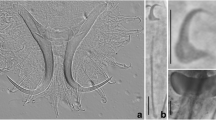
Abstract
Monogeneans identified as Sinodiplectanotrema malayanum n. sp. were collected from the fish Pennahia anea (Sciaenidae) off the west coast of Peninsular Malaysia. The new species is recognised on the basis of morphometrical differences in the anchors, marginal hooks and eggs and apparent differences in the 28S rDNA sequence data. The new species possesses features (ovary looping the intestinal caecum, body spines, a vagina and haptoral reservoirs) not noted in the original description of the type and only other species of the genus, S. argyrosomus Zhang, 2001, necessitating the re-assignment of the genus to the Diplectanidae Monticelli, 1903, a move which is supported by 28S rDNA evidence. Sinodiplectanotrema is redefined on the basis of the observation of several features not included in the original diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During 2008 and 2009 we had the opportunity to collect specimens of the greyfin croaker Pennahia anea (Bloch) [also known as Argyrosomus aneus (Bloch)] off Pulau Langkawi, Malaysia, in the Andaman Sea, and off Sungai Buloh, Malaysia, in the Straits of Malacca. The Sciaenidae is a large, diverse teleost family with 70 genera and 270 species (Froese & Pauly, 2010). A literature search for monogenean species on Pennahia spp. and on Argyrosomus spp. revealed records of Sinodiplectanotrema argyrosomus Zhang, 2001 on Pennahia anea [as Argyrosomus aneus] (type-host). S. argyrosomus can also be found on Johnius distinctus (Tanaka) [as Wak tingi (Tang)], the labrid Halchoeres nigrescens (Bloch & Schneider) and Nibea albiflora (Richardson) from off the coast of China (Zhang, 2001; Wu et al., 2006, 2007). There are some discrepancies in the publication dates and the spelling of the type-species in the original description of Sinodiplectanotrema. As first revisors (ICZN Article 24.2.2), we propose acceptance of Sinodiplectanotrema argyrosomus Zhang, 2001 as the valid name of the type-species, since the species is clearly named after the generic name of the host [the epithet argyrosomus was used first on page 164 of Zhang (2001)], and we reject the name argyromus as used on pages 164, 363 and 364. Furthermore, the descriptions for the species and genus were officially published in 2001 and not in 1999, as cited on page 363 of the latter work.
Monogeneans recovered from Pennahia anea resembled the description of Sinodiplectanotrema argyrosomus. However, a close examination of these specimens revealed not only differences between them and S. argyrosomus, which suggests that they represent a new species, but also important features, not reported in the original description of the latter species, that necessitate amendments to the generic diagnosis and the re-assignment of Sinodiplectanotrema Zhang, 2001 to a different monogenean family.
Materials and methods
Six specimens of Pennahia anea were collected in Malaysian coastal waters, one off Pulau Langkawi in the Andaman Sea and five off Sungai Buloh in the Straits of Malacca. Their monogeneans were collected and some were preserved in 70% ethanol for molecular studies, whereas others were prepared for morphological studies following Lim & Gibson (2008). Briefly, they were removed from the gills and flattened using coverslip pressure in order to best expose their soft anatomical structures and hard parts. Some were mounted in modified ammonium picrate glycerine (Lim & Gibson, 2008) and later made into unstained permanent mounts in Canada balsam. Other specimens were prepared for staining in Gomori’s triple stain (Humason, 1972). Both stained and unstained specimens were examined using both bright field and phase contrast optics using a Leica DMRB microscope. Images of the hard and soft body parts were captured using a Leica digital camera (3.3 MP) and QWin Plus image analysis software, and drawn on a digitising tablet (WACOM) using Adobe Illustrator software. Measurements of the sclerotised hard-parts (both haptoral and reproductive) were made on flattened stained or unstained specimens cleared in ammonium picrate glycerine using the measuring option in QWin software. The measurements are given as the mean and range (within parentheses) in micrometres, and the number of measurements (n) is given after the metric data in the description. For two-dimensional measurements, length is given before breadth. The marginal hooks are enumerated as in Gusev (1976).
DNA from three specimens preserved in 70% ethanol was extracted using a DNEasy extraction kit from Qiagen. For each specimen, 5 μl of extracted DNA were used as a template in PCR reactions to amplify the partial D1-D2 domain of the 28S rDNA using primers C1 (5′-ACCCGCTGAATTTAAGCAT-3′) and D2 (5′-TGGTCCGTGTTTCAAGAC-3′) (Mollaret et al., 2000). PCR reactions (50 μl) were performed in 1.5 mM MgCl2, PCR buffer (Fermentas), 200 μM of each deoxyribonucleotide triphosphate, 1.0 μM of each PCR primer and 1 U of Taq polymerase (Fermentas) in a thermocycler (Biometra) using the following conditions: an initial denaturation at 95°C for 4 min, followed by 35 cycles of 95°C for 1 min, 50°C for 1 min and 72°C for 1 min, followed by a final extension at 72°C for 10 min. Aliquots (10 μl) from the amplicons were examined in 1.3% agarose gels, stained with ethidium bromide and viewed under a UV illuminator. The remaining 40 μl of each amplicon were purified using a DNA purification kit (Qiagen) and subjected to automated DNA sequencing (ABI 3730 DNA Sequencer, First Base Laboratories) using the same primers as used for PCR amplification.
The 28S rDNA sequences of 26 species of diplectanids and two species of Dactylogyrus Diesing, 1850 (used as outgroups) from GenBank (Table 2) and the 28S rDNA sequences of the three specimens from the present study were edited and aligned with Clustal X (Thompson et al., 1997) using the default parameter and verified/edited visually using BioEdit ver. 7.0.5.3 (Hall, 1999). Phylogenetic trees were inferred using neighbour-joining (NJ), minimum evolution (ME) (Fig. 6) and maximum parsimony (MP) (Fig. 7) with MEGA ver. 4.0b (Kumar et al., 2004). The Kimura 2-parameter model was used to estimate distances for the NJ and ME analyses. The robustness of the inferred phylogeny was assessed using a bootstrap procedure with 1,000 replications for the MP, NJ and ME analyses.
The degree of similarities between the present three specimens of Sinodiplectanotrema and other diplectanids found in the same clade (see below; Figs. 6 and 7, i.e. S. argyrosomus (DQ157673), Sinodiplectanotrema sp. (EF100778), Murraytrema pricei (now M. bychowskyi) (DQ157672), Lobotrema sciaenae (EF100556), Diplectanum umbrinum (EF100560), D. blairense (also referred to as Paradiplectanum blairense) (AY553627) and D. sillagonum (AY553626) (also referred to as P. sillagonum) were calculated using BioEdit and the results tabulated (Table 3). (In Figs. 6 and 7, the species names are as given in GenBank and their current names are indicated in Table 2). Comments on the validity of diplectanid species in GenBank are restricted to those in the same clade as the present material.
Sinodiplectanotrema malayanum n. sp.
Type-host: Pennahia anea (Bloch) (Sciaenidae).
Type-locality: Andaman Sea, off Pulau Langkawi, Peninsular Malaysia (6°28′N; 99°47′E).
Other locality: Straits of Malacca, off Sungai Buloh, Malaysia (3°15′N; 101°17′E).
Site: Gills.
Specimens studied: 58 specimens studied from 6 hosts; 42 specimens measured.
Type-material: Holotype BM(NH) 2010.1.27.1 and 2 paratypes BM(NH) 2010.1.27.2–3 in the Natural History Museum, London; 55 paratypes MZUM(P)1250(P)-1304(P) in the University of Malaya Collection.
Description (Figs. 1–5; Table 1)
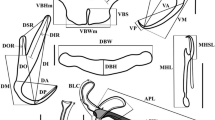
Body large, 1,481 (729–2,027) × 204 (124–320) (n = 42); peduncle long, 341 (177–610) × 135 (81–214) (n = 37), slightly tapered posteriorly, distinguishable anteriorly from body only by absence of vitelline follicles and presence of haptoral reservoirs; haptor comparatively small, 128 (61–222) × 219 (112–348) (n = 41), comprising 2 lateral digitate lobes; squamodiscs/lamellodiscs absent. Anterior region with 3 pairs of prominent head-organs and 4 pigmented eye-spots. Mouth closely posterior to eye-spots; pharynx round, 70 (44–96) × 71 (40–91) (n = 34); oesophagus short; intestine bifurcates just posterior to pharynx; caeca blind, pass posteriorly to end in middle of post-testicular field. Tegumental spines on posterior half of body, distributed sparsely at about testicular level then increasing in density more posteriorly to cover peduncle and entire haptor. Haptor without connecting bars, armed with 7 pairs of marginal hooks and 2 pairs of anchors. Marginal hook pairs 1–6 of similar length, 12 (10–13) (n = 41), located on 6 marginal projections; hooks 7 close to anchors, length 10 (9–11) (n = 38). Anchors with outer root modified as stiff rod with narrow connection (‘hinge’) linking it to main part of anchor, which allows it to flex; smaller ventral anchors have inner length 19 (16–21) (n = 38), inner root 6 (4–7) (n = 38), outer length 26 (20–30; variable due to hinged outer root) (n = 38), outer root 14 (10–16) (n = 38), point curved 12 (9–13) (n = 38); dorsal anchors with inner length 24 (22–26) (n = 35), outer length 39 (31–50; variable due to hinged outer root) (n = 35), inner root 6 (4–7) (n = 35), outer root 26 (17–30) (n = 35), point curved, 13 (8–15) (n = 35). Extrinsic muscles from body attach to each marginal hook. Four pyriform reservoirs in anterior peduncle, with long ducts which extend into haptor to open exteriorly at point of extrusion of anchors.
1. Sinodiplectanotrema malayanum n. sp. (composite dorsal view; tegumental spines omitted from haptor for clarity). 2. Hard-parts (a–e relate to upper scale-bar): a, dorsal anchor; b, ventral anchor; c1, marginal hook (1–6) from haptoral projections; c2, marginal hook (7) located near anchors; d, male copulatory organ; e, spines on body tegument; f, egg
Testis globular, immediately posterior to ovary. Vas deferens leaves antero-sinistral region of testis, loops left intestinal caecum to ventral side, ascends and coils anteriorly expanding slightly, narrows just before entering initial part of male copulatory organ. Male copulatory organ consists of simple, tapered copulatory tube, with proximal (initial) part situated anteriorly and narrow, bent, bill-shaped distal end directed posteriorly, length 84 (57–97) (n = 42), without accessory piece; single prostatic reservoir, with ducts from prostatic glands entering posteriorly; duct from prostatic reservoir opens into initial part of copulatory tube.
Ovary elongate, with narrower antero-distal region looping right intestinal caecum from dorsal to ventral side, then forms oviduct; oviduct receives duct from seminal receptacle and common vitelline duct, passes through indistinct Mehlis’ gland, then continues anteriorly and medially to form uterus; uterine pore opens close to distal extremity of copulatory tube. Single egg a rounded lozenge-shape, 69 (62–75) × 56 (51–65) (n = 5), with long filament, 72 (63–85) (n = 5); distal extremity of filament branched (Fig. 5), forming several thin sclerotised strands with pointed distal tips, which may be reflexed, loosely flexed or in tight bundle. Single vagina sinistral, at mid-body level, subspherical, prominent, muscular, opens via long, coiled duct into seminal receptacle. Vitellarium follicular; follicles extend from just posterior to pharynx to middle of post-testicular field, grouped into compact blocks, particularly in posterior third of body. Lateral vitelline ducts open into indistinct vitelline reservoir.
Differential diagnosis
The present material resembles Sinodiplectanotrema argyrosomus, as described by Zhang (2001), in the unusual shape of the anchors, which have outer roots with a narrow, flexible connection (hinge) with the main part of anchor, the shape of the posteriorly directed copulatory tube, the vitellarium with follicles distributed in compact blocks (particularly in the posterior third of the body), and the elongate shape of the body with a long peduncle and small, digitate haptor. In the original description of S. argyrosomus several features seen in the present material were not described; these are the vagina, the presence of tegumental spines (it is common for haptoral and body spines to be lost in dead specimens), the ovary looping the right intestinal caecum and the fact that the hooks of pair 7 are smaller than the others (i.e. 10 (9–11) and 12 (10–13) μm, respectively) and not on digitate projections.
The present specimens have spines covering the posterior half of the body (Fig. 1) and the haptor (Fig. 4), six pairs of marginal hooks on digitate lateral haptoral lobes and the distal region of the ovary and oviduct looping the right intestinal caecum from the dorsal to the ventral side. The latter is a diplectanid feature, suggesting that Sinodiplectanotrema is not an ancyrocephalid, as designated by Zhang (2001). In addition, body spines have not been reported for ancyrocephalids but are regularly found on diplectanids. Furthermore, the copulatory tube of this species has a typical diplectanid arrangement, with the proximal (initial) part of the copulatory tube situated anteriorly and its distal part directed posteriorly, as in species of Diplectanum Diesing, 1858, Lobotrema Tripathi, 1959 and Murraytrema Price, 1937 (see Oliver, 1987) but not apparently as in ancyrocephalid species (see Yamaguti, 1963; Bychowsky, 1957) (Table 2).
Unfortunately, we were unable to obtain the type-specimens of S. argyrosomus for comparison, despite several attempts, and are uncertain whether they are lost or misplaced. We are left only with the original description of Zhang (2001). However, the morphometric data for the anchors and marginal hooks of the present specimens do differ from those given in the latter description (cf. Table 1). The Malaysian specimens have eggs (mean 69 × 56 μm) with a longer filament (mean 72 μm), compared with 71 × 43 μm and a filament of 29 μm in the Chinese specimens. There is also a terminal branching of the filament (Fig. 5) which was not mentioned for S. argyrosomus. Marginal hooks present on projections of the haptor were noted in the original description of S. argyrosomus, but the present specimens show that only the six comparatively larger pairs are found actually on the projections; these hooks in the Malaysian material are also comparatively smaller (hooks 1–6 = 10–13 μm and hooks 7 = 9–11 μm vs hooks 1–7 = 13–15 μm in S. argyrosomus).
The present molecular analysis shows that the sequences for the three specimens from the present collection were identical (Table 3) and that they form a clade with GenBank data listed under S. argyrosomus, Sinodiplectanotrema sp. and Murraytrema pricei Bychowsky & Nagibina, 1977 nec Caballero, Bravo & Grocott, 1955 (now M. bychowskyi Oliver, 1987) (Figs. 6 and 7) (see below). Had all sequences been S. argyrosomus, then the 28S rDNA sequence of S. argyrosomus (DQ157673) and present three sequences should have been identical (100% similarity). However, the sequences of the present specimens are only 97% and 96% similar to S. argyrosomus and Sinodiplectanotrema sp., respectively (Table 3) (see below). Nevertheless, the actual identity of the Chinese sequences is questionable (see below).
Combined neighbour-joining (NJ) and minimum evolution (ME) tree for the Diplectanidae obtained using partial 28S rDNA sequences (D1–D2 domain), with Dactylogyrus spp. as outgroups. Bootstrap values shown along the branches are based on 1,000 replicates for the NJ and ME analysis. (See Table 2 for data on the material and GenBank accession numbers)
Maximum parsimony (MP) tree for the Diplectanidae obtained using partial 28S rDNA sequences (D1–D2 domain), with Dactylogyrus spp. as outgroups. Bootstrap values shown along the branches are based on 1,000 replicates for the MP analysis. (See Table 2 for data on the material and GenBank accession numbers)
When one considers the number of cryptic species and miniscule morphological differences between species in other groups (e.g. Gyrodactylus spp. in salmonids) and the presence of numerous congeners on the same host species (e.g. Dactylogyrus spp. in cyprinids) (see Shinn, Sommerville & Gibson, 1995; Gibson, Timofeeva & Gerasev, 1996), the lumping of the present material with S. argyrosomus could result in the loss of useful ecological and other data. Therefore, based on the morphometric (albeit small) and molecular (28SrDNA) differences outlined above, and the geographical distance between the records (Indian Ocean vs Chinese Pacific waters), the present species is considered as new to science (rather than as conspecific with S. argyrosomus) and named Sinodiplectanotrema malayanum n. sp.
Discussion
This is the second species of Sinodiplectanotrema to be described from Pennahia anea. Despite not being able to re-examine the type-specimens and in the absence of details of the following characters (vagina, tegumental spines, haptoral reservoirs, ovary looping the right intestinal caecum) in both the original description of the type-species, S. argyrosomus, and in the generic diagnosis, the present specimens are still considered to belong to Sinodiplectanotrema in view of the lack of squamodiscs/lamellodiscs and connecting bars, the unique anchors with ‘hinged’ outer roots, the shape of the posteriorly directed copulatory tube, the vitellarium with follicles distributed in compact blocks and the elongate shape of the body with a long peduncle and small, digitate haptor.
Taxonomic position of Sinodiplectanotrema Zhang, 2001
Sinodiplectanotrema was assigned to the Ancyrocephalidae Bychowsky, 1937 by Zhang (2001). However, Wu et al. (2006) listed it with diplectanids without comment in their molecular studies of Haliotrema spp. and later (2007) transferred it to the Diplectanidae Monticelli, 1903 based solely on molecular evidence. Nevertheless, the genus was not included in the revision of the Diplectanidae given by Domingues & Boeger (2008). As indicated above, the present specimens of Sinodiplectanotrema have features, such as the anterior part of the ovary looping the right intestinal caecum, the posteriorly directed tapering copulatory tube lacking an accessory piece and the presence of spines on the posterior half of the body, all of which are consistent with this genus belonging to the Diplectanidae.
As part of the current study, we analysed the 28S rDNA sequences for three of our specimens of S. malayanum n. sp. and compared them with sequences for other diplectanids available in GenBank, paying particular attention to material of Wu et al. (2006, 2007) listed as S. argyrosomus (DQ157673), Sinodiplectanotrema sp. (EF100778) and Murraytrema pricei [now M. bychowskyi] (DQ157672). The latter three sequences and those from the new species form a sister group with a high bootstrap value (100%) within a clade which also includes Lobotrema sciaenae (Bychowsky & Nagibina, 1977), Diplectanum umbrinum Tripathi, 1955, D. blairense Gupta & Khanna, 1974 (also referred to as Paradiplectanum blairense) and D. sillagonum Tripathi, 1957 (also referred to as P. sillagonum) (Figs. 6 and 7).
Our morphological and molecular data confirm that Sinodiplectanotrema, as represented by the present species, is a diplectanid and support the molecular finding of Wu et al. (2007) that Sinodiplectanotrema is a diplectanid genus. However, in the Chinese study, the molecular data were from Sinodiplectanotrema sp. and S. argyrosomus apparently obtained from Nibea albiflora [in fact sequence DQ157673 is listed (erroneously) as being from both Argyrosomus aneus and Nibea albiflora by Wu et al. (2006) but from N. albiflora only by Wu et al. (2007)] and not from the type-host, Pennahia anea (=Argyrosomus aneus). The 28S rDNA sequences of Sinodiplectanotrema sp. (EF100778) and M. pricei (=M. bychowskyi) (DQ157672), the latter being the usual diplectanid recorded from N. albiflora, are identical, indicating that they are the same species. Hence, the actual identity of DQ157672, listed as M. pricei (=M. bychowskyi), is uncertain and could be Sinodiplectanotrema sp. based on their 28SrDNA (Table 3) or vice versa. However, the close similarity of the conserved 28SrDNA of S. argyrosomus and Sinodiplectanotrema sp. with the present three sequences of S. malayanum n. sp. suggests that the former two species are Sinodiplectanotrema spp. and that the sequence DQ157672 is that of Sinodiplectanotrema sp. It is therefore possible that the host species might have been misidentified, as specimens of Sinodiplectanotrema have not, previous to the work of Wu et al. (2006, 2007), been reported from Nibea spp. There is, therefore, a need to re-examine material from Chinese N. albiflora.
Morphologically, the present specimens of Sinodiplectanotrema are similar to members of the Murraytrematoidinae Oliver, 1982, i.e. Murraytrema Price, 1937, Lobotrema Tripathi, 1959 and Murraytrematoides Yamaguti, 1958, in lacking squamodiscs and lamellodiscs (Oliver, 1987). The anchors of species of Murraytrema, Lobotrema and Murraytrematoides have long outer roots which resemble the rod-like outer roots but lack the narrow, hinge-like connections with the main part of the anchors of S. argyrosomus and the present specimens of S. malayanum. The male copulatory organ of both species of Sinodiplectanotrema is also similar to those of species of Lobotrema and Murraytrematoides (see Oliver, 1987). Current members of the Murraytrematoidinae have either one or two connecting bars, so, as Sinodiplectanotrema has no bars, the diagnosis of the Murraytrematoidinae would need to be amended to accommodate this genus. However, the Murraytrematoidinae was considered to be paraphyletic by Desdevises et al. (2001) and was synonymised with the Diplectaninae Monticelli, 1903 by Domingues & Boeger (2008). The absence of squamodiscs in species of the murraytrematoidine genera and Sinodiplectanotrema could represent a plesiomorphic state for the Diplectanidae and the Murraytrematoidinae could be valid. An alternative, unjustified at present, would be to erect a new subfamily for Sinodiplectanotrema. Herein, however, we confirm the assignment of Sinodiplectanotrema to the Diplectanidae and refrain from a discussion on its subfamilial status until more information on the morphology and molecular biology of its constituent species become available. In order to accommodate Sinodiplectanotrema, the familial diagnosis of Diplectanidae, as given in Domingues & Boeger (2008), needs to be amended to read: Haptor with 3 bars (1 midventral, 2 laterodorsal), 2 bars (Lobotrema), 4 bars (Diplectanocotyla) or 0 bars (Sinodiplectanotrema).
The diagnosis of Sinodiplectanotrema is amended here to include features not given in the original generic diagnosis, such as the anterior part of the ovary looping the right intestinal caecum (a diplectanid character) and the presence of a vagina, haptoral reservoirs and spines on the posterior half of the body.
Sinodiplectanotrema Zhang, 2001 (amend.)
Diagnosis: Diplectanidae. Body elongate, 4 eye-spots and 3 pairs of head organs; alimentary system bifurcate with blind caeca; peduncle long; haptor small, set off from body, wider than long, with 12 digitate projections on lateral lobes; squamodiscs/lamellodiscs absent. Tegument armed with spines on posterior half of body and haptor. Haptor armed with 14 marginal hooks, 6 pairs located on digitate projections, 1 pair near anchors may be smaller; 4 anchors, with outer root modified as stiff rod attached by flexible hinge; dorsal anchors with longer outer root than ventral anchors; no connecting bars. Four pyriform reservoirs in anterior peduncle, with long ducts which extend into haptor and appear to open close to aperture of each anchor. Testis globular, post-ovarian; vas deferens arises from antero-sinistral side of testis, loops around left intestinal caecum to ventral side, coils and opens into male copulatory organ. Male copulatory organ inverted, lacks accessory piece; tapered copulatory tube with narrow distal end directed posteriorly; single prostatic reservoir, with prostatic glands entering posteriorly and prostatic duct opening into initial part of male copulatory organ. Ovary elongate, with anterior region and oviduct looping right caecum from dorsal to ventral side; oviduct continues antero-ventrally to form uterus; vagina sinistral, muscular, opens via thin tube into seminal receptacle; egg rounded lozenge-shaped, with filament which may terminate in distal modification. Vitellarium follicular; follicles grouped, especially posteriorly, into compact blocks, extend from just posterior to pharynx to middle of post-testicular field. On gills of sciaenid fishes.
Type-species: S. argyrosomus Zhang, 2001.
Other species: S. malayanum n. sp.
References
Bychowsky, B. E. (1957). Monogenetic trematodes - their systematics and phylogeny. Moscow-Leningrad: Academy of Sciences, 509 pp. (In Russian; English translation, 1961, Washington: American Institute of Biological Sciences, 626 pp.).
Desdevises, Y., Morand, S., & Oliver, G. (2001). Linking specialization to diversification in the Diplectanidae Bychowsky 1957 (Monogenea, Platyhelminthes). Parasitology Research, 87, 223–230.
Domingues, M. V., & Boeger, W. A. (2008). Phylogeny and revision of Diplectanidae Monticelli, 1903 (Platyhelminthes: Monogenoidea). Zootaxa, 1698, 1–40.
Froese, R., & Pauly, D. (Eds.) (2010). FishBase. World Wide Web electronic publication. www.fishbase.org, version (01/2010).
Gibson, D. I., Timofeeva, T. A., & Gerasev, P. A. (1996). A catalogue of the nominal species of the monogenean genus Dactylogyrus Diesing, 1850 and their host genera. Systematic Parasitology, 35, 3–46.
Gusev, A. V. (1976). Freshwater Indian Monogenoidea. Principles of systematics, analysis of the world faunas and their evolution. Indian Journal of Helminthology, 25 & 26 (1973 & 1974), 1–241.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Humason, G. L. (1972). Animal tissue techniques. San Francisco: W.H. Freeman and Company, 641 pp.
Kumar, S., Tamura, K., & Nei, M. (2004). MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings in Bioinformatics, 5, 150–163.
Lim, L. H. S., & Gibson, D. I. (2008). Species of Triacanthinella Bychowsky & Nagibina, 1968 (Monogenea: Ancyrocephalidae) from triacanthid teleosts off Peninsular Malaysia, with a generic revision, amended diagnosis and key. Systematic Parasitology, 70, 191–213.
Mollaret, I., Lim, L. H. S., & Justine, J.-L. (2000). Phylogenetic position of the monogeneans Sundanonchus, Thaparocleidus, and Cichlidogyrus inferred from 28S rDNA sequences. International Journal for Parasitology, 30, 659–662.
Oliver, G. (1987). Les Diplectanidae Bychowsky, 1957 (Monogenea, Monopisthocotylea, Dactylogyridea). Systématique. Biologie. Ontogénie. Ecologie. Essai de phylogénèse. Thése de Doctorat d’État: Academie de Montpellier, Université des Sciences et Techniques du Languedoc, 433 pp.
Shinn, A. P., Sommerville, C., & Gibson, D. I. (1995). Distribution and characterization of species of Gyrodactylus Nordmann, 1832 (Monogenea) parasitizing salmonids in the UK and their discrimination from G. salaris Malmberg, 1957. Journal of Natural History, 29, 1387–1402.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The Clustal_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 24, 4876–4882.
Wu, X.-Y., Xie, M.-Q., & Li, A.-X. (2007). A preliminary phylogenetic analysis of the Diplectanidae inferred from the C1–D2 Domains of the 28S rDNA sequences. Acta Zootaxonomica Sinica, 32, 593–598. (In Chinese).
Wu, X.-Y., Zhu, X.-Q., Xie, M.-Q., & Li, A.-X. (2006). The radiation of Haliotrema (Monogenea: Dactylogyridae: Ancyrocephalinae): Molecular evidence and explanation inferred from LSU rDNA sequences. Parasitology, 132, 659–668.
Yamaguti, S. (1963). Systema helminthum. Vol. IV. Monogenea and Aspidocotylea. New York: Interscience Publishers, 699 pp.
Zhang, J.-Y. (2001). [Family Ancyrocephalidae Bychowsky & Nagibina, 1978.] In: Zhang, J.-Y., Yang, T.-B., Liu, L., et al. Monogeneans of Chinese marine fishes. Beijing: Agriculture Press, pp. 79–175. (In Chinese, with English descriptions of new taxa).
Acknowledgements
The authors would like to thank Mr KS Liew (UM) for his help in collecting and staining the specimens and Dr Tim Littlewood (NHM) for useful comments. We are also grateful to Prof. Jean-Lou Justine (IRD, Noumea) for the use of his swatches. This study was funded by E-Science Research grant from the Government of Malaysia to the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, L.H.S., Tan, W.B. & Gibson, D.I. Description of Sinodiplectanotrema malayanum n. sp. (Monogenea: Diplectanidae), with comments on the taxonomic position of the genus. Syst Parasitol 76, 145–157 (2010). https://doi.org/10.1007/s11230-010-9242-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-010-9242-2