 (Monogenoidea: Dactylogyridae). Journal of Parasitology, 53, 1016–1022.
Young, P. C. (1969). The taxonomy of some dactylogyrid Monogenoidea from Australian fishes. Zoologischer Anzeiger, 180, 269–279.
Zhang J.-Y. (2001). Ancyrocephalidae Bychowsky & Nagibina, 1978. In: Zhang J.-Y., Yang T., Liu L., et al. (Eds.), Monogeneans of Chinese Marine Fishes. Beijing: Agriculture Press, 400 pp.
Zhang J.-Y., Ding X.-J., Lin L., & Yu X.-D. (1994). [Monogenoidea of Chinese marine fishes. II. One new species and five new Chinese records of Dactylogyridea]. In: Proceedings of the sixtieth Anniversary of the Founding of China Zoological Society 1934–1994. Beijing: Science and Technology Press, pp. 568–573 (In Chinese).
Acknowledgements
The author is deeply appreciative of Dr Thomas Cribb, who invited him to participate in his project on the diversity of parasites infecting the fishes of Moreton Bay. Dr Marina Kazakevich translated the Russian literature. Mr John Page, Mr David Thompson and the many students are gratefully acknowledged for assistance during the collection of the fishes. The support provided by the staff of the Moreton Bay Research Station was instrumental during the field operations and was greatly appreciated. This work was supported by an Australian Biological Resources Study Grant No. RF215-40” to explore the parasites of fishes of Moreton Bay.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as AE3E1D2C-C70C-48FF-A09B-0CE16B724399. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Monogenea.
Rights and permissions
About this article
Cite this article
Kritsky, D.C. Dactylogyrids (Monogenoidea) infecting the gill lamellae of some beloniform fishes from Moreton Bay, Queensland, Australia, with a redescription of Hareocephalus thaisae Young, 1969 and descriptions of six new species of Hemirhamphiculus Bychowsky & Nagibina, 1969. Syst Parasitol 95, 33–54 (2018). https://doi.org/10.1007/s11230-017-9760-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-017-9760-2