Abstract
DNA sequencing of the nuclear ribosomal DNA internal transcribed spacers (ITS) and mitochondrial rrnS and cox2 genes, and analysis of polymorphisms in restriction profiles in the ITS and rrnS, were used to characterise anisakid nematodes belonging to Contracaecum Railliet & Henry, 1912 infecting the brown pelican Pelecanus occidentalis (L.) in Galveston Bay, Texas and Sarasota Bay, Florida. Molecular data led to the detection of two new species: Contracaecum fagerholmi n. sp., which was also supported by clear morphological evidence, and Contracaecum rudolphii F, a new cryptic species within the Contracaecum rudolphii Hartwich, 1964 complex. Bayesian phylogenetic analysis demonstrated that C. fagerholmi and C. rudolphii F form two well-separated clusters, with C. fagerholmi being closely related to Contracaecum bioccai Mattiucci et al., 2008 and C. rudolphii F being included in the C. rudolphii complex. C. fagerholmi can be readily differentiated morphologically from all of its congeners, other than C. microcephalum (Rudolphii 1809) and the five currently recognised members of the C. rudolphii complex (C. rudolphii A, B, C, D and E). C. fagerholmi differs from C. microcephalum in the length of the spicules and the shape of the distal tip of the spicules, and from C. rudolphii (sensu lato) in the shape and size of the ventro-lateral and dorsal lips and by having interlabia which are not distally bifurcate. Further studies are needed to determine which morphological characteristics can be used to distinguish the cryptic species of the C. rudolphii complex in order to assign them with formal names. The recovery of a third species, C. bioccai, from the brown pelican confirms its occurrence in this host and extends its known geographical distribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large number of parasite species of the genus Contracaecum Railliet & Henry, 1912 (Nematoda: Anisakidae) occur as adults in the stomachs of fish-eating birds, including species of Pelecanus (L.) (Mozgovoi, 1953; Hartwich, 1964; Barus et al., 1978; Anderson, 2000; Fagerholm & Overstreet, 2009). The following species of Contracaecum have been reported from the brown pelican P. occidentalis (L.) in the Gulf of Mexico and the Caribbean Sea: C. mexicanum Flores Barroeta, 1957 from off Venezuela and Puerto Rico (Diaz-Ungria, 1978, 1979; Dyer et al., 2002); C. multipapillatum (Drasche, 1882) in the Gulf of Mexico (Courtney & Forrester, 1974; Courtney et al., 1977; Deardorff & Overstreet, 1980; Grimes et al., 1989) and off Puerto Rico (Dyer et al., 2002); C. rudolphii Hartwich, 1964 (sometimes reported as C. spiculigerum Rudolphi, 1809) in the Gulf of Mexico (Hutton, 1964; Huizinga, 1966, 1971; Courtney & Forrester, 1974; Deardorff & Overstreet, 1980) and off Puerto Rico (Bunkley-Williams & Williams, 1994); C. bioccai Mattiucci, Paoletti, Olivero-Verbel, Baldiris, Arroyo-Salgado, Garbin, Navone & Nascetti, 2008 from off northern Colombia. Undetermined species have also been reported, as Contracaecum sp. or spp., from the Gulf of Mexico (Courtney et al., 1977; Humphrey et al., 1978; Deardorff & Overstreet, 1980; Greve et al., 1986; Dronen et al., 2003).
Of particular interest is the study by Deardorff & Overstreet (1980) on nematodes of white and brown pelicans, Pelecanus erythrorhynchus Gmelin and P. occidentalis L., double-crested cormorants Phalacrocorax auritus (Lesson) and least bitterns Ixobrychus exilis (Gmelin) from Mississippi, Louisiana and Florida. These authors reported the presence of specimens of Contracaecum with peculiar morphological characters, i.e. lacking the bifurcate interlabia as in C. microcephalum (Rudolphi, 1809) but having the spicule length and shape of the spicule tip consistent with those described for C. rudolphii, and suggested that these specimens could represent a new species. Possible evidence for a new species from brown and white pelicans in Galveston Bay, Texas, was also indicated by Dronen et al. (2003).
In recent years, nuclear ribosomal and mitochondrial markers have proved to be powerful tools which complement morphology in relation to the identification of new species and infer phylogenetic relationships. The use of the first (ITS-1) and/or second (ITS-2) internal transcribed spacers (ITS) of nuclear ribosomal DNA (rDNA) have provided genetic markers for the accurate identification of a range of species of ascaridoid nematodes (Jacobs et al., 1997; Zhu et al., 1998a, b, 1999, 2000a, b, 2001, 2002; D’Amelio et al., 2000; Hu et al., 2001; Abollo et al., 2003). Also, for species of Contracaecum, molecular methods have provided additional genetic markers and PCR-based practical tools for the identification of two cryptic species within the morphospecies C. rudolphii, which were named as A and B (Li et al., 2005; Zhu et al., 2007). D’Amelio et al. (2007) indicated the existence of a third cryptic species, within the C. rudolphii complex (C. rudolphii C) in double-crested cormorants from west-central Florida based on PCR-RFLP and sequencing of the rrnS mitochondrial gene and nuclear ribosomal spacers. Mattiucci et al. (2008) described a new species, C. bioccai, from brown pelicans off northern Colombia, on the basis of the genetic differentiation at 20 enzyme loci and at the cox2 mitochondrial gene, and also on the basis of morphological evidence. More recently, Shamsi et al. (2008, 2009) described a new morphospecies, C. pyripapillatum Shamsi, Gasser, Beveridge & Shabani, 2008, and two new sibling species within the C. rudolphii complex, designated as D and E, based on both morphology and nuclear ITS1 and ITS2 markers. An additional two new species, previously referred to as C. multipapillatum A and B, were recently described as C. gibsoni Mattiucci, Paoletti, Solorzano & Nascetti, 2010 and C. overstreeti Mattiucci, Paoletti, Solorzano & Nascetti, 2010 by Mattiucci et al. (2010).
The aims of the present paper were to: (1) characterise the different taxa belonging to Contracaecum and infecting the brown pelican P. occidentalis in Galveston Bay, Texas and Sarasota Bay, Florida based on the combined results obtained from DNA sequences of the ITS nuclear ribosomal region, rrnS and cox2 mitochondrial genes; (2) provide molecular markers for their efficient identification based on polymorphisms in restriction profiles in the ITS and rrnS; (3) infer the phylogenetic relationships between these taxa and their congeners; and (4) provide morphological diagnostic characters for use in species descriptions and nomenclatural designations.
Materials and methods
Parasites
A total of 40 adult anisakid nematodes belonging to Contracaecum were collected from the stomach of five Pelecanus occidentalis; one from Sarasota Bay, west-central Florida, and four individual hosts of the same species from Galveston Bay, Texas, were analysed. From each specimen, the anterior and posterior parts of the body were preserved and cleared in lactic acid-phenol (1:1) for morphological studies, whereas the remaining part was used for genetic purposes. Collection data, including number of hosts examined, number of parasite specimens analysed, collecting sites and codes are summarised in Table 1.
Genetic study
DNA was isolated using the Wizard® Genomic DNA purification kit (Promega) according to the manufacturer’s protocol. Genetic characterisation and identification was performed on 40 individuals by PCR-RFLP analysis based on rrnS and ITS markers.
The amplification of the rrnS was performed using 5.0 μl of template DNA(20–40 ng), 10 mM Tris-HCl (pH = 8.3), 50 mM KCl (Applied Biosystems), 3 mM MgCl2 (Applied Biosystems), 40 mM of dNTPs (Promega), 50 pmol/μl of the forward primer MH3 (5′-TTGTTCCAGAATAATCGGCTAGACTT), 50 pmol/μl of the reverse primer MH4.5 (5′-TCTACTTTACTACAACTTACTCC) and 0.5 μl of AmpliTaq Gold™ (Promega) in a 50 μl final volume of reaction. The conditions of PCR were as follows: 10 min at 95°C (initial denaturation), 35 cycles of 30 sec at 95°C (denaturation), 30 sec at 55°C (annealing) and 30 sec at 72°C (extension), and a final elongation step of 7 min at 72°C.
The entire ITS nuclear region was amplified using 5.0 μl of template DNA (20–40 ng), 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2 (Bioline), 40 mM of a nucleotide mix (Promega), 50 pmol/μl each of the forward primer NC5 (5′-GTAGGTGAACCTGCGGAAGGATCAT-3′) and the reverse primer NC2 (5′-TTAGTTTCTTCCTCCGCT-3′) (Zhu et al. 2000b) and 1.0 U of BIOTAQ DNA Polymerase (Bioline) in a final volume of 50 μl. The PCR conditions were: 10 min at 95°C (initial denaturation), 30 cycles of 30 sec at 95°C (denaturation), 40 sec at 52°C (annealing) and 75 sec at 72°C (extension), and a final elongation step of 7 min at 72°C. A negative control (without genomic DNA) was included in each set of amplification reactions. All the PCR reactions were performed in a GeneAmp PCR System 2400 (Applied Biosystems); then aliquots (5 μl) of individual PCR products were separated by electrophoresis using agarose gels (1%), stained with ethidium bromide (0.4 μg/ml) and detected using ultraviolet transillumination. Gel images were captured electronically and analyzed using the program MULTI-ANALYST (v.1.1, Bio-Rad).
The rrnS amplicons were digested with RsaI and DdeI endonucleases and the ITS amplicons were digested with Tsp509I endonuclease, according to D’Amelio et al. (2007). Digests were resolved by electrophoresis in 2% agarose gels, stained with ethidium bromide (0.4 μg/ml), detected upon transillumination and the sizes of fragments determined by comparison with a 100 bp DNA ladder as size marker (Promega).
The cox2 was amplified using the forward primer 211 (5′-TTTTCTAGTTATATAGATTGRTTYAT-3′) and the reverse primer 210 (5′-CACCAACTCTTAAAATTATC-3′) (Nadler & Hudspeth, 2000). PCR amplification was performed using the same reagents of rrnS gene. The conditions of PCR were as follows: 3 min at 94°C (initial denaturation), 34 cycles of 30 sec at 94°C (denaturation), 30 sec at 46°C (annealing) and 90 sec at 72°C (extension), and a final elongation step of 7 min at 72°C.
Phylogenetic analysis
Twenty-seven positive PCR amplicons of the three genomic regions (Table 2), representative of the three taxa identified by restriction profiles analysis, were purified by SureClean Product Insert (Bioline), following the manufacturer’s instructions. The pellets were re-suspended in 30 μl of H2O and subjected to automated sequencing by MWG-Biotech.
Nucleotide mitochondrial sequences (cox2) were aligned using Clustal X implemented in the program MEGA 4.1 (Tamura et al., 2007) and translated into protein to verify that no stop codon or Numt was present. Ribosomal nuclear (ITS) and mitochondrial (rrnS) sequences were aligned using PRANK (Löytynoja & Goldman, 2005). Nucleotide sequences of both nuclear and mitochondrial DNA regions were aligned with verified sequences of Contracaecum species from fish-eating birds available in GenBank (for specimen codes and accession numbers, see Table 2), excluding Contracacum species from phocid seals, which are more closely related to Phocascaris Høst, 1932 (see Nadler et al., 2000).
JModeltest (Posada, 2009) was used to compare the fit of nucleotide substitution models using the Akaike Information Criterion (AIC), under a total of 83 models, corresponding to 11 different schemes; the best-fit ML models and parameters as determined for the rrnS, cox2 and ITS datasets were used for Bayesian analyses. Bayesian analyses were performed using the GTR+G model for ITS1_2, cox2 and rrnS (as selected by ModelTest), using BEAST software (Drummond & Rambaut, 2007); the datasets were run twice for 107 generations. Posterior probability values (BPP) shown in Bayesian consensus trees were determined after discarding trees from the burn-in period. For each dataset, burn-in was estimated to include the first 2 × 105 generations. Phylogenetic trees based on rrnS and ITS regions included Ascaris suum Goeze, 1782 and Toxocara canis (Werner, 1782) as outgroups. To make trees more comparable, Toxascaris leonina (Linstow, 1902) was included rather than Toxocara canis for the cox2 tree, because the ingroup obtained was not monophyletic when rooted by A. suum and T. canis (see Table 2 for GenBank accession numbers). The consistency index was calculated for the three datasets using MEGA 4.1 (Tamura et al., 2007).
Morphological study
Measurements and morphological descriptions were undertaken using a compound microscope equipped with a drawing apparatus at magnifications of ×100–400, with the exception of total body length, which was measured directly. All measurements are in micrometres unless otherwise indicated. The characters studied are those considered of diagnostic value for anisakid nematodes (Fagerholm, 1991) and those used specifically for Contracaecum spp. from fish-eating birds (Barus et al., 1978), including body length and width, labial and interlabial length and shape, oesophageal length, ventriculus and ventricular appendix length, spicule length, shape of the spicule tip, pattern of the male caudal papillae, which were labelled according to the nomenclature proposed by Fagerholm (1991), and tail length. In order to evaluate allometric variation (Fagerholm, 1989), several of the measurements of each male specimen were related to total body length (body length/spicule length; body length/tail length) and oesophageal length (oesophagus length/ventriculus appendix length; oesophagus length/intestinal caecum length).
Results
Genetic characterisation and phylogenetic inference
The analysis of the three DNA regions (rrnS and cox2 mitochondrial genes and ITS nuclear region) with a Bayesian approach provided evidence for the existence of three distinct clades, representing three different taxa: one clade, comprising 11 specimens, is referable to C. bioccai, whereas the other two clades represent two distinct new taxa which are well differentiated with respect to the five cryptic species of the C. rudolphii complex (A, B, C, D and E) and to other previously studied species, such as C. septentrionale Kreis, 1955, C. pyripapillatum, C. bancrofti Johnston & Mawson, 1941, C. microcephalum, C. micropapillatum (Stossich, 1890), C. multipapillatum, C. gibsoni and C. overstreeti. The existence of the two new taxa is very well supported by maximum values of posterior probability.
Considering the two Bayes consensus trees obtained for ribosomal DNA analyses, nuclear ITS and mitochondrial rrnS (Figs. 1, 2), both topologies indicate separate clades well supported by posterior probability values: one clade comprises species belonging to the C. rudolphii complex, including the specimens analysed in the present study and designated below as C. rudolphii F. The other clade is formed by two well separated species, C. bioccai and the second new taxon detected here, which is named below as C. fagerholmi n. sp. in a formal designation. In the ITS tree, C. septentrionale is the sister group of the two clades, whereas C. microcephalum is the sister group of clades 1 and 2 in the rrnS Bayes consensus tree where sequences from C. septentrionale are missing. This evidence is in agreement with the results obtained by D’Amelio et al. (2007) based on Maximum Parsimony analysis of rrnS sequences, where the same species arrangement was defined.
Phylogenetic Bayesian consensus tree based on ribosomal internal transcribed spacer (ITS1 and ITS2) sequences. Numbers at branches represent posterior probability values. Bayesian analysis was performed using the GTR+G model. The dataset was run for 107 generations. The burn-in includes the first 2 × 105 generations. For the taxon names, see Tables 1 and 2
Phylogenetic Bayesian consensus tree based on 12 s mitochondrial small ribosomal subunit gene (rrnS) sequences. Bayesian analysis was performed using the GTR + G model. The dataset was run for 107 generations. The burn-in includes the first 2 × 105 generations. For the taxon names, see Tables 1 and 2
A different topology occurs in the cox2 Bayes consensus tree (Fig. 3): C. rudolphii F specimens are more closely related to members of the C. rudolphii complex, as in the rrnS and ITS trees, and C. septentrionale acts as the sister clade, but C. bioccai and C. fagerholmi do not fall within the same group. It may be important to note that posterior probability values obtained at two nodes of the cox2 Bayes tree are quite low (87% and 76%). The low value of consistency index obtained for cox2 (CI = 0.46) reveals for this gene more homoplasy than occurs in the ITS (CI = 0.78) and rrnS (CI = 0.61). One specimen coded Bp40 was not unequivocally assigned, as it clusters with C. rudolphii D in the ITS tree, with C. rudolphii F in the rrnS tree and with C. rudolphii B in the cox2 tree.
Sequences of the three genomic regions analysed, for the three detected species, were submitted to GenBank. Accession numbers and codes are included in Table 2.
Assessment of genetic markers based on RFLP profiles
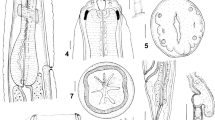
The combination of the restriction profiles obtained after digestion of ITS amplicons with Tsp509I and of rrnS amplicons with RsaI and DdeI allows the identification of the three detected Contracaecum taxa: one profile is referable to C. bioccai and two profiles correspond to the two new taxa named below as C. fagerholmi and C. rudolphii F (Fig. 4), confirming the results obtained by phylogenetic inference.
Virtual restriction profiles in Contracaecum fagerholmi n. sp., C. rudolphii F and C. bioccai after digestion of the ITS region with Tsp509I and the rrnS region with DdeI and RsaI, obtained using the NEBcutter 2.0 interface (Vincze et al., 2003). The scale on the left is a 100 bp ladder
Twenty-three individuals showed, after digestion with Tsp509I, a pattern corresponding to Contracaecum sp. 1 of D’Amelio et al. (2007), with two fragments of 500 and 390 bp, plus fragments of <100 bp; in the same individuals, the restriction with RsaI and DdeI produced, respectively, the following: two main fragments of 340 and 110 bp, plus one fragment of <100 bp; and three fragments of 220, 200 and <100 bp. The combination of cox2 sequences data and the morphological features observed led to their identification as C. bioccai.
Nine individuals showed the same pattern as C. bioccai after Tsp509I digestion but different profiles after digestion with RsaI and DdeI, producing the following: two main fragments of 290 and 110 bp and two minor fragments of <100 bp; and two fragments of 300 and 230 bp, respectively. The morphological evidence, combined with molecular and phylogenetic analyses, enabled their recognition as C. fagerholmi n. sp.
Nine individuals, after digestion with Tsp509I, exhibited a pattern of three fragments of 500, 200 and 170 bp, as observed for C. rudolphii C (of D’Amelio et al., 2007); restriction profiles obtained with DdeI corresponded to the pattern for C. fagerholmi, whereas digestion with RsaI produced a unique pattern of three fragments of 340 and 90 bp, plus two fragments of <100 bp. These specimens had morphological features referable to C. rudolphii (sensu lato) and, considering their molecular and phylogenetic divergence, are indicated as C. rudolphii F. An example of a taxonomic key based on three diagnostic restriction enzymes (Tsp509I, DdeI and RsaI) for the identification of the species under study is presented in Table 3. All restriction profiles were verified and confirmed, by virtual digestion of the relative sequences, using the NEBcutter 2.0 interface (Vincze et al., 2003).
Morphological data
In the present, paper the existence of clear morphological differential characters, strongly supported by genetic evidence, permitted the nomenclatural designation of one of the two new species detected as C. fagerholmi n. sp. For C. rudolphii F, a short description is given, but further studies are needed to distinguish this new species from the other five known cryptic species of the C. rudolphii complex and subsequently assign it a valid species name.
Contracaecum fagerholmi n. sp.
Type-material: Holotype male, allotype female from the stomach of Pelecanus occidentalis (L.) in Galveston Bay, Texas, USA. (type-host and type-locality). Anterior and posterior regions of the holotype are deposited at the Natural History Museum, London (BMNH Reg. No. 2011.7.20.1). Paratypes: four males and three females collected from P. occidentalis in Galveston Bay, Texas and Sarasota Bay, Florida USA. Anterior and posterior regions are deposited in the collection of the Section of Parasitology, Department of Public Health and Infectious Diseases, Sapienza University of Rome.
Etymology: The specific name, fagerholmi, is for Dr Hans-Peter Fagerholm, in acknowledgement of his fundamental contributions to the systematic importance of morphological characters for determining species of Contracaecum.
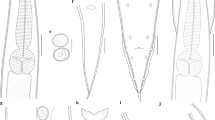
Description (Fig. 5; Table 4)
Body stout. Transverse cuticular striae present on entire length of body except for lips. Maximum width close to, but posterior to, mid-body. Excretory pore closely posterior to ventral interlabium. Nerve-ring at level of end of first fifth of oesophagus. Low, inconspicuous papillate deirids immediately posterior to nerve-ring. Lips prominent without dentigerous ridges; 2 ventro-lateral lips with 2 marked lateral flanges, distinctly longer than wide, and single dorsal lip with slight depression in middle of anterior margin. Ventro-lateral lips with 1 large, double ventro-lateral papilla, 1 externo-lateral papilla and 1 amphid; dorsal lip with 2 large double sublateral papillae. Interlabia large, extend beyond mid-length of lips, with rounded, not bifurcate, distal tip. Ventriculus reduced, globular, with short, solid posterior ventricular appendix. Intestinal caecum present.
Male
[Based on measurements of 5 specimens; holotype in parentheses.] Total length 22–27 (23) mm. Maximum width 800–840 (810). Lip length 100–150 (100); lip width 90–120 (90); lip length/lip width 1.11–1.25 (1.11). Interlabia length 80–100 (90); interlabia length/lip length 0.6–0.9 (0.9). Oesophagus length 2.54–2.94 (2.75) mm. Ventriculus inconspicuous, globular, 100–120 (120) in length, 100–120 (120) in width; ventricular appendix length 430–570 (570); ventriculus + ventricular appendix length 550–680 (680). Intestinal caecum 1.87–2.34 (1.87) mm in length. Oesophagus length/ventriculus length 21.2–29.4 (22.5); oesophagus length/ventricular appendix length 4.7–6 (4.7); oesophagus length/intestinal caecum length 1.2–1.45 (1.45). Spicules roughly equal in length, 4.15–4.85 (4.15–4.50) mm; distal tip of spicule rounded, with the 2 alae that overlap distally and end 20–30 (30) from spicule tip; relative length of the spicules (body length/spicule length) 5–5.5 (5.1–5.5). Tail conical 200–270 (220) in length; body length/tail length 81–125 (104); distal extremity of tail rounded. Caudal papillae (nomenclature according to Fagerholm, 1991) are as follows: proximal papillae numerous (>40) and disposed in single row; 1 median papilla anterior to cloaca; 2 pairs of single proximal papillae (p) short distance posterior to cloaca; 4 minute distal papillae on distal part of tail (d), of which d1 and d2 are more ventral and d3 and d4 more lateral; distal papillae d1 and d2 are slightly posterior to d3 and d4, respectively. Single pair of small papilla-like phasmids situated more dorsally to and just anterior to postero-ventral papilla (d4).
Female
[Based on measurements of 3 specimens, allotype in parentheses.] Total length 30–42 (42) mm. Maximum width 1.21–1.29 (1.29) mm. Lip length 170–190 (190); lip width 140–160 (160); lip length/lip width 1.2 (1.2). Interlabia length 130–140 (130); lip length/interlabia length 1.3–1.35 (1.35). Oesophagus length 3.4–4.4 (4.4) mm. Ventriculus 320–350 (350) in length, 300–350 (350) in width. Ventricular appendix length 0.71–1.05 (0.88) mm. Ventriculus + ventricular appendix length 1.04–1.40 (1.23) mm. Intestinal caecum length 2.70–3.38 (2.90) mm. Vulva at level of junction of first and second quarters of body. Tail conical, 380–410 (410) in length. Eggs rounded, 55-65 × 45-5 (50-65 × 50-55). Pair of papillate phasmids situated sublaterally on tail.
Differential diagnosis
C. fagerholmi n. sp., from Sarasota Bay and Galveston Bay, can be readily differentiated from all congeneric species other than C. microcephalum and the currently recognised members within the C. rudolphii complex. The new species is similar to C. microcephalum in terms of the morphology of the anterior end (length of lips and non-bifurcate interlabia), whereas it resembles C. rudolphii (s. l.) in the length of the spicules, which fall within the range of this morphospecies, and in the similarity of the free distal tip of the spicules.
However, C. fagerholmi differs from C. microcephalum in: (i) the longer length of spicules (4.15–4.85 versus 1.43–3.65 mm; latter data from Hartwich, 1964, and Barus et al., 1978); and (ii) in the shape of the distal end of the spicules, which exhibit a longer free distal tip (i.e. the distance from the most distal insertion of the alae to the rounded distal point of the spicule).
C. fagerholmi differs from C. rudolphii (s. l.) in that: (i) the interlabial tips are rounded and not bifurcate; (ii) the lips are slightly longer than wide; and (iii) the dorsal lip has a slight rather than a deep depression in the middle of its anterior margin.
Moreover, the new species was compared to C. bioccai because of its ecological affinity, i.e. it shares the same host species. C. fagerholmi differs from C. bioccai in that: (i) the interlabial tips are rounded and not bifurcate; and (ii) the arrangement of the male caudal papillae is different.
A comparative list of the morphological and morphometric characters of C. fagerholmi , C. microcephalum, C. rudolphii (s .l.) and C. bioccai is presented in Table 4, with the diagnostic characters given in bold.
Contracaecum rudolphii F
Material examined: Three males and five females from the stomach of Pelecanus occidentalis (L); Galveston Bay, Texas, USA.
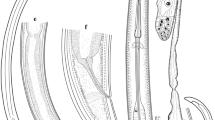
Description (Fig. 6)
Body stout. Transverse cuticular striae present on entire length of body except for lips. Maximum width close to, but posterior to, mid-body. Excretory pore just posterior to ventral interlabium. Nerve-ring at level of end of first fifth of oesophagus. Shallow, inconspicuous, papillate dierids immediately posterior to nerve-ring. Lips prominent, without dentigerous ridges, slightly wider than long; 2 ventro-lateral lips and 1 dorsal lip with marked lateral flanges and marked depression in middle of anterior margin. Ventro-lateral lips with 1 large, ventro-lateral double papilla, 1 externo-lateral papilla and 1 amphid; dorsal lip with 2 sublateral double papillae. Interlabia wider than long, with bifurcate tip, almost as deep as lips. Ventriculus reduced, inconspicuous, globular, with solid postero-ventral appendix. Intestinal caecum present.
Male
[Based on measurements of 3 specimens.] Total length 15–20 mm, width 0.76–1.01 mm. Oesophagus length 2.25–2.72 mm. Ventriculus length 100–140; ventricular appendix length 880–960; ventriculus plus ventricular appendix length 0.99–1.10 mm. Intestinal caecum 2.05–2.17 mm. Spicules equal or sub-equal, alate, length 5.96–7.30 mm; free distal tip of spicules 45–50. Pattern of caudal papillae similar to C. rudolphii (s. l.). Caudal alae absent. Tail markedly pointed, 200–240.
Female
[Based on measurements of 5 specimens.] Total length 45–56 mm, width 1.23–1.64 mm. Oesophagus length 3.16–4.33 mm. Ventriculus length 200–290; ventricular appendix length 0.88–1.32 mm; ventriculus plus ventriculus appendix length 1.13–1.61 mm. Intestinal caecum 2.31–3.03 mm. Vulva at level of junction of first and second quarters of body. Tail 300–400. Eggs 55–70 × 55–70. Pair of papillate phasmids situated sublaterally on tail.
Differential diagnosis
C. rudolphii F from Galveston Bay exhibits morphological characters clearly related to the C. rudolphii complex. Since this complex currently comprises five recognised cryptic species (C. rudolphii A, B, C, D and E), further studies are needed to determine whether morphological characters can be used to distinguish them and assign formal names.
Discussion
Recent molecular systematic approaches have been used to distinguish known species and to discover new species (Nadler & De Leon, 2011). For instance, DNA sequencing of the rrnS gene and PCR-RFLP profiles in the ITS region of specimens of Contracaecum, collected from brown pelicans in west-central Florida, provided evidence for a new, genetically differentiated taxon (D’Amelio et al., 2007). This taxon, designated as Contracaecum sp. 1, proved to be distinct from, but phenetically related to, C. microcephalum and to members of the C. rudolphii complex in both Maximum Parsimony and in UPGMA trees, although the small sample size did not permit a definitive identification.
Further samplings carried out in the present study of pelicans from Galveston Bay, Texas, permitted the recovery of additional specimens for analysis in terms of their taxonomic assignment. The genetic, phylogenetic and morphological analyses of these individuals allowed us to assign them to three distinct taxa: one corresponding to C. bioccai, confirming the presence of this species in brown pelicans and extending its geographic distribution, and two new taxa, C. fagerholmi n. sp. and C. rudolphii F.
Hypotheses based on phylogenetic evidence from nuclear and mitochondrial ribosomal regions (ITS and rrnS) support a close relationship between C. fagerholmi and C. bioccai, whereas C. rudolphii F is clearly included within the C. rudolphii complex cluster.
The consensus tree obtained from cox2 Bayesian analysis shows a quite different relationship between the taxa under study. This topology displays a near maximum statistical support for the monophyly of the C. rudolphii complex, including C. rudolphii F. The positions of C. bioccai and C. fagerholmi appear different from those indicated by rrnS and ITS evidence, probably due to the lower posterior probability values of the most basal internal nodes. In our opinion, cox2 sequence analyses is recommended for identification at the species level (barcoding) and for studying intraspecific variation, but it is less helpful for inferring phylogenetic relationships between congeneric species, because of its low resolution power in describing phylogenetic events or signals. A similar picture was also described in Cavallero et al. (2011) for Anisakis spp., where the Bayesian cox2 consensus tree showed more homoplasy, producing a different topology and demonstrating different relationships between species, as compared to Bayesian consensus trees obtained from the analysis of the ribosomal regions, both nuclear and mitochondrial.
The data obtained using restriction profiles analysis (PCR-RFLP), together with phylogeny and morphology, confirm that the three taxa studied are well defined at the species level. Moreover, the diagnostic key, based on PCR-RFLP diagnostic patterns in the ITS and rrnS regions, represents a practical tool for the detection and delineation of the species under study, which is usable for both sexes and any life-history stage. Overall, these results, together with those reported by D’Amelio et al. (2007), provide a quick, less time consuming and less expensive tool for the genetic identification of those Contracaecum spp. so far characterised.
With regard to the morphological evidence, C. fagerholmi is readily differentiated from all of its congeners other than C. microcephalum and C. rudolphii (s. l.). It differs from C. microcephalum in the length of its spicules and by the shape of the distal tip of the spicules. Thus the morphological study indicates clear structural metrical and non-metrical diagnostic features which distinguish these two taxa. Metrical features, indicated as raw measurements or as ratios, were obtained from fully-grown males to avoid bias due to unequal growth during the development of adult worms. The shape of the distal tip of the spicules is not influenced by allometric growth, since this character is known to be set at the earliest stages of development (Fagerholm, 1989). C. fagerholmi differs from. C. rudolphii (s. l.) in the shape and size of the ventro-lateral and dorsal lips and by the structure of tips of the interlabia, as described in detail above. A bifurcate or non-bifurcate (rounded) interlabial tip is considered a highly significant diagnostic character within Contracaecum (see Barus et al., 1978; Hugot et al., 1991).
The morphological results are consistent with the observations of Deardorff & Overstreet (1980), who reported the presence of Contracaecum specimens, identified as C. microcephalum (Rudolphi, 1809) using the key by Barus et al. (1978), with interlabia which had rounded rather than bifurcate tips, but with spicule lengths (4.7–6.2 mm) which did not correspond to the latter species. These authors recorded spicule lengths that fitted well within the range reported for C. rudolphii and distal spicule tips similar to those of C. rudolphii, but, as the arrangement of the postanal papillae was apparently similar in C. rudolphii and C. microcephalum, this feature was unhelpful. However, the body length range and the spicule lengths observed in the specimens analysed in the present study are consistent with measurements provided by Deardorff & Overstreet (1980) (i.e., 22–27 vs 21–34.5 mm and 4.15–4.85 vs 4.7–6.2 mm, respectively). The correspondence between the morphological characters of the specimens described by Deardorff & Overstreet (1980) and C. fagerholmi, therefore, supports the hypothesis that they belong to one and the same species.
Since the morphological characteristics usable for diagnostic purposes are mainly present in male individuals, genetic markers based on PCR-RFLPs and sequence analyses of informative genomic regions are particularly useful for the correct identification of female and larval specimens.
References
Abollo, E., Paggi, L., Pascual, S., & D’Amelio, S. (2003). Occurrence of recombinant genotypes of Anisakis simplex s.s and Anisakis pegreffii (Nematoda: Anisakidae) in an area of sympatry. Infection, Genetics and Evolution, 3, 175–181.
Anderson, R. C. (2000). Nematode parasite of vertebrates, their development and transmission. (2nd Edit.) Wallingford: CAB International, 650 pp.
Barus, V., Sergeeva, T. P., Sonin, M. D., & Ryzhikov, K. M. (1978). Helminths of fish-eating birds of the Palaearctic Region. I. Nematoda. Prague: Academia, 318 pp.
Bunkley-Williams, L., & Williams, E. H. Jr. (1994). Parasites of Puerto Rican freshwater sport fishes. San Juan, Puerto Rico: Puerto Rico Department of Natural and Environmental Resources; and Mayaguez, Puerto Rico: Department of Marine Sciences, University of Puerto Rico, 164 pp.
Cavallero, S., Nadler, S. A., Paggi, L., Barros, N. B., & D’Amelio, S. (2011). Molecular characterization and phylogeny of anisakid nematodes from cetaceans from southeastern Atlantic coasts of USA, Gulf of Mexico, and Caribbean Sea. Parasitology Research, 108, 781–792.
Courtney, C. H., & Forrester, D. J. (1974). Helminth parasites of the brown pelican in Florida and Louisiana. Journal of the Helminthological Society of Washington, 41, 89–93.
Courtney, C. H., Forrester, D. J., & White, F. H. (1977). Anthelminthic treatment of brown pelicans. Journal of the American Veterinary Medicine Association, 171, 921–922.
D’Amelio, S., Mathiopoulos, K. D., Santos, C. P., Pugachev, O. N., Webb, S. C., Picanço, M., & Paggi, L. (2000). Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase chain reaction-based restriction fragment length polymorphism. International Journal for Parasitology, 30, 223–226.
D’Amelio, S., Barros, N. B., Ingrosso, S., Fauquier, D. A., Russo, R., & Paggi, L. (2007). Genetic characterization of members of the genus Contracaecum (Nematoda: Anisakidae) from fish-eating birds from west central Florida, U.S.A., with evidence of new species. Parasitology, 134, 1041–1051.
Deardorff, T. L., & Overstreet, R. M. (1980). Contracaecum multipapillatum (= C. robustum) from fishes and birds in the northern Gulf of Mexico. Journal of Parasitology, 66, 853–856.
Diaz-Ungria, C. (1978). Helminthos parasitos de vertebrados en el Estado Zulia. Algunas especies nuevas para Venezuela. Kasmera, 6, 207–233.
Diaz-Ungria, C. (1979). Algunas especies de helminthos nuevas para Venezuela. Revista Iberica de Parasitologia, 39, 313–336.
Dronen, N. O., Blend, C. K., & Anderson, C. K. (2003). Endohelminths from the brown pelican, Pelecanus occidentalis, and the American white pelican, Pelecanus erythrorhynchus, from Galveston Bay, Texas, U.S.A., and checklist of pelican parasites. Comparative Parasitology, 70, 140–154.
Drummond, A. J., & Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7, 214.
Dyer, W. G., Williams, E. H., Jr, Mignucci-Giannoni, A. A., Jiménez-Marrero, N. M., Bunkley-Williams, L., Moore, D. P., & Pence, D. B. (2002). Helminth and arthropod parasites of the brown pelican, Pelecanus occidentalis, in Puerto Rico, with a compilation of all metazoan parasites reported from this host in the Western Hemisphere. Avian Pathology, 31, 441–448.
Fagerholm, H.-P. (1989). Intra-specific variability of the morphology in a single population of the seal parasite Contracaecum osculatum (Rudolphi) (Nematoda, Ascaridoidea), with a redescription of the species. Zoologica Scripta, 18, 33–41.
Fagerholm, H.-P. (1991). Systematic implications of male caudal morphology in ascaridoid nematode parasites. Systematic Parasitology, 19, 215–228.
Fagerholm, H.-P., & Overstreet, R. M. (2009). Ascaridoid nematodes: Contracaecum, Porrocaecum, and Baylisascaris. In: Atkinson, C. T., Thomas, N. J., & Hunter, D. B. (Eds) Parasitic diseases of wild birds. Oxford: Wiley-Blackwell, pp. 413–433.
Greve, J. H., Albers, H. F., Suto, B., & Grimes, J. (1986). Pathology of gastrointestinal helminthiasis in the brown pelican (Pelecanus occidentalis). Avian Diseases, 30, 482–487.
Grimes, J., Suto, B., Greve, J. H., & Albers, H. F. (1989). Effect of selected anthelminthics on three common helminths in the brown pelican (Pelecanus occidentalis). Journal of Wildlife Diseases, 25, 139–142.
Hartwich, G. (1964). Revision der vogel parasitischen Nematoden Mitteleuropas. II. Die Gattung Contracaecum Railliet & Henry, 1912 (Ascaridoidea). Mitteilungen aus dem Zoologischen Museum in Berlin, 40, 15–53.
Hu, M., D’Amelio, S., Zhu, X. Q., Paggi, L., & Gasser, R. B. (2001). Mutation scanning for sequence variation in three mitochondrial DNA regions for members of the Contracaecum osculatum (Nematoda: Ascaridoidea) complex. Electrophoresis, 22, 1069–1075.
Hugot, J. P., Morand, S., & Vassart, M. (1991). Morphological study of Contracaecum magnicollare (Nematoda, Anisakidae) from Anous minutus (Aves, Laridae). Systematic Parasitology, 20, 229–236.
Huizinga, H. W. (1966). Studies on the life cycle and development of Contracaecum spiculigerum (Rudolphi, 1809) (Ascaroidea: Heterocheilidae) from marine piscivorous birds. Journal of the Elisha Mitchell Scientific Society, 82, 181–195.
Huizinga, H. W. (1971). Contracaeciasis in pelecaniform birds. Journal of Wildlife Diseases, 7, 198–204.
Humphrey, S. R., Courtney, C. H., & Forrester, D. J. (1978). Community ecology of the helminth parasites of the brown pelican. The Wilson Bulletin, 90, 587–598.
Hutton, R. F. (1964). A second list of parasites from marine and coastal animals of Florida. Transactions of the American Microscopical Society, 83, 439–444.
Jacobs, D. E., Zhu, X. Q., Gasser, R. B., & Chilton, N. B. (1997). PCR-based methods for identification of potentially zoonotic ascaridoid parasites of the dog, fox and cat. Acta Tropica, 68, 191–200.
Li, A., D’Amelio, S., Paggi, L., He, F., Gasser, R. B., Lun, Z. R., Abollo, E., Turchetto, M., & Zhu, X. Q. (2005). Genetic evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) and the validity of Contracaecum septentrionale (Kreis, 1955) (Nematoda: Anisakidae). Parasitology Research, 96, 361–366.
Löytynoja, A., & Goldman, N. (2005). An algorithm for progressive multiple alignment of sequences with insertions. Proceedings of the National Academy of Sciences of the United States of America, 102, 10557–10562.
Mattiucci, S., Paoletti, M., Olivero-Verbel, J., Baldiris, R., Arroyo-Salgado, B., Garbin, L., Navone, G., & Nascetti, G. (2008). Contracaecum bioccai n. sp. from the brown pelican Pelecanus occidentalis (L.) in Colombia (Nematoda: Anisakidae): morphology, molecular evidence and its genetic relationship with congeners from fish-eating birds. Systematic Parasitology, 69, 101–121.
Mattiucci, S., Paoletti, M., Solorzano, A. C., & Nascetti, G. (2010). Contracaecum gibsoni n. sp. and C. overstreeti n. sp. (Nematoda: Anisakidae) from the Dalmatian pelican Pelecanus crispus (L.) in Greek waters: genetic and morphological evidence. Systematic Parasitology, 75, 207–224.
Mozgovoi, A. A. (1953). [Principles of nematodology II. Ascaridata of animals and man and the diseases caused by them. Part II.]. Moscow: Izdatel’stvo Akademii Nauk SSSR, 616 pp. (in Russian).
Nadler, S. A., D’Amelio, S., Fagerholm, H.-P., Berland, B., & Paggi, L. (2000). Phylogenetic relationships among species of Contracaecum Railliet & Henry, 1912 and Phocascaris Host, 1932 (Nematoda: Ascaridoidea) based on nuclear rDNA sequence data. Parasitology, 121, 455–463.
Nadler, S. A., & De Leon, G. P. (2011). Integrating molecular and morphological approaches for characterizing parasite cryptic species: implications for parasitology. Parasitology, 1, 1–22.
Nadler, S. A., & Hudspeth, D. S. S. (2000). Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. Journal of Parasitology, 86, 380–393.
Posada, D. (2009). Selection of models of DNA evolution with jModelTest. Methods in Molecular Biology, 537, 93–112.
Shamsi, S., Gasser, R., Beveridge, I., & Alizadeh Shabani, A. (2008). Contracaecum pyripapillatum n. sp. (Nematoda: Anisakidae) and a description of C. multipapillatum (von Drasche, 1882) from the Australian pelican, Pelecanus conspicillatus. Parasitology Research, 103, 1031–1039.
Shamsi, S., Norman, R., Gasser, R., & Beveridge, I. (2009). Genetic and morphological evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) (Nematoda: Anisakidae) in Australia. Parasitology Research, 105, 529–538.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Vincze, T., Posfai, J., & Roberts, R. J. (2003). NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acids Research, 31, 3688–3691.
Zhu, X. Q., Chilton, N. B., Jacobs, D. E., Boes, J., & Gasser, R. B. (1999). Characterisation of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. International Journal for Parasitology, 29, 469–478.
Zhu, X. Q., D’Amelio, S., Gasser, R. B., Yang, T. B., Paggi, L., He, F., Lin, R. Q., Song, H. Q., Ai, L., & Li, A. X. (2007). Practical PCR tools for the delineation of Contracaecum rudolphii A and Contracaecum rudolphii B (Ascaridoidea: Anisakidae) using genetic markers in nuclear ribosomal DNA. Molecular and Cellular Probes, 21, 97–102.
Zhu, X. Q., D’Amelio, S., Hu, M., Paggi, L., & Gasser, R. B. (2001). Electrophoretic detection of population variation within Contracaecum ogmorhini (Nematoda: Ascaridoidea: Anisakidae). Electrophoresis, 22, 1930–1934.
Zhu, X. Q., D’Amelio, S., Paggi, L., & Gasser, R. B. (2000a). Assessing sequence variation in the internal transcribed spacers of ribosomal DNA within and among members of the Contracaecum osculatum complex (Nematoda: Ascaridoidea: Anisakidae). Parasitology Research, 86, 667–683.
Zhu, X. Q., D’Amelio, S., Palm, H. W., Paggi, L., George-Nascimento, M., & Gasser, R. B. (2002). SSCP-based identification of members within the Pseudoterranova decipiens complex (Nematoda: Ascaridoidea: Anisakidae) using genetic markers in the internal transcribed spacers of ribosomal DNA. Parasitology, 24, 615–623.
Zhu, X. Q., Gasser, R. B., Jacobs, D. E., Hung, G. C., & Chilton, N. B. (2000b). Relationships among some ascaridoid nematodes based on ribosomal DNA sequence data. Parasitology Research, 86, 738–744.
Zhu, X. Q., Gasser, R. B., Podolska, M., & Chilton, N. B. (1998a). Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. International Journal for Parasitology, 28, 1911–1921.
Zhu, X. Q., Jacobs, D. E., Chilton, N. B., Sani, R. A., Cheng, N. A., & Gasser, R. B. (1998b). Molecular characterization of a Toxocara variant from cats in Kuala Lumpur, Malaysia. Parasitology, 117, 155–164.
Acknowledgement
This paper is dedicated to the memory of one of the authors, a dear friend and colleague, Nélio B. Barros, whose essential contribution made this study possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Amelio, S., Cavallero, S., Dronen, N.O. et al. Two new species of Contracaecum Railliet & Henry, 1912 (Nematoda: Anisakidae), C. fagerholmi n. sp. and C. rudolphii F from the brown pelican Pelecanus occidentalis in the northern Gulf of Mexico. Syst Parasitol 81, 1–16 (2012). https://doi.org/10.1007/s11230-011-9323-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-011-9323-x