Abstract
Neoskrjabinolepis (Neoskrjabinolepidoides) nuda n. sp. is described from the shrews Sorex unguiculatus (type-host), S. gracillimus, S. isodon and S. caecutiens on Sakhalin Island, Russia. The new species is characterised by: rostellar hooks 40–44 μm long and provided with small epiphyseal thickening of the handle; a long (95–100 μm) cirrus consisting of basal region with claw-shaped spines, a parabasal region with thin needle-shaped spines and an unarmed distal region; a cirrus-sac extending well into the median field; and 15–22 eggs per gravid uterus. A review of the species of Neoskrjabinolepis Spasskii, 1947 is presented. Currently, this genus includes nine species and is divided in two subgenera on the basis of strobilar development, which is gradual in the subgenus Neoskrjabinolepis (four species) and serial in the subgenus Neoskrjabinolepidoides Kornienko, Gulyaev & Mel’nikova, 2006 (five species). An amended generic diagnosis and an identification key to Neoskrjabinolepis spp. are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoskrjabinolepis Spasskii, 1947 includes parasites specific to shrews of the genus Sorex L. (Insectivora, Soricidae) (Spasskii, 1947, 1954; Gulyaev, 1991; Czaplinski & Vaucher, 1994). For many years, only two species were recognised, N. schaldybini Spasskii, 1947 and N. singularis (Cholodkowsky, 1912) Spasskii, 1954 (see Spasskii, 1954; Genov, 1984; Czaplinski & Vaucher, 1994). Sometimes, N. schaldybini was considered a junior synonym of N. singularis (see Kobuley, 1953; Zarnowski, 1955; Kisielewska, 1958; Prokopič, 1956, 1958; Pojmanska, 1957; Rybicka, 1959), but its validity was supported by Spasskii (1954), Vaucher (1971) and Genov (1984). In order to assess the validity of N. schaldybini and to examine the variability of the species of Neoskrjabinolepis, recent studies have been undertaken on cestodes belonging to this genus from Sorex spp. originating from various parts of the Palaearctic Region. These studies have resulted in redescriptions of the two known forms and the description of a further six species (Kornienko et al., 2006, 2007). These taxa were divided into two subgenera, Neoskrjabinolepis (Neoskrjabinolepis) Spasskii, 1947, with gradual strobilar development (type-species N. schaldybini), and Neoskrjabinolepis (Neoskrjabinolepidoides) Kornienko, Gulyaev & Mel’nikova, 2006, with strobila consisting of series of proglottides at the same developmental stage (type-species N. (Neoskrjabinolepidoides) singularis) (see Kornienko et al., 2006). Therefore, the current species diversity of Neoskrjabinolepis is much greater than was believed a few years ago. However, much taxonomic work remains in order to achieve a comprehensive alphataxonomy of Neoskrjabinolepis in view of the recently proposed criteria for distinguishing species (Kornienko et al., 2006, 2007).
Recent examinations of cestodes from shrews on Sakhalin Island revealed the occurrence of yet another new species of Neoskrjabinolepis. The aim of the present article is to describe this new species. We also provide a taxonomic review and an identification key to the species of this genus.
Materials and methods
In June, 2005, one of us (VDG) collected cestodes from shrews at three localities on Sakhalin Island. These were at Sokol Biological Station, the suburbs of the town of Poronaysk and the village of Ozerskiy. Four species of Sorex were sampled: S. unguiculatus Dobson (130 specimens), S. gracillimus Thomas (10 specimens), S. isodon Turov (5 specimens) and S. caecutiens Laxmann (16 specimens).
Host specimens were dissected immediately after their death. Cestodes were isolated, washed and relaxed in water, and then fixed in 70% ethanol. They were stained in Ehrlich’s haematoxylin, differentiated in a 3% aqueous solution of ferric ammonium sulphate 12-hydrate, dehydrated in an ethanol series, cleared in clove oil and mounted in Canada balsam. Some specimens were mounted in Berlese’s medium to facilitate the examination of the rostellar hooks and copulatory apparatus.
Type-specimens are deposited in the collections of the Natural History Museum, Geneva, Switzerland (MHNG) and the Zoological Museum at the Institute of Systematics and Ecology of Animals, Novosibirsk (ISEZH).
Measurements are given in micrometres except where otherwise stated. Metrical and meristic data are presented as the range followed by the mean, with the number of the measurements taken (n) in parentheses.
Neoskrjabinolepis (Neoskrjabinolepidoides) nuda n. sp.
Type-host: Sorex unguiculatus Dobson (Insectivora: Soricidae).
Other host: Sorex gracillimus Thomas, S. isodon Turov and S. caecutiens Laxmann.
Type-locality: Sokol Biological Station (SBS), Sakhalin Island, Russia.
Other localities: Village of Ozerskiy and the suburbs of the town of Poronaysk, Sakhalin Island, Russia.
Type-material: Holotype: MHNG INVE 49755, 23.06.2005. Paratypes: MHNG INVE 49756 (2 slides), S. unguiculatus, SBS, 28.06.2005; ISEZH No. 1641–1644, S. unguiculatus, SBS, 9–14.06.2005; ISEZH No. 1645, S. gracillimus, SBS, 16.06.2005; ISEZH No. 1646, S. caecutiens, Poronaysk, 08.07.2005; ISEZH No. 1647–1649, S. unguiculatus, SBS, 19–20.07.2005, 05.07.2005; ISEZH No. 1650–1652, S. unguiculatus, SBS, 01.06.2005, 21.06.2005, 08.09.2005; ISEZH No. 1653, S. unguiculatus, Ozerskiy, 9.07.2005; ISEZH No. 1655, S. isodon, SBS, 12.06.2005.
Prevalence and intensity: 33.8% and 1–32 (av. 7.5) in S. unguiculatus; 20.0% and 1 in S. gracillimus; 20.0% and 2 in S. isodon, 50.0% and 1–20 (av. 9.8) in S. caecutiens.
Etymology: The species name nuda (bare) refers to the lack of armament on the distal part of the cirrus, which is a unique character among its congeners.
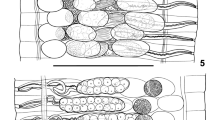
Description (Figs. 1–7)
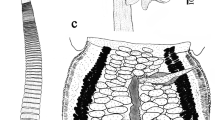
[Based on specimens from the type-host.] Pregravid specimens 5.0–14.0 mm (8.2 mm, n = 10) long, with maximum width at level of postmature proglottides, 280–350 (313, n = 7); strobila flat, consisting of 400–560 (470, n = 7) proglottides. Strobilation serial; pregravid or gravid strobila usually consisting of 4 series of proglottides, each containing proglottides at same developmental stage (first series of juvenile or premature proglottides; second section of hermaphroditic mature proglottides; third series of postmature proglottides; fourth series of pregravid or gravid proglottides); each series consists of c.100–140 proglottides. Strobilar portions containing juvenile, premature or mature proglottides without external segmentation; proglottides externally distinct at level of postmature part of strobila. Scolex 250–270 wide (260, n = 7), clearly wider than neck (Fig. 1). Suckers round, 90–100 × 88–91 (95 × 90, n = 7), with well-developed musculature. Rostellar apparatus complex. Rhynchus short, 60–65 long, 80–85 wide. Rostellum sac-like, 100–140 × 70–75 (121 × 72, n = 7); its walls consist of external layer of longitudinal muscular fibres and internal layer of circular muscular bundles. Rostellar hooks 10 in number, arranged in single row, 40–44 (42, n = 10) long, with characteristic pincers-like shape: axis of blade almost parallel to axis of guard; blade twice length of handle (Fig. 2); handle provided with small epiphyseal thickening. When rostellar apparatus retracted, rostellar hooks with blades directed anteriorly. Rostellar pouch voluminous, 170–230 × 140–180 (204 × 159, n = 5), reaches beyond level of posterior margins of suckers; its wall consists of longitudinal muscular fibres and circular muscular bundles. Neck 110–150 (130, n = 5) wide.
Proglottides acraspedote, transversely elongate. Mature proglottides 12–15 х 260–330 (14 × 296, n = 6) (Fig. 3), with length/width ratio 1:20–22; lateral fields 39–60 wide; gonads densely situated in median field. Postmature proglottides 17–26 × 280–350 (22 × 313, n = 7) (length of proglottides measured as distance between genital atria of adjacent proglottides). Pregravid proglottides 95–110 × 280–300 (102 × 290, n = 5), with length/width ratio c.1:3. Osmoregulatory canals 2 pairs, without transverse anastomoses; ventral canals 7–10 wide; dorsal canals 3–4 wide. Genital pores unilateral. Genital atrium simple, 2–5 deep, 3–6 in diameter.
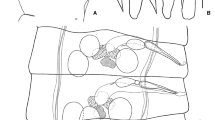
Testes 3, of almost equal size, 18–25 × 28–41 (20 × 35, n = 8), situated in one row; poral testis separated from two antiporal testes by external seminal vesicle (Fig. 3). Diameter of testes larger than proglottis length and, consequently, dense dorsal testicular field is formed in mature strobilar portion. Degenerating testes persist in postmature proglottides (Fig. 4). Cirrus-sac claviform, 110–120 × 9–10 (113 × 9.4, n = 5), slightly winding, passes deeply into median field but does not reach median line of proglottis (Fig. 7). Cirrus long, 95–100 (97, n = 5), whip-shaped; its basal region armed with small claw-shaped spines; parabasal region provided with sparsely distributed, thin, needle-shaped spines; distal region of cirrus unarmed (Fig. 6). Ductus ejaculatorius forms several coils. Internal seminal vesicle small, 20–25 × 7–9 (22 × 8, n = 5), occupies less than quarter of cirrus-sac length even when filled. External seminal vesicle 28–34 × 15–19 (30 × 17, n = 5), connected to cirrus-sac by narrow duct.
Vitellarium subspherical, 13–18 × 18–22 (15 × 20, n = 5), situated antiporally to ovary. Ovary transversely elongate, compact, 19–27 × 65–98 (23 × 83, n = 7), medial or may be in poral half of median field; in latter case, ovary overlaps cirrus-sac, external seminal vesicle and testes ventrally (Fig. 4); ovocytes large. Vagina thin-walled, passes ventally to cirrus-sac.
Uterus not extending into lateral fields of both postmature and gravid proglottides. Uterine wall thin, membranous during all morphogenesis of organ. Number of eggs in uterus 15–22 (Fig. 5). Ripe eggs 49–56 × 32–41; embryophore 29–32 × 17–20; oncosphere 22–26 × 14–15. Embryonic hooks: lateral pairs 10–11 long; medial pair 13–14 long.
Remarks
N. nuda n. sp. is characterised by a serial strobilar development. Therefore, it belongs to the subgenus Neoskrjabinolepis (Neoskrjabinolepidoides) Kornienko, Gulyaev & Mel’nikova, 2006. Currently, this subgenus includes N. singularis (Cholodkowsky, 1912), N. nadtochijae Kornienko, Gulyaev & Mel’nikova, 2006, N. corticirrosa Kornienko, Gulyaev & Mel’nikova, 2007 and N. kedrovensis Kornienko, Gulyaev & Mel’nikova, 2007. The new species can be distinguished from all of these on the basis of the characteristics of the rostellar hooks, the structure of the male copulatory apparatus, the armament and size of the cirrus, and the number of eggs per uterus.
N. nuda differs from N. singularis by having shorter rostellar hooks (40–44 versus 56–65 μm), the small epiphyseal thickening on the handle and the relatively long handle; in contrast, the rostellar hooks of N. singularis posses a short handle and a relatively large epiphyseal thickening (Fig. 13). The new species differs from N. nadtochijae by the shape of the blade and the small epiphyseal thickening (Fig. 16). Its rostellar hooks are intermediate in length between N. corticirrosa (48–53 μm) and N. kedrovensis (36–38 μm).
The new species differs from all the other species of this subgenus in the morphology of the male copulatory apparatus. Compared to N. corticirrosa and N. singularis, which have short cirrus-sacs (70–90 and 90–95 μm long, respectively) that only just cross the poral osmoregulatory canals, those of N. nuda are 100–130 μm long and extend deeply into the median field. In terms of the size of the cirrus-sac, N. nuda is similar to N. nadtochijae (160–180 μm in length) and N. kedrovensis (110–120 μm in length).
The species of this subgenus also differ from each other in their cirral armament (Figs. 8–12), and N. nuda is unique in lacking any armament throughout the entire distal region of the evaginated cirrus. Furthermore, the species of this subgenus have differences in the number of the eggs per gravid uterus: 15–22 in N. nudа; 10–20 in N. corticirrosа; 10–15 in N. kedrovensis; 34–43 in N. singularis; and 20–46 in N. nadtochijae.
On the basis of these differences, we recognise the specimens studied as a species new to science. In view of the results of this study, the genus is redefined below.
Neoskrjabinolepis Spasskii, 1947
Generic diagnosis [modified after Kornienko et al., 2006]. Cestodes of small body size, consisting of numerous acraspedote proglottides. Anterior portion of strobila (to level of postmature proglottides) without external segmentation. Both gradual and serial patterns of strobilar development occur. Mature proglottides considerably wider than long; gravid proglottides almost as long as wide or longer than wide. Scolex relatively large, provided with complex rostellar apparatus with invaginable rostellum. Rostellar hooks 10 in number, pincer-shaped, with epiphyseal thickening of handle. Male and female genital systems with simultaneous development. Testes three, arranged in transverse row situated dorsally to female gonads. Cirrus-sac short or long, from just crossing poral osmoregulatory canals to reaching median line of proglottis. Cirrus armed with spines, often of various shapes. Vitellarium compact, rounded, situated in antiporal half of median field. Ovary oval, transversely elongate, poral to vitellarium. Uterus sac-like, situated in median field during its entire development. Gravid proglottis with strong persisting walls, functioning as oöphore and enabling group dispersion of eggs. Parasites of shrews of genus Sorex (Insectivora: Soricidae) in Palaearctic Region. Type-species: N. schaldybini Spasskii, 1947.
Review of species
Subgenus Neoskrjabinolepis (Neoskrjabinolepis) Spaskii, 1947
Neoskrjabinolepis schaldybini Spasskii, 1947 (type-species)
Syn. Hymenolepis schaldybini (Spasskii, 1947) Vaucher, 1971.
Type-host and locality: “Sorex sp.”, vicinities of the town of Sudzhenka (currently Anzhero-Sudzhensk), Kemerovskaya Oblast’, West Siberia, Russia (Spasskii, 1947).
Other hosts: Sorex araneus (see Genov, 1984; Kornienko et al., 2006; Binkienė, 2006), S. minutus L. (see Kornienko et al., 2006, Genov, 1984; Binkienė, 2006), S. isodon (see Kornienko et al., 2006), S. caecutiens (see Kornienko et al., 2006), S. roboratus Hollister (see Kornienko et al., 2006).
Distribution: West Siberia (Spasskii, 1947; Kornienko et al., 2006), Zabaykalye (Eltyshev, 1975), Bulgaria (Genov, 1984), Lithuania (Binkienė, 2006), Switzerland (Vaucher, 1971), Moldova (Spasskii & Andreyko, 1970), Germany (Vaucher, 1971), United Kingdom (Vaucher, 1971), Finland (Vaucher, 1971; Haukisalmi, 1989), France (Jourdane, 1971; Vaucher, 1971), Netherlands (Vaucher, 1971), Belgium (Vaucher, 1971), Sweden (Vaucher, 1971), Norway (Vaucher, 1971), Poland (Vaucher, 1971), Czech Republic (Vaucher, 1971), Slovakia (Vaucher, 1971; Murai & Mészáros, 1984).
Remarks: Examinations of collections of cestodes of the genus Neoskrjabinolepis from West and East Siberia, Bulgaria and Lithuania revealed the presence of N. schaldybini. The records from the remaining above-mentioned countries are either accompanied with descriptions not corresponding to the current diagnostic characters of the species or no morphological data have been published. Therefore, the geographical distribution of N. schaldybini requires further study.
Sato et al. (1988) reported N. schaldybini from S. unguiculatus, S. gracillimus and S. caecutiens saevus Thomas on the island of Hokkaido, Japan. According to their illustrated description, the rostellar hooks are 38–42 μm long. In our opinion, the shape of these hooks corresponds with that of N. nuda n. sp.: the axis of the blade is almost parallel to the axis of the guard and the blade is twice as long as the handle. On this basis, we consider that the material of Sato et al. (1988) belongs to N. nuda.
Neoskrjabinolepis longicirrosa Kornienko, Gulyaev & Mel’nikova, 2006 (emend.)
Type-host and locality: Sorex araneus, village of Artybash, Altay Mts, Russia.
Other hosts: Sorex minutus, S. isodon, S. caecutiens (see Kornienko et al., 2006).
Distribution: West Siberia (Russia) – Altay Mts, Barabinsk Lowlands, Kuznetskiy Alatau (Kornienko et al., 2006).
Remark: Kornienko et al. (2006) spelled the specific name “longicirrosus”. Since the generic name is feminine in gender, this is hereby emended here to “longicirrosa”.
Neoskrjabinolepis pilosa Kornienko, Gulyaev & Mel’nikova, 2007 (emend.)
Type-host and locality: Sorex araneus, village of Artybash, Altay Mts, Russia.
Other hosts: Sorex isodon (see Kornienko et al., 2007).
Distribution: Altay Mts, West Siberia (Kornienko et al., 2007).
Remark: Kornienko et al. (2007) spelled the specfic name “pilosus”. Since the generic name is feminine in gender, this is hereby emended here to “pilosa”.
Neoskrjabinolepis plagis Kornienko, Gulyaev & Mel’nikova, 2007
Type-host and locality: Sorex caecutiens, village of Shakhterskiy, Chukotka, Russia.
Distribution: The coast of the Anadyr Gulf, Chukotka (Kornienko et al., 2007).
Subgenus Neoskrjabinolepis (Neoskrjabinolepidoides) Kornienko, Gulyaev & Mel’nikova, 2006
Neoskrjabinolepis singularis (Cholodkowsky, 1912 ) Spasskii, 1954 (type-species)
Syn. Hymenolepis singularis Cholodkowsky, 1912
Type-host and locality: “Sorex sp.”, vicinities of the city of Novgorod, Russia (Cholodkowsky, 1912).
Other hosts: Sorex minutus (see Kornienko et al., 2006), S araneus (see Binkienė, 2006; Kornienko et al., 2006), S. caecutiens (see Kornienko et al., 2006), S. isodon (see Kornienko et al., 2006).
Distribution: West Siberia (Russia) (Kornienko et al., 2006), Lithuania (Binkienė, 2006), Poland (Zarnowski, 1955; Soltys, 1952; Pojmanska, 1957; Rybicka, 1959; Vaucher, 1971), Hungary (Kobuley, 1953), Moldova (Spasskii & Andreyko, 1970), France (Vaucher, 1971), Switzerland (Vaucher, 1971), Netherlands (Vaucher, 1971), Germany (Vaucher, 1971), Denmark (Vaucher, 1971), Norway (Vaucher, 1971), Sweden (Vaucher, 1971), Finland (Vaucher, 1971; Haukisalmi, 1989).
Remarks: We found N. singularis in collections from West Siberia, Bulgaria and Lithuania. This species has been recorded from many European countries and from Northern Asia. However, the records have been presented either without morphological data or with data that do not correspond with the current diagnosis of this species. Therefore, its geographical distribution requires further study.
Sato et al. (1988) identified N. singularis from S. unguiculatus, S. gracillimus and S. caecutiens saevus on Hokkaido, Japan. However, their description and drawing of rostellar hooks correspond with none of the known species of Neoskrjabinolepis. The status of this species (probably new) requires further study.
Neoskrjabinolepis nadtochijae Kornienko, Gulyaev & Mel’nikova, 2006
Type-host and locality: Sorex isodon, Bolshekhekhtsirskiy Nature Reserve, Priamurye, Russia.
Other hosts: Sorex caecutiens (see Kornienko et al., 2006), S. unguiculatus (see Kornienko et al., 2006).
Distribution: Kedrovaya Pad Nature Reserve, Lazovsky Nature Reserve (Sikhote-Alin Mts), Primorye; Bolshekhekhtsirskiy Nature Reserve, Priamurye, Russia (Kornienko et al., 2006).
Neoskrjabinolepis kedrovensis Kornienko, Gulyaev & Mel’nikova, 2007
Type-host and locality: Sorex unguiculatus, Kedrovaya Pad Nature Reserve, Primorye, Russia.
Other hosts: Sorex isodon (see Kornienko et al., 2007), S. caecutiens (see Kornienko et al., 2007).
Distribution: Primorye, Chernye Gory Mts, Sikhote-Alin Mts, Kamchatka, Russia (Kornienko et al., 2007).
Neoskrjabinolepis corticirrosа Kornienko, Gulyaev & Mel’nikova, 2007 (emend.)
Type-host and locality: Sorex caecutiens, village of Shakhtersky, the coast of Anadyr Gulf, Chukotka, Russia.
Other hosts: Sorex tundrensis (see Kornienko et al., 2007), S. portenkoi Stroganov (see Kornienko et al., 2007).
Distribution: Village of Esso, Kamchatka; village of Shakhterskiy on the coast of the Anadyr Gulf, Chukotka; Bolshekhekhtsirskiy Nature Reserve, Priamurye, Russia (Kornienko et al., 2007).
Remark: The original spelling of the specific name was “corticirrosus” (see Kornienko et al., 2007). Since the generic name is feminine in gender, this is hereby emended here to “corticirrosa”.
Neoskrjabinolepis nuda n. sp.
See above.
Key to the species of Neoskrjabinolepis
-
1a.
Strobilar development gradual .... [subgenus Neoskrjabinolepis (Neoskrjabinolepis)] .... 2
-
1b.
Strobilar development serial……………………………………………………… ………………………..…...[subgenus Neoskrjabinolepis (Neoskrjabinolepidoides)] ... 5
-
2a.
Fully-everted cirrus short, cylindrical . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
-
2b.
Fully-everted cirrus long, whip-shaped . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
-
3a.
Fully-everted cirrus 40–42 μm long, its parabasal region armed with several large, claw-shaped spines; middle and distal regions of cirrus armed with sparsely distributed, sabre-shaped spines. Rostellar hooks 38–43 μm long . . .. . N. schaldybini
-
3b.
Fully-everted cirrus 45–50 μm long, its median region armed with thin, sabre-shaped spines, with size decreasing in distal direction; distal region of cirrus unarmed. Rostellar hooks 52–55 μm long; handle short; epiphyseal thickening large ............………………………………………………………………………... . . N. plagis
-
4a.
Fully-everted cirrus 120–125 μm long, armed with small, relatively scarce spines whose size decreases in distal direction, becoming indistinct on distalmost region of cirrus. Rostellar hooks 41–45 μm long; axes of blade and guard form acute angle. Eggs per proglottis 16–20 in number . . . . . . . . . . . . . . . . . . . . . . N. longicirrosa
-
4b.
Fully-everted cirrus 100–110 μm long, armed of small, dense spines along its entire length. Rostellar hooks 45–49 μm long; axes of blade and guard almost parallel. Eggs per proglottis 35–47 in number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . N. pilosa
-
5a.
Fully-everted cirrus short, cylindrical . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
-
5b.
Fully-everted cirrus long, whip-shaped . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . 8
-
6a.
Handle of rostellar hooks about half length of epiphyseal thickening. Rostellar hooks 56–65 μm long . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . N. singularis
-
6b.
Handle of rostellar hooks longer than epiphyseal thickening or of comparable length. Rostellar hooks < 56 μm. . . . . . . . . . ……... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
-
7a.
Rostellar hooks 40–45 μm long. Cirrus 71–74 μm long. Eggs per proglottis 20–46 in number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . N. nadtochijae
-
7b.
Rostellar hooks 48–53 μm long. Cirrus 60–65 μm long. Eggs per proglottis 10–20 in number . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . N. corticirrosa
-
8a.
Fully-everted cirrus 90–110 μm long; its distal region with scarce, sabre-shaped spines. Rostellar hooks 36–38 μm long . . . . . . .. . . . . . . . . . . . . . . . N. kedrovensis
-
8b.
Fully-everted cirrus 95–100 μm long; its distal region smooth. Rostellar hooks 40–44 μm long . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . N. nuda n. sp.
Discussion
The validity of the two species, N. singularis and N. schaldybini, corresponding to the generic diagnosis of Neoskrjabinolepis was widely recognised for many years (see Spasskii, 1954; Vaucher, 1971; Genov, 1984). An attempt to verify morphological criteria useful for distinguishing these two species showed that, in Russia at least, each of these names had been used for a complex of species (Kornienko et al., 2006, 2007). The same is probably true for the majority of the records of these two species from European countries and Japan (Soltys, 1952; Kobuley, 1953; Zarnowski, 1955; Pojmanska, 1957; Kisielewska, 1958; Prokopič, 1956, 1958; Rybicka, 1959; Shaldybin, 1964; Spasskii & Andreyko, 1970; Vaucher, 1971; Genov, 1984; Sato et al., 1988; Haukisalmi, 1989). Currently, nine species of Neoskrjabinolepis are recognised (see Kornienko et al., 2006, 2007; present study), but there are likely still undescribed species belonging to this group.
Traditionally, the species within Neoskrjabinolepis were differentiated on the basis of the length of the rostellar hooks and the size of the scolex and the strobila (Spasskii, 1954; Vaucher, 1971). In addition, our study has revealed that the pattern of strobilar development (serial versus gradual), the size and the armament of the cirrus, the morphology of the rostellar hooks (Figs. 8–25), the position of the cirrus-sac in relation to the poral osmoregulatory canals and the number of eggs per gravid proglottis (Tables 1 and 2) as reliable differentiating characters (Kornienko et al., 2006, 2007; present study).
There are records of four sympatric species of Neoskrjabinolepis in the Altay Mountains (Kornienko et al., 2006, 2007), three species in Primorye (Kornienko et al., 2006, 2007) and four species in Bulgaria (Kornienko, unpublished data) and Lithuania (Kornienko & Binkienė, unpublished data). On the basis of the examination of our samples and a reconsideration of the published descriptions, we suggest that in many areas of the Palaearctic Region, between three and five sympatric species of Neoskrjabinolepis occur in Sorex spp. For example, Kobuley (1953) described as N. singularis cestodes from shrews in Hungary, with illustrations better fitting N. schaldybini. However, the rostellar hooks were longer (42–46 μm) than those of N. schaldybini (38–43 μm), which indicates that his material was heterogeneous. According to Karpenko (1989), the length of the rostellar hooks of N. schaldybini ranges between 31 and 53 μm. The proposed synonymy of N. schaldybini and N. singularis (see Zarnowski, 1955; Prokopič, 1956, 1958; Rybicka 1959) resulted in the loss of useful criteria for distinguishing species within Neoskrjabinolepis. For this reason, it is currently difficult to characterise the geographical and host ranges of N. schaldybini and N. singularis on the basis of the published descriptions only.
References
Binkienė, R. (2006). Helminth fauna of shrews (Sorex spp.) in Lithuania. Acta Zoologica Lituanica, 16, 241–245.
Cholodkowsky, N. (1912). Cestodes nouveaux ou peu connus. Deuxiéme ser. Annales Musée Zoologique de l’Academie Impériale des Sciences de St. Petersburg, 18, 221–232.
Czaplinski, B., & Vaucher, C. (1994). Family Hymenolepididae Ariola, 1899. In L. F. Khalil, A. Jones, & R. A. Bray (Eds), Keys to the cestode parasites of vertebrates (pp. 595–663). Wallingford, UK: CAB International.
Eltyshev, Yu. A. (1975). [Helminth fauna in mammals from Barguzinskaya Lowland and an attempt for its geographical analysis. 1. Systematic survey of helminths.] In V. L. Kontrimavichus & A. V. Roytman (Eds.), Paraziticheskie Organizmy Severo-Vostoka Azii (pp. 135–167). Vladivostok: Dal’nevostochniy Nauchniy Tsentr (In Russian).
Genov, T. (1984). [Helminths of insectivores and rodents in Bulgaria.] Sofia: Izdatelstvo na Bulgarskata akademiya na naukite, pp. 348 (In Bulgarian).
Gulyaev, V. D. (1991). [Morphology and taxonomy of the Ditestolepidini–cestodes (Cyclophyllidea) of shrews with a serial metameric structure of the strobila.] Zoologicheskiy Zhurnal, 70, 44–53 (In Russian).
Haukisalmi, V. (1989). Intestinal helminth communities of Sorex shrews in Finland. Annales Zoologici Fennici, 26, 401–409.
Jourdane, J. (1971). Helminthes parasites des Micromammifères des Pyrénéés-Orientales. II Les Plathelminthes de Soricinae. Annales de Parasitologie Humaine et Comparée, 46, 553–574.
Karpenko, S. V. (1989). [Ecology and morphology of the cestode Neoskrjabinolepis schaldybini Spassky, 1947 (Hymenolepididae).] In K. P. Fedorov (Ed.), Ekologiya gel’mintov pozvonochnykh Sibiri (pp. 27–44). Novosibirsk: Nauka (Siberian Branch) (In Russian).
Kisielewska, K. (1958). Cysticercoid of the tapeworm Neoskrjabinolepis singularis (Cholodkowsky 1912) Spassky, 1954 in a beetle of the family Catopidae. Bulletin de l’Académie Polonaise de Sciences (Cl. 2), 6, 206–208.
Kobuley, Т. (1953). On the anatomy and systematics of poorly known cestodes from shrews. Acta Veterinaria Academiae Scientarum Hungaricae, 3, 431–438 (In Russian).
Kornienko, S. A., Gulyaev, V. D., & Mel’nikova, Yu. A. (2006). [On the morphology and systematics of cestodes of the genus Neoskrjabinolepis Spassky, 1947 (Cyclophyllidea, Hymenolepididae).] Zoologicheskiy Zhurnal, 85, 134–145 (In Russian).
Kornienko, S. A., Gulyaev, V. D., & Mel’nikova, Yu. A. (2007). [New species of cestodes of the genus Neoskrjabinolepis Spassky, 1947 (Cyclophyllidea, Hymenolepididae) from shrews of Russia.] Zoologicheskiy Zhurnal, 86, 259–269 (In Russian).
Murai, É., & Mészáros, F. (1984). Helminths from small mammals in the Čergov Mountains (Western Carpathians, Czechoslovakia). Miscellanea Zoologica Hungarica, 2, 17–28.
Pojmanska, T. (1957). Pasożyty wewnetrzne (Cestoda, Trematoda) drobnych ssakówpolnych z okoliz Turwi kolo Posnana. Acta Parasitologica Polonica, 5, 117–161.
Prokopič, J. (1956). Helmintofauna rejska obecneho (Sorex araneus) v ČSSR. Československa Parasitologie, 3, 109–131.
Prokopič, J. (1958). [On the helminth fauna of the genus Sorex in Czechoslovakia.] Zoologicheskiy Zhurnal, 38(2), 174–183 (In Russian).
Rybicka, K. (1959). Tapeworms of forest micromammals (Rodentia and Insectivora) from Kampinos Wilderness. Acta Parasitologica Polonica, 7, 393–420.
Sato, H., Kamiya, H., & Ohbayashi, M. (1988). Hymenolepidid and dilepidid cestodes with armed rostellum in shrews, Sorex spp., from Hokkaido, Japan. Japanese Journal of Veterinary Research, 36, 119–131.
Shaldybin, L. S. (1964). [Helminth fauna of mammals of Mordovsky State Nature Reserve.] Uchenye Zapiski Gor’kovskogo Gosudarstvenogo Pedagogichekogo Instituta, Seriya Zoologicheskaya, 48, 50–81 (In Russian).
Soltys, A. (1952). Pasozyty wewnetrzne ryjowki aksamitmej (Sorex araneus L.) Bialowieskiege Parku Narodowego. Annales de l’Université Marie Curie-Sklodowska, 6, 165–209.
Spasskii, A. A. (1947). [The phenomenon of confluence of proglottides and uteri in cestodes.] Doklady Akademii Nauk SSSR, 58, 723–724 (In Russian).
Spasskii, A. A. (1954). [Classification of hymenolepidids of mammals.] Trudy Gel’mintologcheskoi Laboratorii, 7, 120–167 (In Russian).
Spasskii, A. A., & Andreyko, O. F. (1970). [Cestodes of Insectivora of Moldavia.] Parazity Zhivotnykh i Rastenii, 5, 44–59 (In Russian).
Vaucher, С. (1971). Les cestodes parasites des Soricidae d′Europe. Étude аnatomique, révision taxonomique et biologie. Revue Suisse de Zoologie, 78, 1–113.
Zarnowski, E. (1955). Robaki pasożytnicze drobnych ssaków leśnych (Rodentia i Insectivora) okolicy Pulaw (woj. Lubelskie). I. Cestoda. Acta Parasitologica Polonica, 3, 279–368.
Acknowledgements
We are grateful to V.E. Dokuchaev for his help in the field work. D.I. Gibson kindly advised us on a nomenclatural problem. This study was facilitated by the Research Co-operation Agreement between the Russian Academy of Sciences and the Bulgarian Academy of Sciences, 2006–2008. Substantial part of the work was funded by the Russian Fund of Fundamental Research, Grant (05–04-49010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kornienko, S.A., Gulyaev, V.D., Mel’nikova, Y.A. et al. Neoskrjabinolepis nuda n. sp. from shrews on Sakhalin Island, Russia, with a taxonomic review of Neoskrjabinolepis Spasskii, 1947 (Cestoda: Cyclophyllidea: Hymenolepididae). Syst Parasitol 70, 147–158 (2008). https://doi.org/10.1007/s11230-008-9140-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-008-9140-z