Abstract
Euparyphium capitaneum Dietz, 1909, the type-species of the genus Euparyphium Dietz, 1909, is described on the basis of material collected from the type-host Anhinga anhinga (L.) from Pascagoula River, which drains into the northern coast of the Gulf of Mexico. Combination of light and scanning electron microscopy observations of freshly collected and properly fixed specimens in our study has allowed us to provide novel information on the morphology and topology of the reproductive systems and other morphological features of the species. A Bayesian inference analysis based on the newly-obtained partial sequence of the nuclear 28S rRNA gene for E. capitaneum and 24 previously published sequences from the superfamily Echinostomatoidea Looss, 1899 provided evidence supporting the distinct status of the genera Euparyphium and Isthmiophora Lühe, 1909.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Euparyphium Dietz, 1909 is a small genus of the Echinostomatidae Looss, 1899 including intestinal parasites of birds and mammals (Kostadinova, 2005). The species composition of Euparyphium has been unstable and a subject of several taxonomic revisions since the erection of the genus by Dietz (1909); this partially reflects the peculiar morphology of the type-species Euparyphium capitaneum Dietz, 1909. Some of the species that at different times belonged to Euparyphium were moved between several genera, most notably Isthmiophora Lühe, 1909 and Echinocirrus Mendheim, 1943. Skrjabin & Bashkirova (1956) synonymised Echinocirrus and Isthmiophora with Euparyphium and erroneously listed Euparyphium melis (Schrank, 1788) as the type-species of Euparyphium; this has been followed by some other authors. Kostadinova & Gibson (2002) have corrected the situation and separated the genera Euparyphium (type-species E. capitaneum) and Isthmiophora (type-species I. melis). The detailed account of these systematic and nomenclatural changes has been provided by Kostadinova & Gibson (2002). According to this most recent revision of Euparyphium, the genus is currently represented by four nominal species: E. capitaneum (syn. E. anhingae), E. murinum Tubangui, 1931, E. guerreroi Tubangui, 1931 and E. albuferensis Esteban, Toledo, Sanchez & Munoz-Antoli, 1997. Except for the type-species, Euparyphium spp. were originally described from the mammalian host Rattus norvegicus (Berkenhout).
The type-species, E. capitaneum Dietz, 1909, was briefly described based on material from Anhinga anhinga (L.) in Brazil (Dietz, 1909, 1910). For more than a century this species was recorded twice. Pérez Vigueras (1944) reported E. capitaneum from A. anhinga in Cuba and Premvati (1968) described E. anhingae Premvati, 1968 from A. anhinga in Florida; this species has been later synonymised with E. capitaneum by Kostadinova & Gibson (2002). Unfortunately, the description of Premvati was based on clearly misshapen specimens that could not be later located, lacks many details of the organisation of the reproductive organs and shows inconsistencies with respect to the description of the collar spines. Thus to date, no confirmation of the unusual morphology of the type-material of E. capitaneum examined by Dietz (1909, 1910) that resulted in a somewhat composite diagnosis of Euparyphium (see Kostadinova, 2005) exists.
In the course of helminthological investigation of aquatic birds in Mississippi between 2007 and 2012 we found numerous specimens of E. capitaneum in several A. anhinga. This new, well-fixed material enabled us to re-describe this species and provide previously unknown details of its anatomy based on light and scanning electron microscopy observations. Furthermore, the DNA sequence obtained from this material was used to estimate the phylogenetic relationships of E. capitaneum and other members of the Echinostomatoidea Looss, 1899 with available sequences.
Materials and methods
Numerous specimens of E. capitaneum were found in five A. anhinga collected by EEP and VVT from oxbow lakes in the Pascagoula River drainage area (Mississippi, USA) in December of 2007, August of 2010, and March of 2012. Details of the localities are provided below. Digeneans were removed from host intestines, rinsed in saline, heat-killed with hot water and preserved in 70% ethanol. The specimens for light microscopy examination were stained with aqueous alum carmine, dehydrated in a graded ethanol series, cleared in clove oil and mounted permanently in Damar gum. Drawings were made on a DIC-equipped compound Olympus BX51 microscope with the aid of a drawing tube. All measurements in the text and tables are in micrometres. The following abbreviations were used in the tables: BL, body length; BW, maximum body width; CL, collar length; CW, collar width; OSL, oral sucker length; OSW, oral sucker width; PL, prepharynx length; PHL, pharynx length; PHW, pharynx width; OL, oesophagus length; CSL, cirrus-sac length; CSW, maximum cirrus-sac width; VSL, ventral sucker length; VSW, ventral sucker width; ATL, anterior testis length; ATW, anterior testis width; PTL, posterior testis length; PTW, posterior testis width; OVL, ovary length; OVW, ovary width; MEL, Mehlis’ gland length; MEW, Mehlis’ gland width; EL, egg-length; EW, egg-width; FORE, forebody length; UFL, uterine field length (distance between the ovary and posterior margin of the ventral sucker); PTFL, post-testicular field length (distance between the posterior margin of the posterior testis and posterior extremity of body). In addition to the standard measurements the following relative proportions were calculated after Kostadinova (2005): BW(%), maximum body width as a proportion of body length; FO(%), length of the forebody as a proportion of body length; U(%), length of the uterine field posterior to ventral sucker (used as an approximation for the uterine length) as a proportion of body length; T(%), length of the post-testicular field as a proportion of body length. The specimens studied are deposited in the collection of the Harold W. Manter Laboratory of Parasitology at the University of Nebraska, Lincoln (HWML).
The specimens observed under scanning electron microscopy (SEM) were fixed in 70% ethanol, dehydrated in a graded ethanol series and dried with hexamethyldisilazane (Ted Pella Inc., Redding, California) as a transition fluid. The specimens were mounted on an aluminum stub using conductive double-sided tape, coated with gold-palladium, and examined with the use of a Hitachi 4700 scanning electron microscope (Hitachi U.S.A., Mountain View, California) at an accelerating voltage of 5–10 kV.
Genomic DNA was extracted from five specimens of E. capitaneum according to the protocol described by Tkach & Pawlowski (1999). About 1,350 bp long fragment at the 5′ end of the 28S rRNA gene was amplified from three of these specimens by polymerase chain reaction on Eppendorf EP gradient thermal cycler using the forward primer dig12 (5′-AAG CAT ATC ACT AAG CGG-3′) and the reverse primer 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) (Tkach et al., 2003). About 2,600 bp long fragment spanning the 3′ end of the 18S gene, internal transcribed spacer region (ITS1 + 5.8S gene + ITS2) and partial 28S gene were amplified from the remaining two specimens using the forward primer ITSF (5′-CGC CCG TCG CTA CTA CCG ATT G-3′) and the reverse primer 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) (Tkach et al., 2003). PCR reactions were prepared using One-Taq quick load PCR mix (New England Biolabs). The PCR products were purified using QIAquick PCR purification Kit (Qiagen, Germany). PCR primers and an additional internal forward primer 300F (5′- CAA GTA CCG TGA GGG AAA GTT G-3′) and reverse primers 300R (5′-CAA CTT TCC CTC ACG GTA CTT G-3′) and diglr (5′-CCG CTT AGT GAT ATG CTT-3′) were used in the sequencing reactions. PCR products were cycle-sequenced directly using ABI BigDyeTM (Applied Biosystems, Foster City, California) chemistry, alcohol-precipitated, and run on an ABI Prism 3100™ automated capillary sequencer (Applied Biosystems). Contiguous sequences were assembled using Sequencher (GeneCodes Corp., ver. 4.2), and submitted to GenBank under accession numbers KP009616–KP009620.
The newly-generated sequences for E. capitaneum and matching sequences of the 28S rRNA gene of other echinostomatoidean digeneans available on GenBank (see Table 3 for details) were used in the phylogenetic analysis. A sequence of Notocotylus attenuatus (Rudolphi, 1809) (AF184259) was used as an outgroup based on the topologies in the phylogenetic trees of the Digenea published by Olson et al. (2003). For phylogenetic analyses the sequences were aligned using ClustalX as implemented in the BioEdit program, version 7.0.1 (Hall, 1999). The alignment was then trimmed to the length of the shortest sequence, manually refined using BioEdit, saved in FASTA format and imported into the MacClade ver. 4.02 software (http://macclade.org/macclade.html). Upon selection of the exclusion sets the alignments were saved in NEXUS format for subsequent analyses. Positions with ambiguous alignment were excluded from the analysis.
Phylogenetic analysis was carried out using Bayesian inference (BI) as implemented in the MrBayes software (ver. 3.1) (Huelsenbeck & Ronquist, 2001). The Bayesian analyses were run with the following nucleotide substitution model settings: lset nst = 6, rates = invgamma, samplefreq = 100, ncat = 4, shape = estimate, inferrates = yes and basefreq = empirical, that correspond to a general time reversible (GTR) model including estimates of the proportion of invariant sites (I) and gamma (G) distributed among-site rate variation. The nucleotide substitution model was determined using MrModelTest 2.3 software (Nylander, 2004). Markov chain Monte Carlo (MCMC) chains were run for 3,000,000 generations, log-likelihood scores were plotted and only the final 75% of trees were used to produce the consensus trees by setting the ‘‘burnin’’ parameter at 750. This number of generations was considered sufficient because the standard deviation dropped well below 0.01 at the end of the run. Trees were visualised using the FigTree ver. 1.4 software (Rambaut, 2012).
Family Echinostomatidae Looss, 1899
Genus Euparyphium Dietz, 1909
Euparyphium capitaneum Dietz, 1909
Host: Anhinga anhinga (Linnaeus) (Suliformes: Anhingidae).
Localities: Three lakes in the drainage area of the Pascagoula River in Jackson County (30°45′22″N, 88°39′14″W and 30°37′05″N, 88°38′14″W) and George County (30°53′41″N, 88°44′42″W), Mississippi, USA.
Site in host: Intestine.
Prevalence and abundance: All five birds examined were infected with several to several dozen E. capitaneum.
Voucher material: Deposited in HWML, accession numbers HWML 75112–75114 (15 specimens).
Representative DNA sequences: KP009616–KP009617 (partial 18S; complete ITS1, 5.8S and ITS2; partial 28S sRNA gene); KP009618–KP009620 (partial 28S rRNA gene).
Redescription (Figs. 1–3)
[Based on 15 specimens; metrical data in Tables 1, 2.] Body very elongate (BW = 7–14%), with maximum width at level of ventral sucker. Forebody flattened dorsoventrally, long to very long, representing 20–32% of body length; hindbody subcylindrical. Tegument armed with large spines arranged in alternating transverse rows extending from close to posterior margin of collar to level of anterior testis ventrally (Figs. 2A, 3A) and level of ventral sucker dorsally; transverse rows dense in forebody (Fig. 2A, D, E), progressively more widely spaced in hindbody (Figs. 2A, 3A); tegument in ventral median field from mid-level of forebody to ventral sucker, including its anterior margin, devoid of spines (Fig. 2A, D).
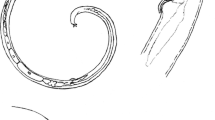
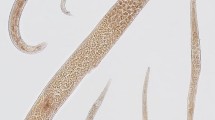
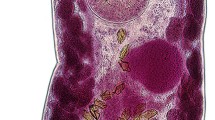
Scanning electron micrographs of Euparyphium capitaneum Dietz, 1909 ex Anhinga anhinga. A, Entire specimen, ventral view; B, C, Head collar; D, Forebody, lateral view (note everted cirrus); E, Head collar, lateral view; F, G, Region of ventral sucker [note large papillae (arrows) and small sensory receptors (arrowheads)]. Scale-bars: A, 1000 μm; B, C, E, 100 μm; D, 200 μm; F, G, 50 μm
Head collar reniform, well developed, with distinct ventral ridge. Collar spines 27; 4 angle spines on each ventral lappet (2 dorsal and 2 ventral), longer than marginal spines, one angle spine distinctly larger (Table 2); lateral spines in single row, first lateral spine smallest (Table 2); dorsal spines in double row (Figs. 1B, 2B–E), aboral spines slightly longer than oral. Oral sucker slightly transversely oval. Ventral sucker large, cup-shaped, with deep cavity and strongly muscular walls, located in first quarter of body; inner rim of ventral sucker with c.20 small sensory receptors (Fig. 2G) in a semicircle on upper half; outer rim with large papillae at base (Fig. 2F, G). Prepharynx distinct. Pharynx large, elongate-oval. Oesophagus long, thick-walled. Intestinal bifurcation just anterior to ventral sucker. Caeca thick-walled, reach close to posterior extremity of body.
Testes 2, tandem, contiguous, elongate-oval, wavy; anterior testis pre-equatorial; posterior testis post-equatorial. Post-testicular field very long, representing 27–50% of body length. Cirrus-sac elongate-oval, anterodorsal to ventral sucker, between intestinal bifurcation and mid-level of ventral sucker. Internal seminal vesicle saccular, elongate-oval, with attenuated distal portion. Pars prostatica moderately developed. Cirrus relatively long, tubular, unspined, with sponge-like surface (Fig. 3B–D) and sensory papillae at base (Fig. 3B). Genital pore small, median, just posterior to intestinal bifurcation.
Ovary elongate-oval, entire, dextral, pre-equatorial. Mehlis’ gland large, diffuse, elongate-oval, sub-median, contiguous with ovary and anterior testis. Uterine seminal receptacle not observed. Uterine field very short (U = 0–7%), with few intercaecal loops. Metraterm muscular, somewhat shorter than cirrus-sac. Eggs not numerous. Vitellarium follicular; follicles large, distributed in 2 lateral fields between level of ovary and posterior extremity; approach median line in post-testicular field; vitelline reservoir median, dorsal to Mehlis’ gland.
Excretory vesicle Y-shaped; pore ventro-subterminal.
Remarks
Although sufficient for the erection of the genus, the original description of E. capitaneum is incomplete, with little information provided on the morphology of terminal genitalia. Of particular importance is the lack of data on the presence/absence of spines on the cirrus, one of the major characteristics differentiating the closest genera Euparyphium and Isthmiophora. The only other description of the species also provided what appears to be erroneous information on the number of collar spines that may be due to either loss of some spines or the orientation of the collars in the mounted specimens that prevented accurate counting of spines (see Premvati, 1968).
The newly-collected material of E. capitaneum both agrees well with the original description of the species and confirms its affiliation with Euparyphium, especially in relation to the features listed as characteristic of the type-species by Kostadinova (2005) (i.e. collar with 27 spines; contiguous, equatorial, strongly elongate, wavy testes; and very short uterus) as well as of the features of generic importance (i.e. two groups of four angle spines that are longer than the marginal spines; dorsal spines in the aboral row longer than those in the oral row; and unspined cirrus).
Importantly, SEM observations of several specimens demonstrated the lack of spines on the cirrus in E. capitaneum (Fig. 3B–D), rather the cirrus has a sponge-like surface with rhomboid “cells” stretching in the everted cirrus. The ovary in the present material is elongate-oval as opposed to spherical in the original description. The topology of the cirrus-sac was mentioned and illustrated in the description by Premvati (1968), but no metrical data were provided. Additional morphological features not mentioned or illustrated in either of the previous descriptions and characterised here are the metraterm, Mehlis’ gland, vitelline reservoir and armed tegument. The SEM examination revealed that tegumental spines only reach to the first third of the hindbody (Figs. 2A, 3A).
Regarding the detailed morphometric characterisation achieved here based on abundant, adequately fixed material, our data extend the range of variation of the metrical data for E. capitaneum, i.e. wider range and higher upper limits for the size of the body, collar spines, most organs and eggs, and lower upper limits for the size of the testes; see Table 1). Of particular relevance are the deviations observed in E. capitaneum from the states and data in the generic diagnosis of Kostadinova (2005): very elongate body [BW = 7–14% (mean 10%) vs 12–18%]; long to very long forebody [FO = 20–32% (mean 25%) vs 12–20%]; very long post-testicular field [T = 27–50% (mean 33%) vs 20–30%]; and very short uterine field [U = 0–7% (mean 3%) vs 3–20%]. The metrical data in the description by Premvati (1968) fall within the range for E. capitaneum except for the much larger testes width (Table 1).
Phylogenetic analysis
Five sequenced specimens of E. capitaneum showed no intraspecific sequence variability. Our phylogenetic analysis included representatives of four families belonging to the superfamily Echinostomatoidea according to the latest systematic revision by Kostadinova (2005). The partial 28S rRNA gene sequence (1,243 bp) of E. capitaneum was included in the phylogenetic analysis together with 24 sequences of the Echinostomatoidea available on GenBank (Table 3). A sequence of Cyclocoelum mutabile Zeder, 1800 was also included for consistency with the results of the phylogenetic analysis by Olson et al. (2003). The final alignment was 1,161 bp long including several introduced gaps. Thirty-two ambiguously aligned sites were excluded from the analysis.
The BI analysis resulted in a tree with overall well-supported topologies. Disregarding weakly-supported branches/clades, the tree contained six strongly (100%) supported clades which are numbered 1–6 in Fig. 4. The largest clade (Clade 1) included representatives of the genera Echinostoma Rudolphi, 1809, Echinoparyphium Dietz, 1909, Ishthmiophora, Petasiger Dietz, 1909, Drepanocephalus Dietz, 1909 and Euparyphium, all belonging to the subfamily Echinostomatinae Looss, 1899 of the family Echinostomatidae. Within this large clade, Echinostoma + Echinoparyphium clustered in a rather weakly-supported clade whereas the members of the remaining genera formed a 100% supported grouping. Interrelationships among genera within the latter group are not well defined.
Phylogenetic relationships among 25 taxa of the Echinostomatoidea resulting from Bayesian analysis (3,000,000 generations) based on the nuclear ribosomal sequences of the 28S rRNA gene. Only bootstrap values > 70 are shown. Dotted rectangles indicate six strongly supported clades. Branch length scale bar indicates number of substitutions per site
Clade 2 (Fig. 4) included representatives of four fasciolid genera; see below for comments on their interrelationships. Clade 3 (Fig. 4) comprised four species representing at least two genera of the Philophthalmidae Looss, 1899. The internal topology of this clade was fully resolved with 100% support of its two sub-clades. The cyclocoelid C. mutabile formed its own independent branch (Clade 4 in Fig. 4). One of the two remaining 100% supported clades (Fig. 4) included two species of Echinochasmus Dietz, 1909 (Clade 5) whereas Clade 6 consisted of representatives of two genera of the Psilostomidae Looss, 1900.
Discussion
In this study we provide the first adequately detailed description of E. capitaneum based on well-fixed, high quality specimens observed on total mounts under light microscope as well as under scanning electron microscope. This allowed the observation of previously unreported details of the species morphology. The SEM study allowed description of the pattern of the tegumental spination, particularly the posterior extent of the spination and the presence of the spine-free area on the ventral surface of the forebody. Among other morphological features observed under SEM were the sensory papillae on the ventral sucker and the basal sensory papillae on the cirrus (Figs. 2F, 3B). The morphological observations of the surface ultrastructure of the cirrus confirmed the lack of spines; this further corroborates the value of this morphological character as a distinguishing feature between the two closely related genera, Isthmiophora and Euparyphium.
The content of the genus Euparyphium, including its type-species, has been called into question by several authors (Mendheim, 1943; Skrjabin & Bashkirova, 1956; Yamaguti, 1958) and more recently by Kostadinova & Gibson (2002). The number of the species within this genus has changed several times, mostly due to the convoluted taxonomic history of the genera Isthmiophora and Echinocirrus. The main reason for the lack of systematic and nomenclatural stability among these digeneans has been the relative paucity of morphological variation among these genera and the lack of phylogenetic data. Based on the examination of the type-species of Euparyphium and Isthmiophora and newly-collected material, plus a critical evaluation of the previously published data, Kostadinova & Gibson (2002) re-established the validity of Isthmiophora with Isthmiophora melis as the type-species. Our molecular phylogenetic analysis has confirmed the systematic conclusions by Kostadinova & Gibson (2002) regarding the separation of Euparyphium and Isthmiophora and the utility of the morphological characters proposed by these authors for generic differentiation. In the phylogenetic tree (Fig. 4) Isthmiophora and Euparyphium appear as distinct genus-level clades within the cluster comprising the Echinostomatinae. Inclusion of the type-species of both genera in our analysis gives credibility to our results and provides the basis for future detailed systematic revision of the content of these genera.
Of the remaining species of Euparyphium only a short (618 bp) sequence of 28S rRNA gene of Euparyphium albuferensis Esteban, Toledo, Sanchez & Munoz-Antoli, 1997 was available on GenBank (AY219697) and compatible with our dataset, albeit too short to be included in the phylogenetic analysis. However, we found high degree of sequence divergence (8.5%) in the overlapping, generally conserved 28S region of E. capitaneum and E. albuferensis suggesting that these species are not very closely related and most likely not congeneric. Moreover, a BLAST search using the sequence of E. albuferensis has demonstrated that this species is much closer to members of the genera Hypoderaeum Dietz, 1909 and Echinoparyphium (1% divergence) than to E. capitaneum, the type-species of Euparyphium. Therefore, either E. albuferensis does not belong to Euparyphium or the specimen that was used as a source for the sequence has been misidentified. The clustering of this species with Echinoparyphium and Hypoderaeum in the tree based on ITS2 sequences of nuclear ribosomal DNA published by Heneberg (2013) supports the above considerations. It is worth noting that in the original description of E. albuferensis Esteban et al. (1997) indicated that this species may belong to either Euparyphium or Echinoparyphium and distinguished the new species from all members of the Echinoparyphium ‘recurvatum’ species complex. Some features in the description by Esteban et al. (1997), i.e. the size of the dorsal spines (dorsal oral spines being longer than aboral), the postequatorial location of the testes and the more anterior extent of the vitelline fields, also agree better with the diagnosis of Echinoparyphium (see Kostadinova, 2005). Therefore, although E. albuferensis may appear valid (and the specimen sequenced identified correctly), additional morphological and molecular phylogenetic analyses are required before a definitive conclusion for its generic affiliation is reached. Further, the morphological and molecular similarity of E. albuferense with Echinoparyphium spp. and the novel data for the type-species of Euparyphium stress the rather mechanistic nature of the diagnosis of the latter genus by Kostadinova (2005). The molecular and morphological results of our study indicate that Euparyphium requires further revision. Sequencing of the Asian species currently allocated to Euparyphium, i.e. E. guerreroi and E. murinum, would help resolve this issue; we do not exclude the possibility of Euparyphium being monotypic.
Although in the most recent systematic treatment of the Cyclocoelidae Stossich, 1902 by Kanev et al. (2002) this family was included into its own superfamily, the subsequent molecular phylogenetic analyses by Olson et al. (2003) firmly placed it within a well-supported clade with other echinostomatoideans. A more detailed study including broader representation of cyclocoelids is necessary to evaluate its interrelationships with other families within the superfamily.
Among the other taxa of the Echinostomatoidea included in the present phylogenetic analysis the position of Fascioloides jacksoni (Cobbold, 1869) deserves a comment. This species is positioned among the Fasciolidae Railliet, 1895 (Clade 2) as the basal taxon to Fascioloides magna (Bassi, 1875) and two species of Fasciola Linnaeus, 1758 in a 100% supported clade. The position of F. jacksoni in this clade and the high level of support of all branches in it do not unequivocally support the conclusion of Heneberg (2013) that phylogenetic data suggest inclusion of F. jacksoni in Fascioloides Ward, 1917. Our result based on 28S sequences is identical to that obtained by Heneberg (2013) who used the same gene as well as ITS1 region of the nuclear ribosomal DNA. On the other hand, the ribosomal ITS2 region and nicotinamide adenine dinucleotide dehydrogenase subunit 1 (nad1) gene in Heneberg’s study favoured the inclusion of F. jacksoni in Fascioloides. Considering the above inconsistencies in the outcome of phylogenetic analyses, the systematic position of F. jacksoni as well as morphological and biological characters used to delineate genera in the Fasciolidae deserve further consideration.
The only somewhat unexpected result in our phylogenetic analysis was the separation of the clade of Echinochasmus spp. (subfamily Echinochasminae Odhner, 1910, Clade 5) from the remaining representives of the family Echinostomatidae (subfamily Echinostomatinae, Clade 1) in its own family-level group. This result indicates that the systematic position of Echinochasmus may deserve a detailed analysis and possibly, re-consideration. It should be mentioned, however, that the sequence for one of these species, Echinochasmus sp. (JQ088098), was based on a cercarial isolate and that the sequence of Echinochasmus japonicus Tanabe, 1926 (JQ890579) is not otherwise published and does not have associated host data. Therefore, at this time we prefer to consider this result with some caution until more Echinochasmus spp. (and other members of the subfamily) are available for phylogenetic analysis.
Our results are in complete agreement with those obtained by Heneberg (2013) who used essentially the same set of taxa with the exception of E. capitaneum and a different outgroup. The pattern was also largely preserved in the tree based on ITS1 sequences presented by Heneberg (2013). At the same time, the ITS2 tree in Heneberg (2013) shows some striking differences in the position of several taxa. These include the position of Isthmiophora as a sister group of the Cathemasiidae Fuhrmann, 1928 and Psilostomidae Looss, 1900 and the nested position of Sphaeridiotrema Odhner, 1913, basal to several echinostomatine genera. Considering these obvious inconsistencies and low support of many topologies in the ITS2 tree in the study of Heneberg (2013) this DNA region seems to be unsuitable for higher level phylogenetics and classification within the Echinostomatoidea.
References
Bergmame, L., Hyffman, J., Cole, R., Dayanandan, S., Tkach, V., & McLaughlin, J. D. (2011). Sphaeridiotrema globulus and Sphaeridiotrema pseudoglobulus (Digenea): species differentiation based on mtDNA (Barcode) and partial LSU-rDNA sequences. Journal of Parasitology, 97, 1132–1136.
Church, M. L., Barrett, P. M., Swenson, J., Kinsella, J. M., & Tkach, V. V. (2013). Outbreak of Philophthalmus gralli in four greater rheas (Rhea americana). Veterinary Ophthalmology, 16, 65–72.
Dietz, E. (1909). Die Echinostomiden der Vögel. Zoologischer Anzeiger, 34, 180–192.
Dietz, E. (1910). Die Echinostomiden der Vögel. Zoologische Jahrbücher, 12, 256–512.
Esteban, J. G., Toledo, R., Sánchez, L., & Muñoz-Antolí, C. (1997). Life-cycle of Euparyphium albuferensis n. sp. (Trematoda: Echinostomatidae) from rats in Spain. Systematic Parasitology, 38, 211–219.
Georgieva, S., Kostadinova, A., & Skírnisson, K. (2012). The life-cycle of Petasiger islandicus Kostadinova & Skirnisson, 2007 (Digenea: Echinostomatidae) elucidated with the aid of molecular data. Systematic Parasitology, 82, 177–183.
Griffin, M. J., Khoo, L. H., Quiniou, S. M., O’Hear, M. M., Pote, L. M., Greenway, T. E., Wise, D. J. (2012). Genetic sequence data identifies the cercaria of Drepanocephalus spathans (Digenea: Echinostomatidae), a parasite of the double-crested cormorant (Phalacrocorax auritus), with notes on its pathology in juvenile channel catfish (Ictalurus punctatus). Journal of Parasitology, 98, 967–972.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor v. 5.0.9. Nucleic Acids Symposium, 41, 95–98.
Heneberg, P. (2013). Phylogenetic data suggest the reclassification of Fasciola jacksoni (Digenea: Fasciolidae) as Fascioloides jacksoni comb. nov. Parasitology Research, 112, 1679–1689.
Huelsenbeck, J. P., & Ronquist, F. (2001). MrBayes: Bayesian inference of phylogeny. Bioinformatics, 17, 754–755.
Kanev, I., Radev, V., & Fried, B. (2002). Family Cyclocoelidae Stossich, 1902. In: Gibson, D. I., Jones, A. & Bray, R. A. (Eds) Keys to the Trematoda, Volume 1. Wallingford: CAB International, pp. 131–145.
Kostadinova, A. (2005). Family Echinostomatidae Looss, 1899. In: Jones, A., Bray, R. A. & D. I. Gibson, D. I. (Eds) Keys to the Trematoda, Volume 2. Wallingford: CAB International and The Natural History Museum, pp. 9–64.
Kostadinova, A., & Gibson, D. I. (2002). Isthmiophora Lühe, 1909 and Euparyphium Dietz, 1909 (Digenea: Echinostomatidae) re-defined, with comments on their nominal species. Systematic Parasitology, 52, 205–217.
Literák, I., Heneberg, P., Sitko, J., Wetzel, E. J., Cardenas Callirgos, J. M., Čapek, M., Valle Basto, D., & Papoušek, I. (2013). Eye trematode infection in small passerines in Peru caused by Philophthalmus lucipetus, an agent with a zoonotic potential spread by an invasive freshwater snail. Parasitology International, 62, 390–396.
Lotfy, W. M., Brant, S. V., DeJong, R. J., Le, T. H., Demiaszkiewicz, A., Rajapakse, R. P., Perera, V. B., Laursen, J. R., & Loker, E. S. (2008). Evolutionary origins, diversification, and biogeography of liver flukes (Digenea, Fasciolidae). American Journal of Tropical Medicine and Hygiene, 79, 248–255.
Mendheim, H. (1943). Beiträge zur Systematik und Biologie der Familie Echinostomatidae (Trematoda). Archiv für Naturgeschichte, 12, 175–302.
Nylander, J. A. A. (2004). MrModelTest 2.3. Program Distributed by the Author. Uppsala University; Uppsala, Switzerland.
Olson, P. D., Cribb, T. H., Tkach, V. V., Bray, R. A., & Littlewood, D. T. J. (2003). Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755.
Pérez Vigueras, I. (1944). Trematodes de la super-familia Echinostomatidae, con descripción de siete especies nuevas de Cuba. Revista de la Universidad de la Habana, 57, 221–234.
Premvati, G. (1968). Echinostome trematodes from Florida birds. Proceedings of the Helminthological Society of Washington, 35, 197–200.
Rambaut, A. (2012). FigTree v. 1.4. Molecular evolution, phylogenetics and epidemiology. Edinburgh, UK: University of Edinburgh, Institute of Evolutionary Biology, http://tree.bio.ed.ac.uk/software/figtree/.
Sato, H., & Suzuki, K. (2006). Gastrointestinal helminthes of feral raccoons (Procyon lotor) in Wakayama Prefecture, Japan. Journal of Veterinary Medical Science, 68, 311–318.
Skrjabin, K. I., & Bashkirova, E. Y. (1956). Family Echinostomatidae. Osnovy Trematodologii, 12, 53–930 (In Russian).
Tkach, V. V., Littlewood, D. T. J., Olson, P. D., Kinsella, J. M., & Świderski, Z. (2003). Molecular phylogenetic analysis of the Microphalloidea Ward, 1901. Systematic Parasitology, 56, 1–15.
Tkach, V. V., & Pawlowski, J. (1999). A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitologica, 44, 147–148.
Tkach, V., Pawlowski, J., & Mariaux, J. (2000). Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. International Journal for Parasitology, 30, 83–93.
Tkach, V., Pawlowski, J., Mariaux, J., & Swiderski, Z. (2001). Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In: Littlewood, D. T. J. & Bray, R. A. (Eds) Interrelationships of the Platyhelminthes. London: Taylor & Francis, pp. 186–193.
Tkach, V. V., Schroeder, J. A., Greiman, S. E., & Vaughan, J. A. (2012). New genetic lineages, host associations and circulation pathways of Neorickettsia endosymbionts of digeneans. Acta Parasitologica, 57, 285–292.
Yamaguti, S. (1958) Systema helminthum. Vol. 1. The digenetic trematodes of vertebrates. New York: Interscience Publishers, 1,575 pp.
Acknowledgements
The authors thank Dr. Robin Overstreet (Gulf Coast Research Laboratory, University of Southern Mississippi, Ocean Springs, USA) who generously provided laboratory facility and equipment for collecting and processing of birds. Donna Laturnus (Department of Anatomy and Cell Biology, School of Medicine and Health Sciences, University of North Dakota) kindly provided technical assistance with preparation of samples for scanning electron microscopy. Birds were collected under USFWS permit MB681207-0 and Mississippi “Administrative Scientific Collecting Permit” issued annually to Robin M. Overstreet by the Mississippi Museum of Natural Science. This study was supported in part by the National Sciences Foundation award DEB1050525 to VVT and the European Union Structural Funds project “Postdoctoral Fellowship Implementation in Lithuania” (VP1-3.1-ŠMM-01-V-02-004) to OK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudlai, O., Tkach, V.V., Pulis, E.E. et al. Redescription and phylogenetic relationships of Euparyphium capitaneum Dietz, 1909, the type-species of Euparyphium Dietz, 1909 (Digenea: Echinostomatidae). Syst Parasitol 90, 53–65 (2015). https://doi.org/10.1007/s11230-014-9533-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-014-9533-0