Abstract
Three species of parasitic nematodes are redescribed based on light and scanning electron microscopical (SEM) examinations of newly collected specimens in fishes of South Carolina, USA: Dichelyne (Cucullanellus) bullocki Stromberg & Crites, 1972 from Fundulus heteroclitus (Linnaeus) (Fundulidae); Dichelyne (Dichelyne) diplocaecum Chandler, 1935 from Ictalurus furcatus (Valenciennes) (Ictaluridae); and Hysterothylacium pelagicum Deardorff & Overstreet, 1982 from Coryphaena hippurus Linnaeus (Coryphaenidae). For the first time, intraspecific variations in the number of intestinal caeca were observed in D. bullocki, as well as previously unknown males and gravid females of D. diplocaecum are described; this enabled to synonymise D. mexicanus Caspeta-Mandujano, Moravec & Salgado-Maldonado, 1999 with D. diplocaecum. Unlike most congeneric species, H. pelagicum has no double postanal papillae, as confirmed by SEM. A key to the species of Dichelyne Jägerskiöld, 1902 parasitic in freshwater and brackish-water fishes in North America is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fauna of nematodes parasitising fishes in North American waters is to this day insufficiently known. Some species remain inadequately described, which makes their identification problematic and, consequently, data on their hosts, distribution, biology, etc., unreliable (Moravec et al., 2011). Occasional examinations of fishes carried out from 2006 to 2018 in South Carolina, USA, yielded specimens of three insufficiently described nematode species. Based on light (LM) and scanning electron microscopical (SEM) examinations, these taxa are redescribed herein.
Materials and methods
Fish were collected by trapping (mummichogs), electrofishing (catfish) or trawling (dolphinfish) with the assistance of the staff of the South Carolina Department of Natural Resources. Fish were dissected within 12 hours post-capture. Nematodes collected were washed and heat killed in physiological saline, and then preserved either in 4% formalin or in 95% ethanol. For LM examination, the nematodes were cleared using glycerine. Drawings were made with the aid of a Zeiss drawing attachment. Formalin-fixed specimens used for SEM were postfixed in 1% osmium tetroxide (in phosphate buffer), dehydrated through a graded acetone series, critical-point-dried and sputter-coated with gold; they were examined using a JEOL JSM-7401F scanning electron microscope at an accelerating voltage of 4 kV (GB low mode). All measurements are in micrometres unless otherwise indicated. The fish nomenclature adopted follows FishBase (Froese & Pauly, 2018).
Family Cucullanidae Cobbold, 1864
Genus Dichelyne Jägerskiöld, 1902
Dichelyne ( Cucullanellus ) bullocki Stromberg & Crites, 1972
Host: Fundulus heteroclitus (Linnaeus) (Cyprinodontiformes, Fundulidae), mummichog; total body length range 6–9 cm.
Locality: Dog House Creek (32°44′46.00″N, 79°54′12.68″W), South Carolina, USA (collected 2.ix.2017, 29.xi.2017 and 8.v.2018).
Site in host: Intestine (posterior region).
Prevalence and intensity: 43% (15 fish infected/35 fish examined); 1–4 (mean 2) nematode specimens per infected fish.
Voucher material: Smithsonian National Museum of Natural History, Washington, USA (Cat. No. USNM 1510425); Helminthological Collection, Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic (Cat. No. N-1165).
Redescription (Figs. 1–3)
General. Small, whitish nematodes with relatively thin, finely transversely striated cuticle (Fig. 2D). Lateral alae absent (Fig. 3A). Oral aperture dorsoventrally elongate, surrounded by raised narrow membranous ala (collarette) supported by row of c.100 minute basal teeth (Figs. 1D, 2A–C, 3A). Four submedian double cephalic papillae and pair of lateral amphids present (Figs. 1D, E, 2A–C, 3A). Oesophagus muscular, relatively short, expanded at anterior end to form bulbous pseudobuccal capsule (oesophastome); posterior part of oesophagus also expanded, as wide as oesophastome or somewhat wider in lateral view (Fig. 1A–C, E). Oesophagus opens into intestine through valve. Intestine forming one, dorsal, anterior caecum usually extending anteriorly to short distance posterior to nerve-ring, but may even exceed level of nerve-ring; exceptionally, much smaller anterior ventral caecum may be also present (Fig. 1A–C). Nerve-ring encircles oesophagus slightly anteriorly to its mid-length. Deirids small, situated short distance posterior to nerve-ring (Figs. 1A–C, 3A, D). Postdeirids not observed. Excretory pore at level or somewhat posterior to oesophago-intestinal junction (Fig. 1A–C). Tail of both sexes conical, sharply pointed at tip.
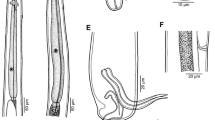
Dichelyne bullocki Stromberg & Crites, 1972 from Fundulus heteroclitus. A, B, Anterior extremity of non-gravid and gravid female, respectively, lateral views; C, Anterior extremity of male with two intestinal caeca, lateral view; D, Cephalic region of female, apical view; E, Cephalic region of male, lateral view; F, Egg; G, Tail of gravid female, lateral view; H, I, Posterior extremity of male, ventral and lateral views, respectively; J, Distal end of spicule, lateral view; K, Gubernaculum, lateral view; L, M, Caudal end of a small and a large male, respectively, lateral views
Dichelyne bullocki Stromberg & Crites, 1972, scanning electron micrographs. A, B, Cephalic region, apical and lateral views, respectively; C, Cephalic region, sub-dorsoventral view; D, Region of cloaca, ventral view; E, Posterior extremity of male, ventral view (arrow indicates median precloacal elevation); F, Posterior extremity of male, lateral view (arrow indicates phasmid); G, Posterior part of male body, ventral view. Abbreviations: a, amphid; b, double cephalic papilla; c, cloacal aperture; d, precloacal sucker; e, median precloacal elevation
Dichelyne bullocki Stromberg & Crites, 1972, scanning electron micrographs. A, Anterior extremity of body, lateral view (arrow indicates deirid); B, Posterior extremity of male body, lateral view (arrow indicates precloacal sucker); C, Caudal end of male, ventroapical view (arrows indicate phasmids); D, Deirid. Abbreviation: c, cloacal aperture
Male [Based on 5 specimens.] Length of body 2.18–3.54 mm, maximum width 177–286. Length of entire oesophagus 449–517, representing 14–24% of total body length; length of oesophastome 99–156, its width 84–96 (204); minimum width of oesophagus 45–48; maximum width of posterior part of oesophagus 78–96. Distance of nerve-ring from anterior extremity 195–245, representing 42–53% of oesophageal length. Deirids and excretory pore 300–394 and 449–707, respectively, from anterior extremity of body. Only one, dorsal, caecum present in 3 larger specimens, whereas 2 smaller specimens possessed 2 caeca, 1 dorsal and 1 smaller ventral; dorsal caecum 204–261 long and 21–45 wide, ventral caecum 123–156 long, 12–15 wide (Fig. 1C). Posterior end of body curved ventrally. Ventral sucker present (Figs. 1I, 2G, 3B). Cloacal region slightly elevated (Figs. 1L, M, 2F). Transverse-oval, median precloacal elevation with 2 minute papillae present (Figs. 1H, L, M, 2D, E, 3C). Caudal papillae 10 pairs: 5 pairs of large subventral preanal papillae, l pair of subventral adanal papillae and 4 pairs (2 subventral, 1 lateral and 1 dorsolateral) of postanal papillae present (Figs. 1I, L,M, 2E-G, 3B, C). Lateral papilla-like phasmids situated just posterior to level of first pair of subventral postanal papillae (Figs. 1I, L, M, 2E, F, 3C). Spicules alate, equal, 598–915 long, representing 23–36% of body length; distal tips of spicules sharply pointed (Fig. 1I, J, M). Gubernaculum small, rod-like in lateral view, 66–87 long (Fig. 1I, K–M). Length of tail 109–141.
Female [Based on 5 ovigerous specimens; measurements of additional 5 specimens without eggs in parentheses.] Length of body 3.03–4.61 (3.11–3.86) mm, maximum width 218–286 (218–286). Length of entire oesophagus 503–558 (530–585), representing 11–17 (16–18)% of total body length; length of oesophastome 150–165 (147–156), its width 105–120 (96–105); minimum width of oesophagus 45–69 (not measured); maximum width of posterior part of oesophagus 87–123 (99–144). Distance of nerve-ring from anterior extremity 216–232 (207–272), representing 41–44 (39–46)% of oesophageal length. Deirids and excretory pore 246–422 (408–490) and 476–748 (639–802), respectively, from anterior extremity of body. Only one, dorsal, caecum 204–273 (252–422) long and 30–57 (24–57) wide present. Vulva postequatorial, 1.61–2.76 (1.85–2.45) mm from anterior extremity, at 53–62 (56–66)% of body length; vulval lips not elevated. Vagina directed anteriorly from vulva. Anterior ovary reaching anteriorly to short distance posterior to end of oesophagus, posterior ovary reaching to short distance anterior to anus. Uteri opposed, containing many eggs (eggs absent). Mature eggs oval, thin-walled, with uncleaved content; size of eggs 84–90 × 57–60 (–) (Fig. 1F). Tail conical, 108–186 (136–177) long, provided with lateral phasmids at its posterior half (Fig. 1G).
Remarks
According to the conception of Petter (1974), the genus Dichelyne comprises three subgenera, Cucullanellus Törnquist, 1931, Dichelyne Jägerskiöld, 1902 and Neocucullanellus Yamaguti, 1941. In possessing a ventral sucker and ten pairs of caudal papillae, Dichelyne bullocki is a representative of the first subgenus. Based solely on LM, this nematode species was described by Stromberg & Crites (1972) from Fundulus heteroclitus collected in the coast of New Hampshire, USA; the authors mention that this nematode was also present in the samples of F. heteroclitus from three other unspecified localities in the Atlantic coast. This parasite was then only reported twice; in a survey of the parasites of mummichogs from the York River in Virginia (Harris & Vogelbein, 2006) and more recently in the ecological study of Anderson & Sukhdeo (2013) from F. heteroclitus in estuarine marshes of New England, i.e. from the same geographical region from where D. bullocki was described. In addition, Stromberg & Crites (1972) considered Cucullanus sp. of Linton (1901) from F. heteroclitus in the Woods Hole region, Massachusetts, as possibly belonging to D. bullocki. The present finding of D. bullocki in South Carolina extends the range southward for this nematode species.
The morphology and measurements of the present specimens are more or less in agreement with the original description of D. bullocki and, since the present host species is the same as the type-host, this further supports our contention that they belong to D. bullocki. Stromberg & Crites (1972) reported only one, dorsal, intestinal caecum in this species, as was found in almost all specimens of the present material. However, the two smallest males (body lengths 2.18 and 2.26 mm) possessed two caeca, the larger dorsal caecum and a much smaller ventral one (Fig. 1C), indicating certain intraspecific variability regarding this feature. The intraspecific variation in the number of caeca (one, two or none) has recently been reported in D. (C.) pleuronectidis (Yamaguti, 1935), a parasite of marine flatfishes (Pleuronectiformes) from off Japan and China (Li et al., 2014).
The SEM examination of D. bullocki, used in this species for the first time, made it possible to observe some morphological features more detailed, such as the structure of the cephalic end, deirids, and the number and distribution of caudal papillae. It also showed, for the first time, the presence of a small median precloacal formation (an elevation with two minute papillae) in this species. Similar organs represent important taxonomic features in some species of the related genus Cucullanus Müller, 1777 (see Moravec & Justine, 2018). Stromberg & Crites (1972) reported 11 pairs of caudal papillae, but they did not distinguish between genital papillae and phasmids. Although a single, unpaired lateral papilla, situated anteriorly to paired caudal papillae, was reported for this nematode by Stromberg & Crites (1972), no similar structure was observed in the present study. Distinction of D. bullocki from other congeneric species parasitic in freshwater and brackish-water fishes of North America is apparent from the key in Discussion.
Within this study, two specimens of D. bullocki from the same host species and the same locality were collected in November 2017 and May 2018. Whereas the November sample consisted of three small males (body length 2.20–2.73 mm) and five non-gravid females (body length 3.11–3.50 mm), the May sample included four larger males (body length 3.05–3.54 mm) and 11 females, five of which were gravid specimens with many eggs (body length 3.03–4.61 mm). This indicates that D. bullocki most likely has a seasonal cycle of maturation, probably similar to that of the closely related species D. cotylophora (Ward & Magath, 1917), as described by Baker (1984a). Worms of this species are acquired by the definitive host from late summer to winter, but most larvae acquired at this time do not develop past the fourth larval stage until the following spring. Worms rapidly develop into adults in the spring and females begin to produce eggs in early summer; these adults disappear in late summer (Baker, 1984a). However, the maturation cycle of D. cotylophora was followed in a colder locality (Lake Erie, Canada), so that it can be expected that the maturation and oviposition of D. bullocki in warmer South Carolina occur somewhat earlier.
Dichelyne ( Dichelyne ) diplocaecum Chandler, 1935
Syn. Dichelyne mexicanus Caspeta-Mandujano, Moravec & Salgado-Maldonado, 1999 (new synonym)
Host: Ictalurus furcatus (Valenciennes) (Siluriformes, Ictaluridae), blue catfish; total body length 64 cm.
Locality: Edisto River, South Carolina, USA (collected 25.iv.2007).
Site in host: Intestine.
Prevalence and intensity: 1 fish infected/2 fish examined; 123 nematode specimens in one infected fish.
Voucher material: Smithsonian National Museum of Natural History, Washington, USA (Cat. No. USNM 1510426); Helminthological Collection, Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic (Cat. No. N-1166).
Redescription (Figs. 4, 5)
General. Small, whitish nematodes with markedly thick cuticle, especially at cephalic region (Fig. 4A–C, G–K). Lateral alae absent. Oral aperture dorsoventrally elongate, surrounded by raised narrow membranous ala (collarette) supported by row of c.140 minute basal teeth (Figs. 4D, 5A, B). Four submedian double cephalic papillae and pair of lateral amphids present (Figs. 4D, 5A, B). Oesophagus muscular, relatively short, expanded at anterior end to form funnel-shaped pseudobuccal capsule (oesophastome); posterior part of oesophagus also somewhat expanded, narrower than oesophastome (Fig. 4A–C). Oesophagus opens into intestine through valve. Intestine forming 2, dorsal and ventral, anterior caeca extending anteriorly to level of nerve-ring or somewhat posterior to it (Fig. 4A–C). Nerve-ring encircles oesophagus approximately at its mid-length. Deirids small, situated approximately in mid-way between nerve-ring and posterior end of oesophagus (Figs. 4A–C, E, 5E); 1, dorsal, postdeirid situated in posterior half of body present in females (Fig. 4F), but not observed in males. Excretory pore slightly posterior to level of deirids (Fig. 4A, C). Tail of both sexes conical, pointed; base of its tip surrounded by minute cuticular spines (Figs. 4K, M, N, 5C, D, H).
Dichelyne diplocaecum Chandler, 1935 from Ictalurus furcatus. A, B, Anterior extremity of male, lateral and dorsoventral views, respectively; C, Anterior extremity of female, lateral view; D, Cephalic region, apical view; E, F, Deirid and postdeirid, respectively, lateral views; G, Cephalic region of male, ventral view; H, Vulva, lateral view; I, J, Posterior extremity of male, ventral and lateral views, respectively; K, Tail of female, lateral view; L, Egg; M, Tail of male, lateral view; N, Tail tip of female with cuticular spines
Dichelyne diplocaecum Chandler, 1935, scanning electron micrographs. A, B, Cephalic region, lateral and apical views, respectively; C, Tail of female, ventroapical view; D, Tail tip of female with cuticular spines, apical view (arrows indicate spines); E, Deirid; F, Caudal end of male, ventrolateral view (arrow indicates median precloacal elevation); G, Tail tip of male with cuticular spines, lateral view (arrows indicate spines); H, Tail of female, lateral view (arrow indicates cuticular spines). Abbreviations: a, amphid; b, double cephalic papilla; c, cloacal aperture; d, anus; p, phasmid
Male [Based on 5 specimens.] Body markedly narrower at its posterior half. Length of body 2.86–3.58 mm, maximum width 286–354. Maximum width of cuticle at oesophageal region 45–60. Length of entire oesophagus 598–694, representing 19–23% of total body length; length of oesophastome 216–225, its width 96–132; minimum width of oesophagus 45–54; maximum width of posterior part of oesophagus 78–87. Distance of nerve-ring from anterior extremity 294–340, representing 45–53% of oesophageal length. Deirids and excretory pore 435–489 and 462–503, respectively, from anterior extremity of body. Dorsal postdeirid not observed. Two caeca present: dorsal caecum 225–340 long and 36–90 wide, ventral caecum 190–272 long and 36–66 wide. Posterior extremity of body slightly and ventrally curved. Ventral sucker absent (Fig. 4I, J). Anterior cloacal lip with small median dome-shaped elevation with papilla (Figs. 4I, J, M, 5F). Caudal papillae 9 pairs. Preanal papillae: 6 pairs present, 5 subventral and 1 lateral, latter situated at level of last subventral pair; postanal papillae: 3 pairs of papillae present, 2 subventral and 1 dorsolateral (Figs. 4I, J, M, 5F). Lateral papilla-like phasmids, situated between 2 pairs of subventral postanal papillae, present (Figs. 4I, J, M, 5F). Spicules unequally long: right spicule conspicuously shorter, 435–476 long, somewhat less sclerotised than left spicule 696–780 long; length ratio of spicules 1:1.50–1.69 (Fig. 4I, J). Length of longer (left) spicule represents 21–27% of body length. Gubernaculum small, rod-like in lateral view, 93–99 long (Fig. 4I, J, M). Tail 136–150 long, with numerous minute cuticular spines around base of its tip (Figs. 4I, J, M, 5F, G).
Female [Based on 5 ovigerous specimens.] Length of body 3.41–3.82 mm; maximum width at level of oesophagus, 476–530. Maximum width of cuticle at oesophageal region 54–63. Length of entire oesophagus 775–816, representing 21–23% of whole body length; length of oesophastome 255–309, its width 141–156; minimum width of oesophagus 63–66; maximum width of posterior part of oesophagus 99–102. Distance of nerve-ring from anterior extremity 367–394, representing 45–52% of oesophageal length. Deirids and excretory pore 476–544 and 544–625, respectively, from anterior extremity of body. Distance of dorsal postdeirid (Fig. 4F) from anterior extremity 1.60–2.00 mm. Dorsal caecum 408–476 long, 36–95 wide; ventral caecum 120–435 long, 33–117 wide. Vulva postequatorial, 1.77–1.97 mm from anterior extremity, at 52–56% of body length; vulval lips not elevated (Fig. 4H). Vagina directed anteriorly from vulva (Fig. 4H). Coils of anterior ovary may exceed anteriorly posterior end of oesophagus (Fig. 4C), posterior ovary extends posteriorly nearly to end of intestine. Uteri opposed, containing many eggs. Mature eggs oval, thin-walled, with uncleaved content; size of eggs 63–75 × 45–48 (Fig. 4L). Tail conical, 150–171 long, provided with pair of lateral papilla-like phasmids situated approximately at its middle; base of tail tip surrounded by numerous minute cuticular spines (Figs.4 K, N, 5C, D, H).
Remarks
Based on two available young females, Chandler (1935) described Dichelyne diplocaecum Chandler, 1935 from the intestine of Ictalurus furcatus in Texas, characterised by the presence of two, dorsal and ventral, anterior intestinal caeca. This species has not been recorded since. Specimens of the present material from the same host species, but in South Carolina are morphologically similar to D. diplocaecum and, therefore, they are considered to belong to this species. Accordingly, it is for the first time that males and gravid females of D. diplocaecum are described, thus enabling a comparison of this nematode with related congeneric species.
In addition to the presence of two well-developed intestinal caeca, a remarkable feature of D. diplocaecum is the conspicuously unequally long spicules; in other congeneric species, both spicules are usually equally long or one of them may be slightly longer. Another characteristic feature of D. diplocaecum is the presence of minute cuticular spines on the tail tip of both sexes. Of the North-American species of Dichelyne, this feature has so far been reported only in D. mexicanus Caspeta-Mandujano, Moravec & Salgado-Maldonado, 1999, a species described from Dajaus monticola (Bancroft) (Mugilidae), Ictalurus balsanus (Jordan & Snyder) (Ictaluridae) and Mayaheros beani (Jordan) (Cichlidae) from central Mexico (States of Guerrero, Nayarit and Veracruz) (Caspeta-Mandujano et al., 1999). The presence of two intestinal caeca and unequally long spicules are also characteristics of D. mexicanus. This species was later reported from Ictalurus furcatus and I. punctatus (Rafinesque) (Ictaluridae) from other states in central Mexico (States of Oaxaca, Tamaulipas and Veracruz) (Pérez & Choudhury, 2002; see also Salgado-Maldonado, 2006).
The morphology and measurements of D. diplocaecum and D. mexicanus are much the same. Moreover, the latter species is reported from the type-host (I. furcatus) of D. diplocaecum and both these forms occur in nearby regions (south of the USA and central Mexico). Therefore, D. mexicanus should be considered a junior synonym of D. diplocaecum. Although Caspeta-Mandujano et al. (1999) designated Da. monticola (reported as Agonostomus monticola) as the type-host of D. mexicanus, based on our findings it is apparent that the main definitive hosts of D. diplocaecum (syn. D. mexicanus) are ictalurid catfishes, whereas its occasional records in mugilids or cichlids may represent findings in paradefinitive or postcyclic hosts.
By its general morphology, D. diplocaecum somewhat resembles another North American congeneric species, D. robustus (Van Cleave & Mueller, 1932), reported from ictalurid catfishes Ameiurus nebulosus (Lesueur), A. melas (Rafinesque), A. natalis (Lesueur) and I. punctatus (see Van Cleave & Mueller, 1932; Hoffman, 1999; Choudhury & Nadler, 2016). In both species the precloacal sucker is absent and the cuticle is markedly thick; also, individuals of both species possess a postdeirid, the number and arrangement of caudal papillae are identical, and they were collected from hosts belonging to the Ictaluridae. However, as it is apparent from the key in Discussion, individuals of D. robustus have allegedly only one intestinal caecum, and no cuticular spines are reported to be present on the tail tip. While the length of spicules was not given in the original description, it can be derived from the respective illustration (figure 4 in Van Cleave & Mueller, 1932) and the accompanying scale to be approximately 450 µm (i.e. the size of the short spicule in D. diplocaecum).
It may well be that only the shorter, well-sclerotised right spicule was observed and illustrated by Van Cleave & Mueller (1932), whereas the longer, less sclerotised left spicule was possibly overlooked, as well as the presence of minute cuticular spines on the tail tip; figure 3 of their paper shows the nematode anterior end of the body in dorsoventral view, but in this position, the two caeca, if present, may overlap, appearing as single; the same can be seen in specimens of D. diplocaecum in dorsoventral view (Fig. 4B). Therefore, it cannot be excluded that future studies will demonstrate that the inadequately described species D. robustus is the same as D. diplocaecum. Unfortunately, as mentioned by Caspeta-Mandujano et al. (1999), the type-specimens of D. robustus were lost and a newly collected topotypic material will be necessary to solve this problem.
Family Anisakidae Railliet & Henry, 1912
Genus Hysterothylacium Ward & Magath, 1917
Hysterothylacium pelagicum Deardorff & Overstreet, 1982
Host: Coryphaena hippurus Linnaeus (Coryphaenidae, Perciformes), common dolphinfish.
Locality: Off South Carolina coast, USA (collected 13.vi.2016).
Site in host: Stomach.
Prevalence and intensity: 1 fish infected/1 fish examined; 6 nematode specimens in the only fish infected.
Vouchers: Smithsonian National Museum of Natural History, Washington, USA (Cat. No. USNM 1510424); Helminthological Collection, Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic (Cat. No. N-1164).
Redescription (Figs. 6–8)
General. Robust, elongate nematodes of yellowish colour when fixed. Maximum width near middle of body. Cuticle with fine, dense transverse striations (Fig. 8C, D). Lips shield-shaped, with distinct interlocking processes (Fig. 8A, B), almost equal in size, somewhat shorter than wide, with relatively broad bases; their lateral flanges widest approximately at middle of lips; pulp with four anteriorly protruding lobes; each of 2 inner lobes with 1 distinct porus at base (Fig. 8B). Inner surface of each lip at level of anterior lobes with 2 distinct oval pits; small granular protuberance present just posterior to each pit (Fig. 8A). Dorsal lip with 2 subdorsal double papillae; each subventral lip with 1 double subventral papilla, 1 small single papilla and amphid situated laterally (Figs. 6B, 7A–D, 8A). Very narrow lateral alae present, extending from base of subventral lips posteriorly almost to posterior extremity (Figs. 7D–F, 8C). Interlabia well developed, cone-shaped (Figs. 6B, 7A–D). Oesophagus muscular, long, ending in small spherical ventriculus; posterior ventricular appendix narrow, relatively short. Anterior intestinal caecum very short, slightly longer or shorter than length of ventriculus (Fig. 6A); caecum to ventricular appendix length ratio 1:1–3. Nerve-ring encircles oesophagus at about 1/7 of its length. Excretory pore at level of nerve-ring or just posterior to it (Fig. 6A). Tail of both sexes conical.
Hysterothylacium pelagicum Deardorff & Overstreet, 1982 from Coryphaena hippurus. A, Anterior extremity of female, lateral view; B, Cephalic region of male, dorsal view; C, Tail of female, lateral view; D, Distal end of spicule, lateral view; E, Posterior extremity of male, lateral view; F, Egg; G, Tail of male, ventral view
Hysterothylacium pelagicum Deardorff & Overstreet, 1982, scanning electron micrographs. A, B, Cephalic region, sublateral and apical views, respectively; C, Dorsal lip; D, Subventral lip (arrow indicates lateral ala); E, Tail of female, lateral view (arrow indicates lateral ala); F, Caudal end of male, ventroapical view (arrow indicates lateral ala). Abbreviations: a, amphid; b, double labial papilla; c, single labial papilla; d, dorsal lip; e, ventrolateral lip; f, anus; i, interlabium; p, phasmid; s, spicule
Hysterothylacium pelagicum Deardorff & Overstreet, 1982, scanning electron micrographs. A, Enlarged distal ends of lips, sublateral view (arrow indicates inner granular elevation); B, Enlarged distal ends of lips, apical view (arrows indicate poral openings); C, Enlarged postanal region of male, ventral view (arrow indicates lateral ala); D, Posterior extremity of male, subventral view; E, Tail of male, ventral view (another specimen); F, Distal end of spicule, lateral view. Abbreviations: a, amphid; b, double labial papilla; c, single labial papilla; p, phasmid; s, spicule
Male [Based on 2 specimens.] Length of body 50.01–64.19 mm, maximum width 1.61–1.81 mm, at base of lips 503–577. Lips 340–408 long; length of interlabia 150–163. Length of oesophagus 6.38–7.15 mm, representing 11–13% of body length; maximum width 449–462. Nerve-ring and excretory pore 979 and 1,088–1,102, respectively, from anterior extremity. Ventriculus 245–354 × 313–408; ventricular appendix 422–476 long, maximum width 109–150. Intestinal caecum 136–476 long, maximum width 136. Caecum to ventricular appendix length ratio 1:1.0–3.1. Posterior end of body curved ventrally. Spicules alate, somewhat unequal, 2.34–2.59 mm and 2.04–2.31 mm long, larger spicule representing 3.6–5.2% of body length; distal ends of spicules pointed (Figs. 6D, E, 8D, F). Total of 30–34 pairs of caudal papillae obseved, 20–23 being preanals, 2–3 adanals and 8–11 postanals; postanal pairs arranged in 2 longitudinal rows; numbers of postanal papillae on right and left side of tail not always identical (Figs. 6E, G, 7F, 8C–E). Pair of lateral phasmids located posterior to genital papillae (Figs. 6G, 7F, 8C). Papilla-like ventromedian organ on anterior cloacal lip present (Fig. 6E, G). Tail conical, 190–272 long, with smooth, pointed tip (Figs. 6E, G, 7F, 8C–E).
Female [Based on 4 ovigerous specimens.] Length of body 69.46–81.68 mm, maximum width 1.41–2.15 mm, at base of lips 588–625. Lips 394–408 long; length of interlabia 150–177. Length of oesophagus 6.25–7.62 mm, representing 9–11% of body length; maximum width 408–585. Nerve-ring and excretory pore 884–156 and 1,006–1,306, respectively, from anterior extremity. Ventriculus 272–408 × 340–408; ventricular appendix 544–1,088 long, maximum width 109–177. Intestinal caecum 354–571 long, maximum width 163–272. Caecum to ventricular appendix length ratio 1:1.3–2.7. Vulva preequatorial, 26.93–30.46 mm from anterior end of body, at 34–42% of body length. Uterus contains numerous, almost spherical eggs 68–95 in diameter (Fig. 6F). Tail conical, 748-843 long, with small knob-like formation at tip (Fig. 6C).
Remarks
Based solely on LM, this nematode species was described by Deardorff & Overstreet (1982) from specimens collected in Coryphaena hippurus from off Hawaii, off the continental USA (Alabama, South Carolina) and in the Gulf of Panama; the authors also mention its occurrence in the same host species from off Papua New Guinea. Later Williams & Bunkley-Williams (1996) found this nematode in C. hippurus in the Caribbean Sea from around Puerto Rico, mentioning that it also occurs in this host off the Gulf and Atlantic USA coasts from Texas to North Carolina, including the Florida Keys. Although it seems to be a rather common, specific parasite of C. hippurus in tropical and temperate zones of the western Atlantic and western and eastern Pacific (Williams & Bunkley-Williams, 1996), it is remarkable that it was not recorded from the many specimens of C. hippurus examined from the regions of the eastern Atlantic (off Balearic Islands in the western Mediterranean and off Canary Islands in the central-eastern Atlantic) (Carbonell et al., 1999).
From a different host species, Istiompax indica (Cuvier) (reported as Makaira indica), H. pelagicum was also reported from Australian waters (off Queensland) by Bruce & Cannon (1989), who had provided an incomplete description of their few specimens, based on LM and SEM. However, the presence of numerous small cuticular spines on the tail tip of their specimens, a different structure of lips as visible from SEM micrographs (absence of four anterior lobes and a pair of granular protuberances on their inner surface), and the fact that this material was collected from the host belonging to a different fish family (Istiophoridae vs Coryphaenidae), show clearly that, in fact, the Australian specimens in question were misidentified and belonged to a different species of Hysterothylacium.
Consequently, the only description of H. pelagicum is that originally provided by Deardorff & Overstreet (1982). The present, first study of this species by SEM revealed some taxonomically important, not previously reported morphological features or those that could not be described in detail. For the first time is reported the presence of amphids, four anterior lobes of lips and lip inner structures, labial pores and the presence and location of phasmids in males. The shape and structure of the distal ends of spicules as well as the arrangement of caudal papillae are described more accurately; caudal papillae were found to be less numerous (30–34 vs 43–47 pairs) and eggs somewhat larger (68–95 vs 49–61 µm in diameter) than reported in the original description by Deardorff & Overstreet (1982).
Discussion
The following nominal species of Dichelyne have so far been reported from freshwater and brackish-water fishes in North America (Canada, USA and Mexico) (see Hoffman, 1999; Salgado-Maldonado, 2006; Arai & Smith, 2016): D. bullocki from F. heteroclitus in the Atlantic coast of the USA (Stromberg & Crites, 1972; Harris & Vogelbein, 2006; Anderson & Sukhdeo, 2013); D. cotylophora from Percidae and some other fishes in several localities in the USA and Canada (Ward & Magath, 1917; Baker 1984b; Hoffman, 1999); D. diplocaecum from I. furcatus in Texas, USA (Chandler, 1935); D. fasciatus Chandler, 1935 from Sciaenops ocellatus (Linnaeus) in Texas and South Carolina, USA (Chandler, 1935; Moravec et al., 2011); D. lepisosteus Casto & McDaniel, 1967 in Texas, USA (see Casto & McDaniel, 1967); D. mexicanus Caspeta-Mandujano, Moravec & Salgado-Maldonado, 1999 from Ictalurus spp., D. monticola and M. beani in central Mexico (Caspeta-Mandujano et al., 1999; Pérez & Choudhury, 2002); and D. robustus from Ameiurus spp. and I. punctatus from several localities in the USA and Manitoba, Canada (Van Cleave & Mueller, 1932; Hoffman, 1999; Arai & Smith, 2016; Choudhury & Nadler, 2016).
Of them, D. robustus was designated a species inquirenda by Caspeta-Mandujano et al. (1999) and D. mexicanus has been synonymised with D. diplocaecum in this paper (see above). In addition, Barreto (1922) established D. lintoni Barreto, 1922 for specifically unidentified cucullanids from several species of marine fishes reported by Linton (1901, 1905, 1907) from the USA and Bermuda, including F. heteroclitus and S. ocellatus, hosts of D. bullocki and D. fasciatus, respectively. According to Chandler (1935), Linton’s various records do not apply to a single species but probably to several; since there is no description or type-specimen designated, Chandler (1935) and Stromberg & Crites (1972) suggested to discard this name as a nomen nudum.
Although most species of Dichelyne possess just one intestinal caecum, dorsal or ventral (Ivashkin & Khromova, 1976), the present study confirms that there are some congeneric species with two, sometimes equally long caeca. In addition to D. diplocaecum and D. japonicus Moravec, Nagasawa & Ogawa, 2001 for which two caeca were reported, two caeca were illustrated, but not described, e.g. for D. diminutus (Rasheed, 1968), D. exiguus (Yamaguti, 1954), D. hartwichi Moravec, Wolter & Körting, 1999, D. indentatus (Rasheed, 1968) and D. tripapillatus (Gendre, 1927) (see Ivashkin & Khromova, 1976; Moravec et al., 1999, 2001). Moreover, the present study shows the intraspecific variations in the number of intestinal caeca in D. bullocki, as was previously observed in D. pleuronectidis or D. spinigerus Moravec, Khosheghbal & Pazooki, 2014 (see Li et al., 2014; Moravec et al., 2014).
A taxonomically important feature in some Dichelyne spp. is the presence and structure of the tail tip, but this can be properly studied only with the use of SEM. In some species, e.g. D. japonicus and D. spinigerus, the tail tip terminates into two, one dorsal and one ventral, sharply pointed spikes, with a pair of minute lateral cuticular spines at its base; in other species the tail tip ends in three spikes, such as in D. longispiculatus Wang & Ling, 1975 or D. spinicaudatus Petter, 1974, sometimes proximally surrounded by many tiny spines (D. spinicaudatus); or the tail tip is simply conical, surrounded by numerous small cuticular spines, e.g. in D. alatae De & Maity, 1995, D. diplocaecum, D. hartwichi or D. rasheedae Petter, 1974 (see Wang & Ling, 1975; De & Maity, 1995; Gibbons & Saayman, 1996; Moravec et al., 1999, 2001, 2014, present study).
Key to the species of Dichelyne parasitising frashwater and brackish-water fishes of North America
-
1a
Ventral precloacal sucker in males present … subgenus Cucullanellus ………………………… 2
-
1b
Ventral precloacal sucker in males absent … subgenus Dichelyne ………………………………. 4
-
2a
Cuticle thin (5–9 µm). Spicules 598–915 µm long, gubernaculum 51–87 µm long. Body of males at most 3.6 mm long, that of females at most 4.7 mm. Parasitic in Fundulidae (Fundulus) …………………………………………………………………………………………….. D. bullocki
-
2b
Cuticle thick (20–68 µm). Spicules up to1.31 mm long, gubernaculum up to174 µm. Body of males up to 6.5 mm long, that of females up to 9.5 mm. Parasitic in Perciformes ……… 3
-
3a
Male with 4 pairs of postanal papillae and 1 pair of phasmids. Cloacal region elevated. Spicules 1–1.31 mm long; gubernaculum120–174 µm long. Parasitic in Sciaenidae (Sciaenops) ………………………………………………………………………………………… D. fastigatus
-
3b
Male with 5 pairs of postanal papillae and 1 pair of phasmids. Cloacal region not elevated. Spicules 0.233–1.024 mm long; gubernaculum 50–98 µm long. Parasitic in Percidae and Centrarchidae (Perca, Micropterus, Sander) ………………………………………. D. cotylophora
-
4a
Body large; length of males 9.3–10.5 mm, of females 12.5–13.2 mm. Spicules equally long, 2.3–2.6 mm. Postanal papillae: 4 pairs. Parasitic in Lepisosteidae (Atractosteus) ………………………………………………………………………………………………………… D. lepisosteus
-
4b
Body small, at most 5 mm long. Spicules at most 780 µm long. Postanal papillae 3 pairs and 1 pair of phasmids. Parasitic in Siluriformes …………………………………………………………… 5
-
5a
Two anterior intestinal caeca. Spicules distinctly unequally long, left spicule measuring 696–780 µm, right spicule 435–476 µm; length ratio of spicules 1:1.5–1.7; gubernaculum 93–99 µm long. Base of tail tip surrounded by many minute cuticular spines. Males 2.86–3.58 mm long, length of gravid females 3.37–3.82 mm. Parasitic in Ictaluridae (Ictalurus) ……………………………………………………………………………………………………… D. diplocaecum
-
5b
One intestinal cacum present. Spicules about 450 µm long; gubernaculum allegedly absent. Tail tip without cuticular spines. Males 4.25 mm long, length of females about 5 mm. Parasitic in Ictaluridae (Ameiurus spp. and Ictalurus punctatus) ……………………………………….…………………………………………………………………………………. D. robustus (species inquirenda)
References
Anderson, T. K., & Sukhdeo, M. V. K. (2013). Qualitative community stability determinates parasite establishment and richness in estuarine marshes. PeerJ, 1, e92.
Arai, H. P., & Smith, J. W. (2016). Guide to the parasites of fishes of Canada. Part V: Nematoda. Zootaxa, 4185, 1–274.
Baker, M. R. (1984a). On the biology of Dichelyne (Cucullanellus) cotylophora (Ward & Magath, 1917) (Nematoda, Cucullanidae) in perch (Perca flavescens) from Lake Erie, Ontario. Canadian Journal of Zoology, 62, 2062–2073.
Baker, M. R. (1984b). Redescription of Dichelyne (Cucullanellus) cotylophora (Ward & Magath, 1917) (Nematoda: Cucullanidae) parasitic in freshwater fishes of eastern North America. Canadian Journal of Zoology, 62, 2053–2061.
Barreto, A. L. B. (1922). Revisão de familia Cucullanidae Barreto, 1916. Memórias do Instituto Oswaldo Cruz, 14, 68–87, Plts. 33–46.
Bruce, N. L., & Cannon, L. R. G. (1989). Hysterothylacium, Iheringascaris and Maricostula new genus, nematodes (Ascaridoidea) from Australian pelagic marine fishes. Journal of Natural History, 23, 1397–1441.
Carbonell, E., Massutí, E., Castro, J. J., & García, R. M. (1999). Parasitism of dolphinfishes, Coryphaena hippurus and Coryphaena equiselis, in the western Mediterranean (Balearic Islands) and central-eastern Atlantic (Canary Islands). Scientia Marina, 63, 343–354.
Caspeta-Mandujano, J. M., Moravec, F., & Salgado-Maldonado, G. (1999). Observations on cucullanid nematodes from freshwater fishes in Mexico, including Dichelyne mexicanus sp. n. Folia Parasitologica, 46, 289–295.
Casto, S., & McDaniel, B. (1967). Helminth parasitism in gars from south Texas with a description of Dichelyne lepisosteus n. sp. (Nematoda: Cucullanidae). Proceedings of the Helminthological Society of Washington, 34, 187–194.
Chandler, A. C. (1935). Parasites of fishes in Galveston Bay. Proceedings of the United States National Museum, 83, 123–157, Plts. 6–12.
Choudhury, A., & Nadler, S. A. (2016). Phylogenetic relationships of Cucullanidae (Nematoda), with observations on Seuratoidea and the monophyly of Cucullanus, Dichelyne and Truttaedacnitis. Journal of Parasitology, 102, 87–93.
De, N. C., & Maity, R. N. (1995). A new nematode, Dichelyne alatae sp. n. (Cucullanidae), from Sillaginopsis panijus (Pisces) of West Bengal, India. Folia Parasitologica, 42, 220–226.
Deardorff, T. L., & Overstreet, R. M. (1982). Hysterothylacium pelagicum sp. n. and H. cornutum (Stossich, 1904) (Nematoda: Anisakidae) from marine fishes. Proceedings of the Helminthological Society of Washington, 49, 246–251.
Froese, R., & Pauly, D. (Eds) (2018). FishBase. World Wide Web electronic publication. http://www.fishbase.org, version 07/2018.
Gibbons, L. M., & Saayman, J. E. (1996). Redescriptions of Dichelyne (Dichelyne) rasheedae Petter, 1974 and Spirocamallanus olseni Campana-Rouget & Rayarihelissoa, 1965, recorded for the first time from fish in Lake St. Lucia, South Africa. Onderstepoort Journal of Veterinary Research, 63, 39–46.
Harris, C. E., & Vogelbein, W. K. (2006). Parasites of mummichogs, Fundulus heteroclitus, from the York River, Virginia, U.S.A., with a checklist of parasites of Atlantic coast Fundulus spp. Comparative Parasitology, 73, 72–110.
Hoffman, G. L. (1999). Parasites of North American freshwater fishes. Second edition. Ithaca and London: Cornell University Press, 539 pp.
Ivashkin, V. M., & Khromova, L. A. (1976). [Cucullanata and Gnathostomatata of animals and man and the diseases caused by them.] Osnovy Nematodologii 27. Moscow: Nauka, 436 pp (In Russian).
Li, L., Du, L.-Q., Xu, Z., Guo, Y.-N., Wang, S.-X., & Zhang, L.-P. (2014). Morphological variability and molecular characterisation of Dichelyne (Cucullanellus) pleuronectidis (Yamaguti, 1935) (Ascaridida: Cucullanidae) from the flatfish Pleuronichthys cornutus (Temminck & Schlegel) (Pleuronectiformes: Pleuronectidae) in the East China Sea. Systematic Parasitology, 87, 87–98.
Linton, E. (1901). Parasites of fishes of the Woods Hole region. Bulletin of the United States Fish Commission, 19, 405–492.
Linton, E. (1905). Parasites of fishes of Beaufort, North Carolina. Bulletin of the United States Bureau of Fisheries, 24, 321–428.
Linton, E. (1907). Notes on parasites of Bermuda fishes. Proceedings of the United States National Museum, 33, 85–126.
Moravec, F., & Justine, J.-L. (2018). Three new species of Cucullanus (Nematoda: Cucullanidae) from marine fishes off New Caledonia, with a key to species of Cucullanus from Anguilliformes. Parasite, 25, 51.
Moravec, F., Khosheghbal, M., & Pazooki, J. (2014). Dichelyne (Dichelyne) spinigerus sp. nov. (Nematoda: Cucullanidae) from the marine fish Otolithes ruber (Sciaenidae) off Iran and first description of the male of Philometra otolithi Moravec et Manoharan, 2013 (Nematoda: Philometridae). Acta Parasitologica, 59, 229–237.
Moravec, F., Levron, C., & de Buron, I. (2011). Morphology and taxonomic status of two little-known nematode species parasitizing North American fishes. Journal of Parasitology, 97, 297–304.
Moravec, F., Nagasawa, K., & Ogawa, K. (2001). Dichelyne japonicus sp. n. (Nematoda: Cucullanidae), a new intestinal parasite of the marine sciaenid fish Argyrosomus argentatus in Japan. Helminthologia, 38, 43–45.
Moravec, F., Wolter, J., & Körting, W. (1999). Some nematodes and acanthocephalans from exotic ornamental freshwater fishes imported into Germany. Folia Parasitologica, 46, 296–310.
Peréz, G., & Choudhury, A. (2002). Adult endohelminth parasites of ictalurid fishes (Osteichthyes: Ictaluridae) in Mexico: Empirical evidence for biographical patterns. Comparative Parasitology, 69, 10–19.
Petter, A. J. (1974). Essai de classification de la famille des Cucullanidae. Bulletin du Muséum National d’Histoire Naturelle, 3e série. Zoologie, 177, 1469–1491.
Salgado-Maldonado, G. (2006). Checklist of helminth parasites of freshwater fishes from Mexico. Zootaxa, 1324, 1–357.
Stromberg, P. C., & Crites, J. L. (1972). A new nematode Dichelyne bullocki sp. n. (Cucullanidae) from Fundulus heteroclitus (Linnaeus). Proceedings of the Helminthological Society of Washington, 39, 131–134.
Van Cleave, H. J., & Mueller, J. F. (1932). Parasites of the Oneida Lake fishes. Part I. Descriptions of new genera and new species. Roosevelt Wild Life Annals, 3, 9–71.
Wang, P., & Ling, X. (1975). Some nematodes of the suborder Camallanata from Fujian Province, with notes on their life histories. Acta Zoologica Sinica, 21, 350–358 (In Chinese with English summary).
Ward, H. B., & Magath, T. B. (1917). Notes on some nematodes from fresh water fishes. Journal of Parasitology, 3(Year 1916), 57–64.
Williams, E. H., Jr., & Bunkley-Williams, L. (1996). Parasites of offshore big game fishes of Puerto Rico and the western Atlantic. Mayagüez, Puerto Rico: Antillean College Press, 382 pp.
Acknowledgements
We thank the staff of the SC DNR, Charleston SC for providing fish specimens. Thanks are also due to the Laboratory of Electron Microscopy, Institute of Parasitology, Biology Centre CAS, institution supported by the MEYS CR (LM2015062 Czech-BioImaging) for their support with obtaining scientific data presented in this paper, and to Blanka Škoríková of the same Institute for help with the illustrations.
Funding
This study was partly supported by the Czech Science Foundation (Grant. No. P505/12/G112), and by institutional support (RVO:60077344, Institute of Parasitology, BC AS CR; Department of Biology, College of Charleston, SC, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed. When live fish were collected (Mummichogs), they were euthanized according to the approved ethical codex of the College of Charleston IACUC-2018-003.
Additional information
This article is part of the Topical Collection Nematoda.
Rights and permissions
About this article
Cite this article
Moravec, F., de Buron, I. & González-Solís, D. Redescription of three species of nematodes (Nematoda) parasitising fishes in the USA, with a key to the species of Dichelyne Jägerskiöld, 1902 parasitic in freshwater and brackish-water fishes of North America. Syst Parasitol 96, 79–94 (2019). https://doi.org/10.1007/s11230-018-9829-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-018-9829-6