Abstract
Based on light and scanning electron microscopical studies, two new species of nematode parasites are described from freshwater fishes in Thailand: Orientatractis mekongensis n. sp. (Atractidae) from the intestine of Pangasius bocourti Sauvage (type-host) and Helicophagus leptorhynchus Ng & Kottelat (both Pangasiidae, Siluriformes), and Neosynodontisia suratthaniensis n. sp. (Pharyngodonidae) from the intestine of Labiobarbus siamensis (Sauvage) (Cyprinidae, Cypriniformes), for which a new genus Neosynodontisia n. g. is established. Orientatractis mekongensis is mainly characterised by the number and distribution of caudal papillae (2 preanal, 1 adanal and 5 postanal pairs), the length of the left spicule (306–384 µm) and large body sizes (length of males and gravid females 5.4–6.7 mm and 7.8–9.0 mm, respectively). Neosynodontisia differs from other pharyngodonid genera with representatives parasitic in fishes not only by some morphological features (mouth withdrawn into the cephalic end with inflated cuticle, structure of the male caudal end, filamented eggs), but mainly by the occurrence of males inside the body of females. A key to the genera of the Pharyngodonidae with representatives parasitising fishes is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The territory of Thailand is noted for a variety of types of waters, the presence of some endemic water basins and the wealth of fish species. However, despite this, the fauna of nematodes parasitising freshwater fishes in this country remains little known and the reported parasites often were not identified to species. The available data are rather scarce (e.g. Pearse, 1933; Ratanasritong & Kliks, 1972; Sirikanchana, 1982; Moravec et al., 1999, 2004, 2013; Boonchot & Wongsawad, 2005; Lerssutthichawal & Supamattaya 2005; Purivirojkul, 2009; Moravec & Yooyen, 2011a, b; Moravec & Kamchoo, 2012; Sriwongpuk, 2013) and no atractid or pharyngodonid nematodes have so far been reported there from fish hosts in natural waters.
During recent investigations of parasites of freshwater fishes in Thailand, carried out by the Thai co-authors of this paper, nematodes belonging to two remarkable cosmocercoid and oxyuroid species were collected from two species of pangasiid catfishes, Pangasius bocourti Sauvage and Helicophagus leptorhynchus Ng & Kottelat, and a cyprinid fish Labiobarbus siamensis (Sauvage), respectively. A detailed study of these nematodes using light and scanning electron microscopy have shown that they represent a new species of Orientatractis Petter, 1966 (Atractidae) and another new species of the family Pharyngodonidae, for which a new genus is established. Results of this study are presented herein.
All host fishes, H. leptorhynchus (maximum body length 47 cm), P. bocourti (maximum length 120 cm) (both Pangasiidae, Siluriformes) and L. siamensis (maximum length 22 cm) (Cyprinidae, Cypriniformes) are tropical freshwater fishes distributed in the Chao Phraya and Mekong River basins (Froese & Pauly, 2015).
Materials and methods
Both species of pangasiid catfish, P. bocourti and H. leptorhynchus, were collected by local fishermen using nets along the Mekong River in the Muang District, Nakhon Phanom Province, northern Thailand from March to August 2011, whereas specimens of the cyprinid L. siamensis were collected from the Khun Thalae Swamp, Khun Thalae Subdistrict, Muang District, Surat Thani Province, southern Thailand in May 2011. The nematodes recovered from the intestine of fishes were washed in physiological saline and fixed in hot 4 % formalin. For light microscopical examination, the nematodes were cleared with glycerine. Drawings were made with the aid of a Zeiss drawing attachment. Specimens used for scanning electron microscopy (SEM) were postfixed in 1 % osmium tetroxide (in phosphate buffer), dehydrated through a graded acetone series, critical-point-dried and sputter-coated with gold; they were examined using a JEOL JSM-7401F scanning electron microscope at an accelerating voltage of 4 kV (GB low mode). All measurements are in micrometres unless otherwise indicated. The fish nomenclature adopted follows FishBase (Froese & Pauly, 2015).
Superfamily Cosmocercoidea Railliet, 1916
Family Atractidae Railliet, 1917
Orientatractis mekongensis n. sp.
Type-host: Pangasius bocourti Sauvage (Siluriformes: Pangasiidae) (body length 16–22 cm, weight 45–85 g).
Other host: Helicophagus leptorhynchus Ng & Kottelat (Siluriformes, Pangasiidae) (body length 11–30 cm, weight 15–225 g).
Site in host: Intestine.
Type-locality: Mekong River, Muang District, Nakhon Phanom Province, Northeast Thailand (P. bocourti collected in March–April 2011; H. leptorhynchus collected in May–August 2011).
Prevalence and intensity: Ex P. bocourti: 33% (20 fish intected/60 fish examined), 10–170 (mean 27 nematodes per fish); ex H. leptorhynchus: 55% (41/74), 81–1,286 (mean 58).
Type-material: Helminthological Collection, Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice (Cat. No. N–1092).
Etymology: The specific name of this nematode relates to the Mekong River, from where the fish hosts of this parasite were collected.
Description (Figs. 1, 2)
General [Based on specimens from P. bocourti.] Small-sized nematodes. Cuticle with fine transverse striations (Fig. 2A–C, F). Cephalic end blunt, posterior end with very slender, long, pointed tail. Oral opening irregular, almost circular, surrounded by 6 lip-like formations fused together and bearing 4 submedian cephalic papillae and pair of lateral amphids; each of 4 submedian lip-like formations armed with one Y-shaped, well-sclerotised piece, consisting of 2 horns extending outward and downward and immediately anterior to a single-horned structure (Figs. 1C–E, 2A–C, E). Deirids small, knob-like, situated somewhat anterior to end of oesophageal corpus (Figs. 1B, 2E, F). Oesophagus consisting of almost cylindrical corpus with wealky outlined “pharynx” and somewhat distended posterior end, isthmus and poorly differentiated bulb. Nerve-ring slightly posterior to posterior end of corpus. Excretory pore slightly posterior to level of mid-way between nerve-ring and posterior end of oesophagus (Fig. 1A, B). Intestine straight, narrow. Tail of both sexes slender, long, sharply pointed.
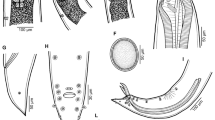
Orientatractis mekongensis n. sp. A, Female, general view; B, Anterior end of female, lateral view; C, Cephalic end of female, lateral and apical views, respectively; E, Cephalic end of female, lateral view (another specimen); F, Posterior end of female, lateral view; G, Posterior end of male, lateral view; H, Small spicule; I, Large spicule
Orientatractis mekongensis n. sp., scanning electron micrographs. A, B, C, Cephalic end, lateral, apical and subdorsoventral views, respectively; D, Posterior end of male, ventrolateral view; E, Anterior end of body, lateral view (arrow indicates deirid); F, Deirid; G, H, Tail of male, lateral and ventrolateral views, respectively (arrows indicate genital papillae). Abbreviations: a, amphid; b, cephalic papilla; c, cloacal aperture; e, horns of submedian Y-shaped sclerotised structures; f, simple submedian horn
Male [Based on 10 specimens; measurements of holotype in parentheses.] Body 5.35–6.66 (5.96) mm long, maximum width 150–190 (163). Length of lip-like formations 18–24 (21). Size of buccal cavity 12–15 × 15–18 (15 × 18). Length of sclerotised horns 12 (12). Length of entire oesophagus 786–879 (788); anterior part of oesophagus (corpus) 312–360 (326) long, maximum width 39–45 (45); posterior part including bulb 462–519 (462) long, maximum width of bulb 78–90 (90). Deirids, nerve-ring and excretory pore 228–381 (303), 384–453 (405) and 612–694 (625), respectively, from anterior extremity. Tail slender, 435–680 (544) long. Caudal papillae: 8 pairs present; 2 pairs subventral preanal, 1 pair subventral adanal and 5 pairs (3 subventral and 2 lateral) postanal (Figs. 1G, 2D, G, H). Median preanal papilla absent. Spicules unequal, well sclerotised; larger (left) spicule 306–384 (306) long, smaller (right) spicule 90–105 (93) long; length ratio of spicules 1.3.1–3.6 (1:3.3); proximal ends of spicules blunt, distal ends sharply pointed (Figs. 1G–I, 2D). Gubernaculum simple, weakly sclerotised, 33–51 (33) long.
Female [Based on 10 gravid specimens; measurements of allotype in parentheses; measurement of 1 nongravid specimen in brackets.] Body 7.75–8.95 (8.49) [6.54] mm long, maximum width 326–367 (326) [204]. Lip-like formations 18–24 (18) [21] long. Size of buccal cavity 15 (15) [12] × 18–24 (21) [18]. Length of sclerotised horns 12–15 (15) [15]. Length of entire oesophagus 871–952 (897) [830]; corpus 354–381 (367) 340] long, 51–54 (51) [42] wide; posterior part of oesophagus including bulb 517–571 (530) [490], maximum width of bulb 54–102 (54) [75]. Deirids, nerve-ring and excretory pore 233–255 (233) [279], 435–462 (462) [394] and 680–748 (680) [639], respectively, from anterior extremity. Vulva slightly elevated, situated 6.71–7.75 (7.36) [5.62] mm from anterior end, comprising 86–87 % (87 %) [86 %] of body length; distance of vulva from anus 108–150 (150) [129]. Vagina short, muscular, directed anteriorly from vulva (Fig. 1A, F). Monodelphic. Uterus containing 1 or 2 oval, thin-walled eggs, 313–408 × 204–245 (313 × 231), and few larvae. Ovary short, reflexed, situated far below end of oesophagus (Fig. 1A). Length of tail 952–1,074 (952) [789] (Fig. 1A, F).
Remarks
The general morphology of the present nematodes, especially the structure of their cephalic end, shows that they belong to the atractid genus Orientatractis which includes parasites of fishes, amphibians and reptiles (tortoises). Species of this genus differ from those of the closely related Klossinemella Costa, 1961 mainly in having only four sclerotised bicornuate pieces and four single horns surrounding the mouth, whereas specimens of Klossinemella are characterised by the presence of eight Y-shaped sclerotised structures and four single horns (Moravec & Thatcher, 1997; González-Solís & Moravec, 2004).
To date, the genus Orientatractis contains six species: O. levanhoai Petter, 1966 (type-species) from the tortoise Indotestudo elongata (Blyth) (reported as Testudo elongata) in Vietnam, O. leiperi Buckley, 1969 from the tortoise Podocnemis vogli Müller in Colombia, O. campechensis González-Solís & Moravec, 2004 from cichlid fishes Paraneetroplus bifasciatus (Steindachner) (reported as Vieja bifasciata) and Cichlasoma pearsei (Hubbs) in Mexico, O. chiapasensis González-Solís & Moravec, 2004 from cichlid fishes Theraps intermedius (Günther) (reported as Vieja intermedia) and Tomocichla tuba (Meek) in Mexico and Nicaragua, O. asymmetrica Gibbons & Platt, 2006 from the turtle Rhinoclemmys pulcherrima (Gray) in Costa Rica and O. hamabatrachos Bursey, Goldberg & Kraus, 2014 from the microhylid frog Austrochaperina basipalmata (Kampen) in Papua New Guinea (Petter, 1966; Buckley, 1969; González-Solís & Moravec, 2004; Gibbons & Platt, 2006; Bursey et al., 2014).
The new species is the largest one of all congeners, the length of males being 5.4–6.7 mm (vs 2.7–4.4 mm) and those of gravid females 7.8-9.0 mm (vs 2.7–5.5 mm). By the number of paired genital papillae, O. mekongensis n. sp. resembles only O. hamabatrachos, but in contrast to the latter, the second postanal pair of papillae is lateral (vs subventral) and the unpaired median preanal papilla is absent (vs present); in other species, preanal pairs of papillae are more numerous (3–4 vs 2) and, except for O. levanhoi, they posses an unpaired preanal papilla, which is absent in the new species. Orientatractis mekongensis n. sp. also differs from other congeneric species by the length of the left spicule (306–384 vs ≥ 430 or < 240 µm). Therefore, the present specimens are considered to represent a new species.
Orientatractis mekongensis n. sp. is the third known species of this genus parasitising fishes and the first representative of Orientatractis reported from Thailand. A characteristic feature of these nematodes, as of all Atractidae, is that the eggs hatch and larvae develop to the third stage in utero to autoinfect the current host (Anderson, 2000). However, their transmission from host to host is not known. Costa (1962) believed that larvae of Rondonia Travassos, 1920 (Atractidae) (previously known only from fish) pass from the host to infect other fish directly. However, it may well be that, even though atractids are homoxenous and no intermediate hosts are involved in their life-cycles, some paratenic hosts may play a role in their transmission.
Superfamily Oxyuroidea Cobbold, 1864
Family Pharyngodonidae Travassos, 1919
Neosynodontisia suratthaniensis n. g., n. sp.
Type-host: Labiobarbus siamensis (Sauvage) (Cypriniformes: Cyprinidae) (body length 11–18 cm, weight 12–40 g).
Site in host: Intestine.
Type-locality: Khun Thalae Swamp, Khun Thalae Subdistrict, Muang District, Surat Thani Province, southern Thailand (collected in May 2011).
Prevalence and intensity: 9 % (1 fish infected/11 fish examined), 8 nematode specimens.
Type-material: Helminthological Collection, Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice (Cat. No. N–1093).
Etymology: The specific name suratthaniensis relates to the Surat Thani Province, Thailand, where this new nematode species was collected.
Description (Figs. 3–5)
General. Small nematodes; females distinctly larger than males (Fig. 3A, B). Cuticle with fine transverse striations (Fig. 5A–C). Lateral alae absent. Mouth withdrawn inside anterior end of body, surrounded by four cephalic papillae (Figs. 3B–E, 5A, B); amphids not observed. Buccal capsule absent. Oesophagus consisting of long, almost cylindrical corpus with slightly outlined “pharynx” at its anterior end, short isthmus and bulb. Nerve-ring encircling oesophagus short distance from its anterior end. Excretory pore situated posterior to end of oesophagus or at its level (Fig. 3A–D). Tail conical, ending in sharp cuticular spine.
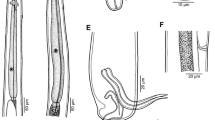
Neosynodontisia suratthaniensis n. sp. A, Male from female’s uterus, lateral view; B, Gravid female, lateral view; C, Female mouth withdrawn into cuticle of anterior end; D, Female with male (arrow) and juvenile female in uterus, lateral view; F, Spicule, lateral view; G, Posterior end of male, lateral view
Male [Based on 2 specimens from female uterus; holotype; measurements of paratype in parentheses.] Length of body 750 (909), maximum width 60 (72) (Fig. 3A). Length of entire oesophagus 162 (150); corpus including “pharynx” 126 (111) long, 21 (18) wide; isthmus 6 (9) long, 18 (15) wide; size of bulb 30 × 27 (30 × 30); “pharynx” 9 (9) long. Nerve-ring and excretory pore 45 (45) and 174 (117), respectively, from anterior extremity. Testis extending anteriorly to short distance posterior to excretory pore (Fig. 1A). Spicule simple, well sclerotised, 45 (42) long and 6 (6) wide, with sharply pointed distal tip (Fig. 3F, G). Gubernaculum absent. Region of cloacal aperture distinctly elevated as genital cone. Caudal papillae: 2 subventral pairs (1 adanal, 1 postanal) close to each other present on genital cone and 1 pair situated ventrally slightly anterior to mid-length of tail (Fig. 3G). Length of tail 111 (108).
Female [Based on 4 gravid specimens; measurements of allotype in parentheses.] Body fusiform, 2.61–3.02 (2.83) mm long, maximum width 218–313 (299) (Fig. 3B). Cuticle of anterior end somewhat inflated (Fig. 1C–E). Mouth, surrounded by four submedian cephalic papillae, withdrawn into anterior end of body (Figs. 3B–E, 5A, B); amphids not observed. Length of entire oesophagus 396–510 (492); corpus including “pharynx” 315–405 (393) long, width 36–45 (39); length of isthmus 15–27 (21), width 24–33 (27); size of bulb 66–87 × 69–87 (78 × 78); “pharynx” 12–21 (18) long. Nerve-ring and excretory pore 114–141 (141) and 558–1,088 (830), respectively, from anterior extremity (Fig. 3B–E). Vulva not elevated, situated near middle of body at 1.29–1.90 (1.41) mm from anterior end, comprising 48–63 (50 %) of body length (Fig. 3B). Vagina short, directed anteriorly from vulva. Uterus opposed; both ovaries situated in vulval region. Uterus of 2 specimens contains 15 eggs, that of 1 specimen 16 eggs and a male, and 1 specimen contains only 1 male and 1 free larva but no eggs in uterus (Fig. 3D). Eggs oval, thin-walled, 186–204 (186–201) long and 72–84 (72–84) wide (Figs. 4A–D, 5D); some eggs (dissected out of nematode body) provided with one long filament at each pole measuring 370–721 in length and with maximum width of 12–18 (Fig. 4A, B); filaments not visible on some larvated eggs inspected inside nematode body (Fig. 4C); hatching larvae observed in 2 eggs in uterus (Fig. 4D). Tail conical, 326–354 (326) long, ending in sharp cuticular point (Figs. 3B, 5C).
Neosynodontisia n. g.
Diagnosis
Pharyngodonidae. Body small, with fine transverse striation of cuticle; lateral alae absent. Four cephalic papillae present. Buccal capsule absent. Tail of both sexes conical. Male: gubernaculum absent; spicule simple, well sclerotised; genital papillae clearly separated into anterior group on protruding genital cone and one posterior pair; caudal alae absent. Female: mouth withdrawn into anterior end of body; vulva approximately equatorial; uterus opposed; eggs larvated, with polar filaments. Larvae may hatch and futher develop in utero; males occur inside female uterus.
Type-species: Neosynodontisia suratthaniensis n. sp.
Etymology: The generic name is derived from that of the morphologically similar genus Synodontisia Petter, Vassiliadès & Troncy, 1972.
Remarks
The general morphology of these nematodes shows that they belong to the oxyuroid family Pharyngodonidae, including the parasites of cold-blooded vertebrates, rarely of archaic mammals (Anderson et al., 2009). As mentioned by Anderson & Lim (1996), oxyuroids described from fishes are of interest, because they are diverse (at present there are 12 recognised genera) and known only from tropical and subtropical regions. They seem to be most common in catfishes and detritus-feeding fishes; thus the feeding behaviour of most of the hosts would faciliate direct contaminative transmission of oxyuroids. All but one species have been found in freswater fishes.
To date, the following 12 pharyngodonid genera are known to contain species parasitic in fishes: Brasilnema Moravec, Kohn & Fernandes, 1992; Cithariniella Khalil, 1964; Cosmoxynema Travassos, 1949; Cosmoxynemoides Travassos, 1949; Hakynema Moravec & Sey, 1988; Ichthyouris Inglis, 1962; Laurotravassoxyuris Vigueras, 1938; Parasynodontisia Moravec, Kohn & Fernandes, 1992; Royandersonia Moravec & Van As, 2004; Spinoxyuris Petter, 1994; Synodontisia Petter, Vassiliadès & Troncy, 1972; and Travnema Pereira, 1938 (see Anderson & Lim, 1996; Moravec et al. 1992b; Moravec & Thatcher, 2001; Anderson et al., 2009; Gibbons, 2010; Moravec & Van As, 2015).
Of the above-mentioned pharyngodonid genera, representatives of only three, Cosmoxynemoides, Hakynema and Royandersonia, have so far been reported from native freshwater fishes in Asia. Moravec & Sey (1988) described Hakynema vietnamensis Moravec & Sey, 1988 from Spinibarbus (reported as Spinibarbichthys) denticulatus (Oshima) (Cyprinidae, Cypriniformes) in Vietnam and Anderson & Lim (1996) established another new species, Synodontisia moraveci Anderson & Lim, 1996, a parasite of Osteochilus melanopleurus (Bleeker) (Cyprinidae, Cypriniformes), which was subsequently transferred to Royandersonia by Moravec & Van As (2004).
Three nominal species of Cosmoxynemoides were inadequately described solely from females in India, C. nandusii Sood, 1972 from Nandus nandus (Hamilton) (Perciformes: Nandidae), C. indica Gupta & Naqvi, 1984 from Ompok (reported as Callichrous) pabda (Hamilton) (Siluriformes: Siluridae) and C. colisi Gambhir, Gyaneswori & Tarnita, 2006 from Trichogaster labiosa Day (reported as Colisa labiosus) (Perciformes: Osphronemidae) (Sood, 1972; Gupta & Naqvi, 1984; Gambhir et al., 2006). In addition, a record of Cosmoxynemoides sp. was reported from Trichogaster (as Colisa) fasciata Bloch & Schneider in Bangladesh (see Sood, 1989). However, the allocation of all these Indian species in Cosmoxynemoides is evidently wrong, because their female morphology is considerably different from that of the type- and the only species of Cosmoxynemoides, C. aguirrei Travassos, 1949, a parasite of characiform fishes in South America, as redescribed by Moravec et al. (1992a). In contrast to the Indian nematodes, females of latter species are characterised by the bulbously inflated posterior half of the oesophageal corpus, the vulva situated at the anterior half of body and in having very elongate eggs provided with a distinct operculum at one pole. All these three above-mentioned Indian species should be considered species inquirendae and incertae sedis (see also Soota, 1983).
Moreover, one species of Ichthyouris, I. bursata Moravec & Prouza, 1995, a parasite of Neotropical discus fishes (Symphysodon spp., Cichlidae), was found to commonly occur in Symphysodon spp. and hybrids cultured in different discus farms in central Thailand (Moravec & Laoprasert, 2008).
Mainly in the structure of the male caudal end and some other features, the present nematodes are most similar to representatives of Synodontisia and Parasynodontisia. However, in contrast to species of these genera, they possess filamented eggs and the female mouth is withdrawn into the cephalic end with inflated cuticle. But the most striking feature of the Thai nematodes is the presence of males inside the body of females, which is quite unique within all pharyngodonids.
The presence of males inside the female body is quite unusual in nematodes and, in fact, this was observed only in some trichosomoidid nematodes (Trichinelloidea: Trichosomoididae). The males of Trichosomoides crassicauda (Bellingham, 1840), a parasite of the urinary tract (usually the bladder) of rats, are much smaller than females and are typically found within the vagina and uterus of females (Hyman, 1951; Moravec, 2000, 2001a); according to Thomas (1924), copulation in T. crassicauda takes place in various regions of the urogenital tract of the host and some males may leave females after fertilising them, although generally they remain within the female. In Anatrichosoma spp., tissue-dwelling parasites of some mammals, males are extremely slender, but often as long as females, and during copulation the male inserts the posterior part of its body, often up to half its length, into the vagina and uterus of the female (Little & Orihel, 1972).
It may well be that two types of eggs develop in female uteri in N. suratthaniensis n. sp. as, for example, in another didelphic pharyngodonid nematode Gyrinicola batrachiensis (Walton, 1929), a parasite of tadpoles (Adamson, 1981; Anderson, 2000). One of the two uteri of G. batrachiensis contains a single row of thin-shelled eggs (the “shell” of these eggs is the vitelline membrane) in various stages of development and these eggs in the vagina contain fully developed third-stage larvae; on the contrary, the other uterus contains a single row of eggs with thick shells and opercula and these eggs in the vagina are in the one- to eight-cell cleavage stage. Larvae from thin-shelled eggs were autoinfective and did not survive more than one hour in water, whereas thick-shelled eggs required a six-day incubation in water to contain third-stage larvae; these eggs overwintered and were available to infect a new batch of tadpoles the following spring (Adamson, 1981; Anderson, 2000).
Also females of Paracapillaria philippinensis (Chitwood, Velasquez & Salazar, 1968) (Trichinelloidea, Capillariidae), a highly pathogenic parasite causing serious illness in humans in many countries of Asia and Africa, produce two types of eggs. Some females of this monodelphic nematode lay typically Capillaria-type eggs with a thick shell and polar plugs which pass out unembryonated in the host faeces, whereas other females produce eggs with only a vitelline membrane which embryonate and hatch in utero or in the lumen of the host gut. The latter larvae are autoinfective giving rise to a second generation of adult nematodes in the same host individual, in contrast to the thick-shelled eggs that require 10-day incubation in water to become infective for the next host (Cross et al., 1972; Moravec, 2001a, b).
It can be assumed that, as in G. batrachiensis, two types of eggs develop in females of N. suratthaniensis n. sp. (see above); whereas the eggs of one type (probably unembryonated, filamented eggs) are oviposited by females and get along with the host faeces to the external environment, the eggs of the second type (non-filamented, larvated eggs) remain in the female body and serve for autoinfection in the same host individual. The third-stage larvae, which hatch from eggs of the second type, continue to develop and attain maturity inside the body of the worm, where they may copulate before leaving the decomposing body of the dead nematode female. However, subsequent studies are necessary to elucidate the development and transmission of this species. Nevertheless, important morphological differences of this Thai species from representatives of other pharyngodonid genera and the occurrence of males inside the bodies of conspecific females justify the erection of a new genus, Neosynodontisia n. g., to accommodate this new species, N. suratthaniensis n. sp.
Key to the genera of the Pharyngodonidae containing species parasitic in fishes
-
1a
Oesophagus unusually short and stout … 2
-
1b
Oesophagus long … 3
-
2a
Oesophagus consists of stout corpus and much reduced bulb; eggs not operculate … Hakynema
-
2b
Oesophagus divided into corpus and bulb of similar length; eggs operculate at one pole … Travnema
-
3a
Oesophageal corpus conspicuously dilate posteriorly; eggs operculate at one pole … 4
-
3b
Oesophageal corpus almost cylindrical, not markedly dilate posteriorly; eggs not operculate or operculate … 5
-
4a
Large, well-sclerotised buccal capsule armed with basal teeth present … Cosmoxynema
-
4b
Buccal capsule absent … Cosmoxynemoides
-
5a
Male with small caudal alae lateral and posterior to cloaca; caudal appendage without papillae … Ichthyouris
-
5b
Male caudal alae absent; caudal appendage with prominent papillae … 6
-
6a
Spicule absent; vulva at posterior half of body … Royandersonia
-
6b
Spicule present; vulva near middle of body or at short distance anterior to anus … 7
-
7a
Spicule with dorsally oriented capitulum; vulva at short distance anterior to anus … Cithariniella
-
7b
Spicule without capitulum … 8
-
8a
Cephalic end of female with 6 large tooth-like structures protruding prominently from oral opening … Laurotravassoxyuris
-
8b
Cephalic end of female without protruding oral tooth-like structures … 9
-
9a
Eggs without filaments … 10
-
9b
Eggs with long polar filaments … 11
-
10a
Mouth opening triangular; lateral alae absent … Parasynodontisia
-
10b
Mouth opening hexagonal; lateral alae present … Synodontisia
-
11a
Lateral alae in female well developed, ending posteriorly in spine posterior to anus … Spinoxyuris
-
11b
Lateral alae absent; if present, their posterior ends not spine-like … 12
-
12a
Lateral alae present; buccal cavity large with well developed teeth arising from base; male free … Brasilnema
-
12b
Lateral alae absent; mouth of female withdrawn into cephalic end with inflated cuticle, buccal cavity indistinct; male occurs inside female body … Neosynodontisia n. g.
References
Adamson, M. L. (1981). Development and transmission of Gyrinicola batrachiensis (Walton, 1929) (Oxyuroidea: Nematoda). Canadian Journal of Zoology, 59, 1351–1367.
Anderson, R. C. (2000). Nematode parasites of vertebrates. Their development and transmission. 2nd Edition. Wallingford: CABI Publishing, 650 pp.
Anderson, R. C., Chabaud, A. G., & Willmott, S. (Eds) (2009). Keys to the nematode parasites of vertebrates. Archival Volume. Wallingford: CAB International, 463 pp.
Anderson, R. C., & Lim, L. H. S. (1996). Synodontisia moraveci n. sp. (Oxyuroidea: Pharyngodonidae) from Osteochilus melanopleurus (Cyprinidae) of Malaysia, with a review of pinworms in fish and a key to species. Systematic Parasitology, 34, 157–162.
Boonchot, K., & Wongsawad, C. (2005). A survey of helminths in cyprinoid fish from the Mae Ngad Somboonchon Reservoir, Chiang Mai Province, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health, 36, 103–107.
Buckley, J. J. C. (1969). On a remarkable oxyurid nematode, Orientatractis leiperi n. sp., (Atractidae) from a South American tortoise, Podocnemis vogli. Journal of Helminthology, 43, 218–286.
Bursey, C. R., Goldberg, S. R., & Kraus, F. (2014). New species of Orientatractis (Nematoda: Atractidae), new species of Rondonia (Nematoda: Atractidae) and other helminths in Austrochaperina basipalmata (Anura: Microhylidae) from Papua New Guinea. Acta Parasitologica, 59, 115–121.
Costa, S C Gda. (1962). Aspectos biológicos do género Rondonia Travassos, 1920 (Nematoda, Atractidae). Arquivo do Museu Nacional, 52, 75–78.
Cross, J. H., Banzon, T., Clarke, M. D., Basaca-Servilla, V., Watten, R. H., & Dizon, J. J. (1972). Studies on the experimental transmission of Capillaria philippinensis in monkeys. Transactions of the Royal Society of Tropical Medicine and Hygiene, 66, 819–827.
Froese, R., & Pauly, D. (Eds) (2015). FishBase. World Wide Web electronic publication. http://www.fishbase.org, version 05/2015.
Gambhir, R. K., Gyaneswori, I., & Tarnita, T. (2006). A new nematode of the genus Cosmoxynemoides (Nematoda – Cosmocercinae) from intestine of Colisa labiosus in Manipur, India. Flora and Fauna, 12, 105–107.
Gibbons, L. M. (2010). Keys to the nematode parasites of vertebrates. Supplementary Volume. Wallingford: CABI Publishing, 416 pp.
Gibbons, M. L., & Platt, T. R. (2006). Rhinoclemmysnema n. g. and three new species of nematodes of the family Atractidae (Cosmocercoidea), with notes on the helminth fauna of Rhinoclemmys pulcherrrima (Testudines: Bataguridae) in Costa Rica. Journal of Helminthology, 80, 333–340.
González-Solís, D., & Moravec, F. (2004). Two new nematode species, Orientatractis campechensis n. sp. and Orientatractis chiapasensis n. sp. (Nematoda: Atractidae) from cichlid fishes in southern Mexico and Nicaragua. Journal of Parasitology, 90, 1443–1449.
Gupta, S. P., & Naqvi, N. H. (1984). Nematode parasites. 1. Cosmoxynemoides indica sp. nov. from freshwater fish Callichrous pabda from Lucknow. Indian Journal of Helminthology, 36, 68–72.
Hyman, L. H. (1951). The invertebrates: Acanthocephala, Aschelminthes and Entoprocta. The pseudocoelomate Bilateria III . New York: McGraw-Hill Book Company, 572 pp.
Lerssutthichawal, T., & Supamattaya, K. (2005). Diversity and distribution of parasites from potentially cultured freshwater fish in Nakhon Si Thammarat. Songklanakarin Journal for Science and Technology, 27 (Supplement), 333–345.
Little, M. D., & Orihel, T. C. (1972). The mating behaviour of Anatrichosoma (Nematoda: Trichuroidea). Journal of Parasitology, 58, 1019–1020.
Moravec, F. (2000). Review of capillariid and trichosomoidid nematodes from mammals in the Czech Republic and the Slovak Republic. Acta Societatis Zoologicae Bohemicae, 64, 271–304.
Moravec, F. (2001a). Trichinelloid nematodes parasitic in cold-blooded vertebrates. Prague: Academia, 429 pp.
Moravec, F. (2001b). Redescription and systematic status of Capillaria philippinensis, an intestinal parasite of human beings. Journal of Parasitology, 87, 161–164.
Moravec, F., Fiala, I., & Dyková, I. (2004). Philometra thaiensis sp. nov. (Nematoda, Philometridae) from Tetraodon palembangensis and T. fluviatilis (Pisces) from fresh waters in Thailand, with a key to Philometra spp. parasitic in the host’s abdominal cavity. Acta Parasitologica, 49, 319–324.
Moravec, F., & Kamchoo, K. (2012). Description of Rhabdochona (Globochona) rasborae sp. n. (Nematoda: Rhabdochonidae) from the freshwater cyprinid fish Rasbora paviana Tirantin southern Thailand. Folia Parasitologica, 59, 209–215.
Moravec, F., Kohn, A., & Fernandes, B. M. M. (1992a). Nematode parasites of fishes of the Paraná River, Brazil. Part 1. Trichuroidea. Oxyuroidea and Cosmocercoidea. Folia Parasitologica, 39, 327–353.
Moravec, F., Kohn, A., & Fernandes, B. M. M. (1992b). Three new species of oxyuroid nematodes, including two new genera, from freshwater catfishes in Brazil. Systematic Parasitology, 21, 189–201.
Moravec, F., & Laoprasert, T. (2008). Redescription of Ichthyouris bursata Moravec & Prouza, 1995 (Nematoda: Pharyngodonidae), a parasite of wild and aquarium-reared discus Symphysodon spp. (Osteichthyes). Systematic Parasitology, 71, 137–143.
Moravec, F., Pachanawan, A., & Kamchoo, K. (2013). Rhabdochona (Rhabdochona) hypsibarbi n. sp. (Nematoda: Rhabdochonidae) from the freshwater cyprinid fish Hypsibarbus wetmorei (Smith) in northeast Thailand. Journal of Parasitology, 99, 297–302.
Moravec, F., & Sey, O. (1988). Nematodes of freshwater fishes from North Vietnam. Part 3. Cosmocercoidea, Seuratoidea, Atractoidea, Heterakoidea and Ascaridoidea. Acta Societatis Zoologicae Bohemoslovacae, 52, 250–265.
Moravec, F., & Thatcher, V. E. (1997). New data on the morphology and systematic status of Klossinemella iheringi (Nematoda: Atractidae) from an Amazonian serrasalmid fish. Folia Parasitologica, 44, 48–54.
Moravec, F., & Thatcher, V. E. (2001). New oxyuroid nematodes of the genera Ichthyouris and Spinoxyuris from South American freshwater fishes. Folia Parasitologica, 48, 311–320.
Moravec, F., & Van As, J. G. (2004). Nematodes from the squeaker fishes Synodontis nigromaculatus and S. vanderwaali from the Okavango River, Botswana, including three new species. Systematic Parasitology, 59, 169–187.
Moravec, F., & Van As, L. L. (2015). Studies on ascaridid, oxyurid and enoplid nematodes (Nematoda) from fishes of the Okavango River, Botswana. Folia Parasitologica, 62, 039.
Moravec, F., Wolter, J., & Körting, W. (1999). Some nematodes and acanthocephalans from exotic ornamental freshwater fishes imported into Germany. Folia Parasitologica, 46, 296–310.
Moravec, F., & Yooyen, T. (2011a). Two new species of Rhabdochona (Nematoda: Rhabdochonidae) from freshwater fishes in Thailand. Folia Parasitologica, 58, 224–232.
Moravec, F., & Yooyen, T. (2011b). Observations on two nematode species parasitizing freshwater fishes in Thailand, including Spinitectus thaiensis sp. nov. (Cystidicolidae) from Pseudomystus siamensis (Bagridae). Acta Parasitologica, 56, 58–66.
Pearse, A. S. (1933). Parasites of Siamese fishes and crustaceans. Journal of the Siamese Society of Natural History, 9 (Supplement), 179–181.
Petter, A.-J. (1966). Équilibre des espèces dans les populations de nématodes parasites du colon des tortues terrestres. Mémoires du Muséum National d’Histoire Naturelle, Paris, Serie Zoologie, 39, 1–245.
Purivirojkul, W. (2009). Fish parasite diversity in the Mekong River in the north of Thailand. KKU Science Journal, 37 (Supplement), 62–70.
Ratanasritong, S., & Kliks, M. (1972). A survey of the helminth parasites of freshwater fish in Chiang Mai Province. Bulletin of Chiang Mai Medical Technology, 5, 185–200.
Sirikanchana, P. (1982). Checklists of parasites of fishes in Thailand. Bangkok: Kasetsart University Museum of Fisheries, 11 pp.
Sood, M. L. (1972). Two rare oxyurid nematode parasites from fresh water fishes of India. Zoologischer Anzeiger, 188, 106–109.
Sood, M. L. (1989). Fish nematodes from South Asia. New Delhi-Ludhiana: Kalyani Publishers, 703 pp.
Soota, T. D. (1983). Studies on nematode parasites of Indian vertebrates I. Fishes. Records of the Zoological Survey of India. Miscellaneous Publication Occasional Paper no. 54. Calcutta: Zoological Survey of India, 352 pp.
Sriwongpuk, S. (2013). Parasites of knife Àsh (Notopterus notopterus, Pallas) from the Huajarake Mark Reservoir in Buriram province. Khonkaenagr Journal, 41 (Supplement), 446–457.
Thomas, L. J. (1924). Studies on the life history of Trichosomoides crassicauda (Bellingham). Journal of Parasitology, 10, 105–135.
Acknowledgements
We thank Aquatic Animal Biotechnology Research Center, Department of Fishery and Coastal Resources, Faculty of Science and Industrial Technology, Prince of Songkla University; and Department of Fisheries, Faculty of Agriculture and Technology, Nakhon Phanom University for providing necessary facilities. The authors’ thanks are also due to the staff of the Laboratory of Electron Microscopy, Institute of Parasitology, Biology Centre of the CAS, České Budějovice, for their technical assistance, and to Blanka Škoríková of the same Institute for help with the illustrations.
Funding
This study was partly supported by the Czech Science Foundation (Grant. No. P505/12G112) and by institutional support (RVO:60077344, Institute of Parasitology, BC CAS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Moravec, F., Kamchoo, K. & Pachanawan, A. New nematode species, Orientatractis mekongensis n. sp. (Atractidae) and Neosynodontisia suratthaniensis n. g., n. sp. (Pharyngodonidae) from freshwater fishes in Thailand. Syst Parasitol 92, 197–209 (2015). https://doi.org/10.1007/s11230-015-9598-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-015-9598-4