Abstract
Two species of Pseudodiscocotyla Yamaguti, 1965 (Monogenea: Discocotylidae) were collected from crimson jobfish Pristipomoides filamentosus (Valenciennes) (Perciformes: Lutjanidae) off Okinawa-jima island, southern Japan. Pseudodiscocotyla opakapaka is redescribed and represents the first Japanese record. A new species, Pseudodiscocotyla mikiae n. sp., differs from Ps. opakapaka in the absence of spines around the male genital pore, the shape of the vaginal pore, the presence of spines inside the vaginal pore, and the shape of the clamp. The locations of the male genital atrium and the vaginal pore in both species were similar, and the observed armament differences of the male copulatory organ are therefore presumed to establish reproductive isolation. The phylogenetic trees for the Mazocraeidea based on the partial 28S rDNA sequences were created using new sequences of Pseudodiscocotyla mikiae n. sp., and Discocotylidae formed a sister group with the species Diclidophoridae, Macrovalvitrematidae, and Plectanocotylidae. Pristipomoides filamentosus is widely distributed across the Indo-Pacific, and Pseudodiscocotyla mikiae n. sp. could share the distribution of the host.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yamaguti (1965) established a monotypic genus for Pseudodiscocotyla opakapaka Yamaguti, 1965 (Monogenea: Discocotylidae) on the basis of specimens found on the gills of crimson jobfish Pristipomoides filamentosus (Valenciennes) (as Pristipomoides microlepis) (Perciformes: Lutjanidae) and Aphareus rutilans Cuvier in Hawaii, USA. Pseudodiscocotyla is distinguished from the other genera belonging to Discocotylidae by the following characters: the intestinal caeca do not unite, the shape of the male copulatory organ is elliptical and bulbus, and the genital atrium is armed (Yamaguti, 1965, 1968).
Pristipomoides filamentosus is a deep-water etelinae snapper inhabiting the tropical and subtropical Indo-Pacific, ranging from Southern Japan to the South China Sea (Nakabo, 2013) and is very important for commercial fishes in Japan and Hawaii (Taki, 2000). Three species of monogeneans have been reported in Pr. filamentosus from Hawaii: Pseudallobenedenia opakapaka Yamaguti, 1966 (Monopisthocotylea: Capsalidae), Oliveriplectanum opakapaka (Yamaguti, 1968) (Monopisthocotylea: Diplectanidae) (as Diplectanum opakapaka), and Pseudodiscocotyla opakapaka (Yamaguti, 1965, 1968).
During a survey of monogeneans from marine fishes around Okinawa-jima island, specimens of Pseudodiscocotyla were collected from Pr. filamentosus off the coasts of Okinawa City and Yomitan Town on Okinawa-jima island, southern Japan. We present Ps. opakapaka along with its redescription as the first record of this monogenean in Japan and describe a new Pseudodiscocotyla species.
Materials and methods
One specimen of Pr. filamentosus was purchased at the Awase Fish Market in Okinawa City on 21 December 2018 and another at the Yomitan Fishery Port in Yomitan Town on 23 May 2019. The former fish was captured off Okinawa-jima island, and the latter one was captured using a set-net off Yomitan Town (26°21′51.4″N, 127°43′09.2″E). The fish were transported on ice to the laboratory of Ryukyu University Museum (Fujukan), identified following Nakabo (2013) and examined for parasites. Fifteen monogeneans were removed from the gills using forceps under a dissecting microscope (Olympus SZ), then flattened under coverslip pressure and fixed in 99% ethanol or acetic acid–formalin–alcohol (AFA). For molecular analysis, the bodies of two ethanol-fixed specimens were used: a small piece was cut from the right lateral side of the main body using a razor, and was preserved in 99% ethanol. Thirteen specimens fixed in AFA and one specimen fixed in 99% ethanol (the hologenophore) were stained with Heidenhain’s iron hematoxylin. The remaining haptor of the ethanol-fixed specimen (the hologenophore) was not stained. Then, the specimens were dehydrated in an ethanol series, cleared in xylene, and mounted in Canada balsam. Drawings were made with the aid of a drawing tube fitted on an Olympus BX50 compound microscope. Measurements are in micrometers. The following voucher specimens from the parasite collection at the National Museum of Natural History (USNM) and Meguro Parasitological Museum (MPM) were examined for comparative purposes: the holotype of Pseudodiscocotyla opakapaka (USNM 1359220) and the paratypes of Ps. opakapaka (15550, 15551) described by Yamaguti (1965). Voucher specimens were deposited in the collection of the Meguro Parasitological Museum (21967–21969), Tokyo, Japan.
DNA was extracted from the isolated body of two specimens using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) and sequencing protocols followed Kamio & Nitta (2022). The edited sequences were submitted to the DNA Data Bank of Japan (DDBJ). The partial fragments of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1) region were compared with the available sequences for related species in the International Nucleotide Sequence Databases (INSD) using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) software on 14 March 2022.
For phylogenetic analysis, the 28S rDNA region was used. Sequences obtained for the 28S rDNA region in the present study were aligned with 39 other monogenean sequences retrieved from INSD (Table 1). The sequences of the 28S rDNA were aligned using MAFFT version 7 (Katoh et al., 2019) using the “unalignlevel: 0.0” and “Try to align gappy regions anyway” options under the G-INS-i strategy. Ambiguous sites in the aligned datasets were removed with Gblocks ver. 0.91b (Castresana, 2000) using the “Allow gap positions within the final blocks” option. Nucleotide substitution models were analyzed for each molecular marker based on the Bayesian information criterion using IQ-TREE version 2.0.4. (Kalyaanamoorthy et al., 2017; Minh et al., 2020). The maximum likelihood (ML) phylogeny was constructed under the GTR+F+I+G4 model using IQ-TREE version 2.0.4 with 1,000 bootstrap (BS) repeats. Bayesian inference (BI) and Bayesian posterior probabilities (PP) were estimated using MrBayes 3.2.6 (Ronquist et al., 2012) under the GTR+G+I model. Two independent runs of four Markov chains were conducted for 1,000,000 generations and the tree was sampled every 100 generations. Parameter estimates and convergence were checked using Tracer v. 1.6.0 (Rambaut & Drummond, 2013); the first 25,000 samples from each run were discarded as burn-in and the remaining were analyzed.
Family Discocotylidae Price, 1936
Subfamily Pseudodiscotylinae Yamaguti, 1965
Genus Pseudodiscocotyla Yamaguti, 1965
Pseudodiscocotyla opakapaka Yamaguti, 1965
Type-host: Pristipomoides filamentosus (Valenciennes) (Perciformes: Lutjanidae) (as Pristipomoides microlepis: see Yamaguti, 1965).
Other-host: Aphareus rutilans Cuvier (see Yamaguti, 1965).
Type-locality: Hawaii, USA (see Yamaguti, 1965).
Other-locality: East China Sea, off Yomitan, Okinawa City, Okinawa Prefecture, Japan (this study).
Newly collected material examined: 4 specimens from off Yomitan Town (21967).
Comparative museum material examined: Holotype and 13 paratypes (USNM 1359220) deposited in the Smithsonian US National Parasite Collection; 27 paratypes of Pseudodiscocotyla opakapaka (15550, 15551) deposited in the Meguro Parasitological Museum.
Site on host: Gills.
Redescription (Figs. 1, 2, 3, 4)
Pseudodiscocotyla opakapaka Yamaguti, 1965 from Pristipomoides filamentosus (Valenciennes) off Okinawa-jima island, Japan. Whole body (ventral view, 21967). Scale bar: 1 mm.
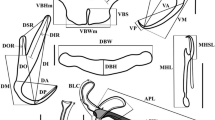
Pseudodiscocotyla opakapaka Yamaguti, 1965. A, Clamp (ventral view, 21967); B, Reproductive organs (ventral view, 21967); C, Male copulatory organ (21967); D, Vaginal pore (21967); E, Egg (21967). Scale bars: A, C, D, 50 μm; B, E, 100 μm.
Holotype (USNM 1359220) of Pseudodiscocotyla opakapaka Yamaguti, 1965 from Pristipomoides filamentosus (Valenciennes). Whole body (dorsal view). Scale bar: 1 mm.
Holotype (USNM 1359220) of Pseudodiscocotyla opakapaka Yamaguti, 1965. A, Clamp (dorsal view); B, Reproductive organs (dorsal view); C, Male copulatory organ; D, Vaginal pore; E, Egg. Scale bars: C, D, 50 μm; A, B, E, 100 μm.
Body elongated, total length 3,200–4,375 (3,875, n = 3), width at level of posterior end of germarium 375–500 (425, n = 3). Haptor ginkgo-leaf-shaped, symmetrical, with 4 pairs of clamps. Clamps (Fig. 2A) of equal structure, typically Discocotyle-type, each clamp 100–145 × 100–205 (118 × 159, n = 3). Clamp consisting of pair of antero-lateral sclerites and postero-lateral sclerites, ventral mid-sclerite and dorsal mid-sclerite. Antero-lateral sclerites and postero-lateral sclerites thin and long. Small sclerites from proximal end of postero-lateral sclerites toward inside. Ventral mid-sclerite long, bifurcated on top. Dorsal mid-sclerite short, bifurcated on top, armed with accessory skeletal piece at its distal end. Accessory skeletal piece small, V-shaped.
Mouth opening anterior terminal. Pair of buccal suckers elliptical, 50–70 × 40–110 (58 × 97, n = 3). Pharynx globular, lying on body midline immediately posterior to buccal suckers, 50–60 × 40–50 (53 × 43, n = 3). Oesophagus short without diverticula, bifurcating anterior or posterior to the male genital pore. Intestinal caeca with numerous lateral diverticula, extending into haptor, not united posteriorly.
Testes (Fig. 2B) with irregular shape, 99–127 (108, n = 3) in number, postgermarial, arranged in the posterior half of body and confined to intercrural field, divided into two lateral groups from posterior end of germarium to level of the shell gland. Vas deferens (Fig. 2B) long, conspicuous, coming from anterior testes on right side of body at level of oötype, ventral to germarium, running forward along body midline, entering base of male genital pore. Male copulatory organ (Fig. 2C), consisting of thick-walled duct and penis, length 99–127 (108, n = 3) from base to tip. Penis bearing numerous minute spines: lateral surface with numerous spines; distal end of penis with minute acicular spines and a pair of long and slender spines. Male genital pore ventral, opening anterior to bifurcating of oesophagus, with muscular rim, armed with two alternating rows of minute spines.
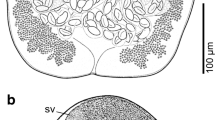
Germarium (Fig. 2B) pretesticular 180–200 × 240–260 (190 × 150, n = 2), inverted U-shaped. Oviduct (Fig. 1) arising from the posterior end on the right side of germarium and opening into genito-intestinal canal. Genito-intestinal canal (Fig. 2B) originating from right intestinal cecum, bifurcating into vitelline reservoir, ventral to testes. Oötype (Fig. 2B) extending from vitelline reservoir to uterus. Uterus (Fig. 2B) originating from oötype, running anteriorly along body midline, ventral to vas deferens, opening anterior to the male genital pore. Vaginal pore (Fig. 2D) elliptical, cuticle uneven, with many peaks and valleys, no spines, paired, parallel, located posterior to level of male copulatory organ, ventrolateral 55–67.5 × 20–35 (62.1 × 29.2, n = 3); length/width ratio 1.83–3.13 (2.19, n = 3). Vaginal duct (Fig. 2B) long, narrow, arising from vaginal pore, connecting vitelline reservoir, ventral to germarium, traveling anteriorly, parallel to the intestinal caeca on its ventral side. Eggs (Fig. 2E) fusiform, 150–175 × 60–70 (162.5 × 65, n = 2) excluding filaments, with filaments at anterior and posterior ends. Vitelline follicles (Fig. 1) coextensive with intestinal branches, extended from behind vaginal pore to haptor. Vitelline duct not observed.
Remarks
The holotype and paratypes are in good state but did not appear to be stained (Figs. 3, 4). The details of male copulatory organ could not be observed and number of minute acicular spines on penis could not be counted in all examined specimens. However, they possess several minute acicular spines and a pair of long spines on penis and male genital pore with a crown of two alternating rows of minute spines (Fig. 4C).
The newly collected specimens from Pr. filamentosus in Japanese waters show the diagnostic morphological characteristics of Pseudodiscocotyla provided by Yamaguti (1965) and agree approximately with the descriptions of Ps. opakapaka by Yamaguti (1965, 1968). We could distinguish differences between the newly collected specimens and the original description in clamp structure. Yamaguti (1965) indicated that the distal ends of both mid-sclerites bifurcated. In the newly collected specimens, the holotype and paratypes, an accessory skeletal piece represented by a projecting V-shaped process was observed on the distal end of the dorsal mid-sclerite that was not mentioned nor illustrated in the original description.
Some differences between the original description and our observation justify the need to emend the generic diagnosis: intestine bifurcation at anterior or posterior end of male copulatory organ, the arms of vitelline reservoir are not absolutely distended with sperm at anterior end.
Pseudodiscocotyla mikiae n. sp.
Type-host: Pristipomoides filamentosus (Valenciennes) (Perciformes: Lutjanidae).
Type-locality: East China Sea, off Awase, Okinawa City, Okinawa Prefecture, Japan.
Type-materials: Holotype (21968) and 10 paratypes (21969).
Site of host: Gills.
Representative DNA sequences: Partial 28S rDNA gene and partial cox1 gene sequences obtained from a paratype (21969) were submitted to the DNA Data Bank of Japan (DDBJ) under the accession numbers LC732579 and LC732580, respectively.
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Pseudodiscocotyla mikiae n. sp. is urn:lsid:zoobank.org:act: EC9D29B3-F0EC-429E-BDA6-774E82C24DEB.
Etymology: The species is named in condolence to Miki Haruna. She was a student of the first author and had conducted research on parasites with the authors during her school years, but died four years ago at a young age due to a sudden illness.
Description (Fig. 5)
Body elongated, total length 4,675–6,600 (5,807, n = 7), width at level of posterior end of germarium 750–1,300 (1,115, n = 8). Haptor ginkgo-leaf-shaped, symmetrical, with 4 pairs of clamps. Clamps (Fig. 6A) of equal structure, typically Discocotyle-type, each clamp 65–260 × 147.5–300 (157 × 228, n = 7). Clamp consisting of pair of antero-lateral sclerites and postero-lateral sclerites, ventral mid-sclerite and dorsal mid-sclerite. Antero-lateral sclerites and postero-lateral sclerites thin and long. Small sclerites from proximal end of postero-lateral sclerites toward inside. Ventral mid-sclerite long, bifurcated on top. Dorsal mid-sclerite short, spanner-shaped.
Mouth opening anterior terminal. Pair of buccal suckers elliptical 70–120 × 50–150 (100 × 133, n = 10). Pharynx globular, lying on body midline immediately posterior to buccal suckers, 70–90 × 50–70 (79 × 59, n = 10). Oesophagus short without diverticula, bifurcating anterior or posterior to the male genital pore. Intestinal caeca with numerous lateral diverticula, extending into haptor, not united posteriorly.
Testes (Fig. 6B) with irregular shape, 93–146 (127, n = 9) in number, postgermarial, arranged in the posterior half of body and confirmed to intercrural field, divided into two lateral groups from posterior end of germarium to level of the shell gland. Vas deferens (Fig. 6B) long, conspicuous, coming from anterior testes on right side of body at level of oötype, ventral to germarium, running forward along body midline, entering base of male genital pore. Male copulatory organ (Fig. 6C), consisting of thin-walled duct and penis, length 50–62.5 × 17.5–32.5 (54 × 25, n = 7) from base to tip. Penis bearing numerous slender spines. Male genital pore ventral, opening anterior to bifurcating of oesophagus, with muscular rim.
Germarium (Fig. 6B) pretesticular 150–350 × 230–570 (303 × 467, n = 7), beginning on right side of body, extending from right to left side, turned anterior to right side of body and then extended toward posterior. Oviduct (Fig. 6B) long, twisted, arising from distal end of germarium and opening into genito-intestinal canal. Genito-intestinal canal (Fig. 6B) originating from right intestinal cecum, bifurcating into vitelline reservoir, ventral to testes. Oötype (Fig. 6B) extending from vitelline reservoir to uterus. Uterus (Fig. 6B) originating from oötype, running anteriorly along body midline, ventral to vas deferens, opening anterior to the male genital pore. Vaginal pore (Fig. 6D) circular, with numerous small spines, paired, parallel, located posterior to level of male copulatory organ, ventral, lateral 25–37.5 × 22.5–32.5 (30.5 × 25.3, n = 10), surrounded by radiate short muscle fibers; length/width ratio 0.83–1.63 (1.23, n = 10). Vaginal duct (Fig. 6B) long, narrow, arising from vaginal pore, connecting vitelline reservoir, ventral to germarium, traveling anteriorly, parallel to the intestinal caeca on its ventral side. Eggs fusiform, 120–192.5 × 60–80 (156.3 × 70, n = 2) excluding filaments, with filaments at anterior and posterior ends.
Vitelline follicles coextensive with intestinal branches, extended from behind vaginal pore to haptor, fused posterior to testes. Vitelline duct not observed.
Remarks
Pseudodiscocotyla mikiae n. sp. shows the diagnostic morphological characteristics of the genus (see Yamaguti, 1965). Currently, only Pseudodiscocotyla opakapaka Yamaguti, 1965 is assigned to this genus (Yamaguti, 1965). This new species is distinguished from Ps. opakapaka by the following characters of the new species: (i) the absence of spines around the male genital pore, (ii) the circular vaginal pore (length/width ratio: 0.83–1.63 vs. 1.83–3.13), (iii) bearing numerous small spines inside, and (iv) the spanner-shaped dorsal mid-sclerite. Morphological measurements of Pseudodiscocotyla species are presented in Table 2.
Molecular data analysis
The result of the BLAST search for the sequences of cox1 gene of Ps. mikiae obtained in this study are shown in Table 3. The close hits were Microcotyle caudata Goto, 1894, Microcotyle sebastis Goto, 1894 (Microcotylidae), and Heterobothrium okamotoi Ogawa, 1991 (Diclidophoridae).
The trimmed multiple sequence alignment length of the 28S rDNA fragments consisted of 980 base pairs including gaps. The topologies of each constructed by ML and BI analysis were almost identical, and the phylogenetic trees based on BI analysis are shown in Fig. 7. The Mazocraeidea are divided into two major clades. The clade includes the Hexostomatidae, Allodiscocotylidae, Mazocreaeidae, Protomicrocotylidae, Chauhaneidae, Gastorocotylidae, Axinidae, Gotocotylidae and Thoracocotylidae. The other clade separates two groups, one consists of the Discocotylidae, Diclidophoridae, Macrovalvitrematidae, and Plectanocotylidae; and the other is comprised of the Heteraxinidae, Microcotylidae, Octomacridae, Heteromicrocotylidae, and Diplozoidae. Each of the families for which multiple species were used in the analysis constituted a monophyletic group, with the exception of Discocotylidae. Species of Discocotylidae was a paraphyletic group including Diclidophoridae and Macrovalvitrematidae in the ML analysis, but the BS value of each branch was relatively low (45–66), and those were shown as multibranching in the BI analysis.
Bayesian inference (BI) trees for the Mazocraeidea based on partial 28S rDNA (980 bp including gaps) data using two species of Hexabothriidae (Diclybothriidea) as the outgroups. The corresponding INSD accession numbers are shown. The tree includes results for ML and Bayesian inference with BS/PP branch support.
Discussion
The Pristipomoides filamentosus collected in the waters off Okinawa-jima island was found to be infected with two species of Pseudodiscocotyla: Ps. mikiae n. sp. and Ps. opakapaka. The host, Pr. filamentosus, is a slow growing and long-lived species and engages in mass spawning of buoyant eggs for five to seven months of the year (Andrews et al., 2011, Meagan et al., 2017, Haight et al., 1993, Leis & Lee, 1994). Additionally, the documented ability of some mature Pr. filamentosus to disperse 400 km across deep water channels indicates that adults can contribute to dispersal in this species, at least on an archipelagic scale (Kobayashi, 2008). Therefore, Pr. filamentosus is distributed widely in the Indo-Pacific (Nakabo, 2013) and Ps. opakapaka and Ps. mikiae n. sp. could share the distribution of the host. However, Ps. mikiae n. sp. was not found in previous studies in Hawaii (Yamaguti, 1965, 1968, Kent et al., 2005). However, after Yamaguti (1965, 1968), no detailed morphological examination of the newly collected specimens has been carried out (see Kent et al., 2005). In future studies the distribution rage of those Pseudodiscocotyla species will need to be examined.
The locations of the male genital atrium and the vaginal pore in both species were similar. However, the structure of the vagina (circular vaginal pore in Ps. mikiae vs. elliptical vaginal pore with corrugated cuticle in Ps. opakapaka) and male genital pore (no spines around male genital pore in Ps. mikiae vs. two alternating rows of spines around male genital pore in Ps. opakapaka) differed. The observed morphological differences are therefore presumed to establish reproductive isolation. Since the sequence of Ps. opakapaka was not obtained in the present study, we are not able to test the reproductive isolation. This would require detailed studies of distribution and molecular analysis for these two species.
Boeger & Kritsky (1993, 2001) indicated by morphological analysis that the Mazocraeidea is divided into five suborders (Mazocraeinea, Discocotylinea, Gastrocotylinea, Hexostomatinea, and Microcotylinea). The phylogenetic analysis based on the 28S rDNA did not support the previous phylogenetic tree proposed by Boeger & Kritsky (2001) (Olson & Littlewood 2002, this study). Olson & Littlewood (2002) presented a phylogenetic tree of the whole monogeneans based on the 28S rDNA sequences and supported the monophyly of suborders Gastrocotylinea and Discocotylinea. The present analysis is broadly in agreement except for the position of the Plectanocotylidae, but monophyly was not supported for all suborders. Olson & Littlewood (2002) suggested the 28S rDNA fragment had lost a great deal of resolving power as more taxa were included, and Hebert et al. (2003) reported the ability of cox1 gene to discriminate animal taxa. Available cox1 sequences of Mazocraeidea species are limited, and the accumulation of molecular studies is needed for the phylogenetic analysis and DNA barcoding of this order.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Acosta, A. A., & Smit, N. J. (2021). A first for Southern Africa: description of a new Heterobothrium (Monogenea: Diclidophoridae) parasitizing the evileye pufferfish Amblyrhynchotes honckenii (Tetraodontiformes: Tetraodontidae). Parasitology Research, 120, 819–830.
Aiken, H. M., Bott, N. J., Mladineo, I., Montero, F. E., Nowak, B. F., & Hayward, C. J. (2007). Molecular evidence for cosmopolitan distribution of platyhelminth parasites of tunas (Thunnus spp.). Fish and Fisheries, 8, 167–180.
Al-Nabati, E., Ali, S., Al-Quraishy, S., Alajmi, R., Al-Shaebi, E. M., Aljawdah, H. M. A., Dkhil, M. A., & Abdel-Gaber, R. (2021). Heteromicrocotyla polyorchis Unnithan, 1961 (Monogenea: Heteromicrocotylidae), a gill parasite of the yellow-spotted trevally, Carangoides fulvoguttatus (Carangidae) from Saudi Arabia: Morphology and phylogeny. Microbial Pathogenesis, 160, 105–165.
Andrews, A. H., Humphreys, R. L., DeMartini, E. E., Nichols, R. S., & Brodziak, J. (2011). Bomb radiocarbon and lead-radium dating of opakapaka (Pristipomoides filamentosus). U.S. Dept. of Commerce, NOAA Pacific Islands Fishery Science Center Administrative Report, H-11-07, 1–82.
Ayadi, Z. E. M., Tazerouti, F., Gastineau, R., & Justine, J.-L. (2022). Redescription, complete mitochondrial genome and phylogenetic relationships of Hexostoma thynni (Delaroche, 1811) Rafinesque, 1815 (Monogenea, Hexostomatidae). Parasite, 29, 29.
Badets, M., Whittington, I., Lalubin, F., Allienne, J.-F., Maspimby, J. L., Bentz, S., Du Preez, L. H., Barton, D., Hasegawa, H., Tandon, V., Imkongwapang, R., Ohler, A., Combes, C., & Verneau, O. (2011). Correlating early evolution of parasitic platyhelminths to Gondwana breakup. Systematic Biology, 60, 762–781.
Baghdadi, H. B., Al-Salem, A. A. M., Ibrahim, M. M., Younes, A. M., Aboelenin, S. M., & Bayoumy, E. M. (2022). Morphomolecular identification and considerations of the infestation site adaptations of Pricea multae (Thoracocotylidae: Priceinae) from Scomberomorus commerson, off Arabian Gulf, Saudi Arabia. Revista Brasileira de Parasitolgia Veterinária, 31, e007122.
Benovics, M., Koubková, B., Civáňová, K., Rahmouni, I., Čermáková, K., & Šimková, A. (2021). Diversity and phylogeny of Paradiplozoon species (Monogenea: Diplozoidae) parasitizing endemic cyprinoids in the peri-Mediterranean area, with a description of three new Paradiplozoon species. Parasitology Research, 120, 481–496.
Boeger, W. A., & Kritsky, D. C. (1993). Phylogeny and a revised classification of the Monogenoidea Bychowsky, 1937 (Platyhelminthes). Systematic Parasitology, 26, 1–32.
Boeger, W. A., & Kritsky, D.C. (2001). Phylogenetic relationships of the Monogenoidea. In: Littlewood, D. T. J. & Bray, R. A. (Eds.) Interrelationships of the Platyhelminthes. London: Taylor & Francis, pp. 92–102.
Camargo, A. C., & Santos, C. P. (2020). Morphological and molecular analyses of Pseudomazocraes sulamericana n. sp., Pseudomazocraes selene (Hargis, 1957), Cemocotyle carangis (MacCallum, 1913) and Zeuxapta seriolae (Meserve, 1938) (Monogenea: Mazocraeidea) from carangid fishes in the south-western Atlantic Ocean. Journal of Helminthology, 94, e28.
Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17, 540–552.
Haight, W. R., Kobayshi, D. R., & Kawamoto, K. E. (1993). Biology and management of deepwater snappers of the Hawaiian archipelago. Marine Fisheries Review, 55, 20–27.
Hebert, P. D. N., Ratnasingham, S., & deWaard, J. R. (2003). Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society, Section B: Biological Sciences, 270, S96–S99.
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K., Haeseler, A. V., & Jermiin, L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589.
Kamio, Y., & Nitta, M. (2022). New records of Gemmaecaputia corrugata (Monogenea: Chauhaneidae) from Sphyraena forsteri (Sphyraenidae) off Yomitan Town, Okinawa-jima island, southern Japan. Species Diversity, 27, 83–90.
Katoh, K., Rozewicki, J., & Yamada, K. D. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166.
Kent, M. L., Heidel, J. R., Marie, A., Moriwake, A., Moriwake, V., Alexander, B., Watral, V., & Kelley, C. D. (2005). Diseases of opakapaka Pristipomoides filamentosus. In: Walker, P., Lester, R., Bondad-Reantaso, M. G. (Eds.) Diseases in Asian Aquaculture V. Manila: Asian Fisheries Society, pp. 183–195.
Kobayashi, D. R. (2008). Spatial Connectivity of Pacific Insular Species: Insights from Modeling and Tagging. Ph.D. dissertation, University of Hawaii, 220 pp.
Leis, J. M., & Lee, K. (1994). Larval development in the lutjanid subfamily Etelinae (Pisces): the genera. Aphareus, Aprion, Etelis and Pristipomoides. Bulletin of Marine Science, 55, 46–125.
Li, R., Zhou, C., Ye, S., Men, L., Liu, Y., & Fu, S. (2019). Mitochondrial genome sequencing of the monogenean Heterobothrium okamotoi isolated from the tiger puffer Takifugu rubripes in North China. Mitochondrial DNA Part B, 4, 3051–3052.
Littlewood, D. T. J., Rohde, K., & Clough, K. A. (1997). Parasite speciation within or between host species? —Phylogenetic evidence from site-specific polystome monogeneans. International Journal for Parasitology, 27, 1289–1297.
Lockyer, A. E., Olson, P. D., & Littlewood, D. T. J. (2003). Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biological Journal of the Linnean Society, 78, 155–171.
Meagan, A. L., Edward, E. D., & Robert, L. H. Jr. (2017). Seasonality, sex ratio, spawning frequency and sexual maturity of the opakapaka Pristipomoides filamentosus (Perciformes: Lutjanidae) from the Main Hawaiian Islands: fundamental input to size-at-retention regulations. Marine and Freshwater Research, 69, 325–335.
Minh, B. Q., Schmidt, H. A., Chernomor, O., Schrempf, D., Woodhams, M. D., von Haeseler, A., & Lanfear, R. (2020). IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution, 37, 1530–1534.
Nakabo, T. (2013). Fishes of Japan with pictorial keys to the species (3rd ed.). Hadano: Tokai University Press, l+xxxii+xvi+2428 pp (In Japanese).
Nam, U.-H., Whang, I., Kim, J.-H. (2020). The complete mitochondrial genome sequence of Microcotyle caudata (Platyhelminthes: Monogenea) from dark-banded rockfish (Sebastes inermis) in Korea. Mitochondrial DNA Part B, 5, 1817–1819.
Nitta, M., Kondo, Y., Ohtsuka, S., Kamarudin, A.S., & Ismail, N. (2022). Kannaphallus leptosomus n. sp. (Monogenea: Heteraxinidae: Cemocotylinae) parasitizing Scyris indica (Carangiformes: Carangidae) from Malaysia. Systematic Parasitology, 99, 587–599.
Olson, P. D., & Littlewood, D. T. J. (2002). Phylogenetics of the Monogenea – evidence from a medley of molecules. International Journal for Parasitology, 32, 233–244.
Ono, N., Matsumoto, R., Nitta, M., & Kamio, Y. (2020). Taxonomic revision of Microcotyle caudata Goto, 1894 parasitic on gills of sebastids (Scorpaeniformes: Sebastidae), with a description of Microcotyle kasago n. sp. (Monogenea: Microcotylidae) from off Japan. Systematic Parasitology, 97, 501–516.
Rambaut, A., & Drummond, A. J. (2013). Tracer v 1.6. Available at http://tree.bio.ed.ac.uk/software/tracer/. Accessed 20 June 2017.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Su, C. (2009). Studies on Species Diversity, Histopathology and Molecular Phylogenetics of Fish Monogeneans in Xiamen Sea Area. Xiamen: Master Thesis, Xiamen University, IV+iv+85 pp (In Chinese).
Taki, Y. (2000). Fish. In: Shitanaka, N. (Ed.), The Encyclopedia of Fish and Seafood. Tokyo: Heibonsha, pp. 90–91 (In Japanese).
Tambireddy, N., Gayatri, T., Gireesh-Babu, P., & Pavan-Kumar, A. (2016). Molecular characterization and phylogeny of some mazocraeidean monogeneans from carangid fish. Acta Parasitologica, 61, 360–368.
Torres-Carrera, G., Ruiz-Escobar, F., García-Prieto, L., & Oceguera-Figueroa, A. (2020). Narcinecotyle longifilamentus n. gen., n. sp. (Monogenea: Hexabothriidae), gill parasite of the numbfish Narcine entemedor (Torpediniformes: Narcinidae) from the Mexican Pacific coast. Parasitology International, 76, 102095.
Verma, A. K., & Verma, J. (2021). Redescription and new host record of Heteraxinoides atlanticus (Monogenea: Heteraxinidae) from the gills of Nemipterus japonicus (Bloch) and its systematics. Agricultural Science Digest-A Research Journal, 42, 203–209.
Víllora-Montero, M., Pérez-Del-Olmo, A., Georgieva, S., Raga, J. A., & Montero, F. E. (2020). Considerations on the taxonomy and morphology of Microcotyle spp.: redescription of M. erythrini van Beneden & Hesse, 1863 (sensu stricto) (Monogenea: Microcotylidae) and the description of a new species from Dentex dentex (L.) (Teleostei: Sparidae). Parasites & Vectors, 13, 45.
Yamaguti, S. (1965). New monogenetic trematodes from Hawaiian fishes. I. Pacific Science, 19, 55–95.
Yamaguti, S. (1966). New monogenetic trematodes from Hawaiian fishes. II. Pacific Science, 20, 419–434.
Yamaguti, S. (1968). Monogenetic Trematodes of the Hawaiian Fishes. Honolulu: University of Hawaii Press, 287 pp.
Acknowledgments
We thank Dr. T. Sasaki (Ryukyu University Museum (Fujukan), University of Ryukyu) and Dr. T. Sato (Kobe University) for providing laboratory facilities. Dr. T. Kuramochi, Dr. T. Iwaki (Meguro Parasitological Museum), and Dr. A. J. Phillips (National Museum of Natural History) for helping us with examination of the type specimens. We give thanks to Dr. T. Nakano (Kyoto University) for borrowing the Holotype and providing laboratory facilities for us, Mr. N. Sawada (Kyoto University) and Ms. R. Horikawa (Hakuryo Junior and Senior High School) for supporting our study. Ms. J. Yukihiro (Hakuryo Junior and Senior High School) kindly improved the English of this paper.
Funding
This study was partially supported by a JSPS KAKENHI Grant (Nos. 20H00986 to YK and 18J00466 to MN).
Author information
Authors and Affiliations
Contributions
Kamio and Inoue wrote the main manuscript text and prepared figures 1-6 and Nitta performed a molecular phylogenetic analysis and prepared figures 7. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as 75847911-5ECF-43D7-9EA9-EF3300F5457D. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamio, Y., Inoue, A. & Nitta, M. Description of a new species, Pseudodiscocotyla mikiae n. sp. (Monogenea: Discocotylidae) parasitic on gills of Pristipomoides filamentosus from off Okinawa-jima island in Japan, with redescription of Pseudodiscocotyla opakapaka. Syst Parasitol 100, 657–671 (2023). https://doi.org/10.1007/s11230-023-10115-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-023-10115-2