Abstract
Cyclopeptide derivatives have attracted great interest in host-guest chemistry during the past decades. In this work, four cyclopeptides including one cyclodecapeptide (CDP) and three modified CDPs (M-CDP involves I-CDP, E-CDP, and H-CDP) are adopted as hosts to differentiate the four guests of the amphetamine (AP) and ibuprofen (IP) enantiomers using a proposed integrated computation protocol. The obtained results demonstrated that the guests of AP and IP enantiomers could be recognized by different cyclopeptides using the certain optimized quantum chemistry methods. Specifically, the AP or IP enantiomers might be identified by the corresponding cyclopepitdes in the five pairs of the inclusion complexes associated with the large differences of binding energies of hosts with guests, that is, the two of H-CDP/AP and H-CDP/IP by B3LYP, the two of I-CDP/IP and H-CDP/IP by CAM-B3LYP, and the other one of I-CDP/IP by M06-2X, which are mainly determined by their corresponding stable conformations, electronic properties, and favorable interactions. The intermolecular hydrogen bonds and NBO analyses of the inclusion complexes further suggest the corresponding differences of binding energies. The visual nonbonded weak interactions for the studied systems gave the reasons why the AP and IP enantiomers are identified by the corresponding cyclopeptides. Molecular dynamics simulated results further support the above conclusions. The investigation provides detailed information at a molecular level about the recognition of the two chiral drug molecules by the four cyclodecapeptides. The integrated computation protocol proposed in this work provides people a feasible way to study interaction of hosts and guests, molecular recognition, and chiral separation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recognition of chiral organic molecules by a molecular host has become a topic of strong interest over the past decades due to its potential implications in biomimetic organic chemistry, supramolecular chemistry, biology, and pharmacy [1–6]. A large number of excellent examples of macrocyclic molecules have been reported acting as host candidates such as cyclodextrins (CDs) [7–11], cucurbituril (CB) [12–14], cationic pillar[n]arenes (CPAs) [15, 16], crown ethers [17], and calixarenes [18]. Noteworthy, for these host molecules, the high symmetry and rigidity are the iconic structural feature. In particular, the novel host molecule CPAs [19], analogous to crown ethers, is a new kind of macrocycles composed of electron-donating 2,5-dialkoxybenzene units which are linked by methylene bridges at their parapositions, forming a unique rigid pillar architecture. Various interesting CPAs-based supramolecular systems have been constructed by adjusting number “n” to selectively bind different kinds of guests [20]. However, the deficiency of conformational flexibility is a limitation regarding efficiency of inclusion complex. It is difficult for the CPAs molecules to adjust their geometries to fit the guest molecules in an optimal interaction mode. Noticeably, the conformational disadvantages of CPAs are exactly good qualities for cyclic peptides which are polypeptide linked by amino acid residues [21]. Recently, in order to modify and improve their stabilities and inclusion selectivity, a variety of cyclic peptide derivatives have been designed and synthesized as anticancer, antibacterial drug carriers, and enzyme inhibitors [22–28].
Understanding interaction of cyclic peptides with guest molecules may help us delineate the features that are responsible for the remarkable potency of cyclic peptides. However, knowledge of the detailed structures of cyclic peptide with enantiomers of a chiral molecule at the molecular level is still very limited [29–33]. Especially, conformations and structures of cyclic peptides are not yet clear experimentally [34–36]. The theoretical chemists have been interested in investigating the structures and properties of cyclopeptides by using theoretical calculations in recent years [37–46], for instance, Poteau and Trinquier’s systematic exploration on all-cis and trans conformations of cyclopolyglycines, cyclopolyalanines, and cyclopolyphenylalanines [47]. Takeda group proposed variable conformations in peptide nanorings and nanotubes by a mathematical conformational analyses [48]. Alireza and his coworkers have investigated the structural characteristics of the inclusion complexes composed by an important type of nanotubular cyclic peptides, cyclo(Ala) n=4∼9, as the host molecules and enantiomers of lactic acid (LA) as the guest molecules based on density function theory (B3LYP and CAM-B3LYP) [49]. These theoretical explorations on the conformational and electronic structures of the cyclopeptides are very significant in guiding the design of desirable host molecules. In particular, our previous theoretical calculations have indicated that the cyclic decapeptide (CDP) could differentiate the enantiomer of 1-phenyl-1-propanol, suggesting that cyclopeptide is a desirable host molecule for chiral and molecular recognition [50, 51].
In the present work, we proposed an integrated protocol to investigate interaction between a host and a guest. The favorable conformations of the 16 inclusion complexes of the cyclic decapeptides with two enantiomers were studied. Specifically, the cyclic decapeptides include one homocyclic CDP and three heterocyclic CDPs (named as I-CDP, E-CDP, and H-CDP) as shown in Fig. 1a. In the known chiral molecules, amphetamine (assigned as AP) and ibuprofen (assigned as IP) as shown in Fig. 1b, who are good candidates for constructing simple models to study molecular chiral recognition, are selected as guest molecules separated by the cyclic decapeptides. In order to provide further insights into the different inclusion properties of the four guest molecules, the conformational and structural features of the 16 inclusion complexes were investigated using three DFT methods. The geometry, binding free energy, electronic property, and molecular orbital of the studied complex are calculated and considered in detail. All obtained results suggest the significance of the cyclodecapeptides acting as excellent host candidates for chiral and molecular recognition.

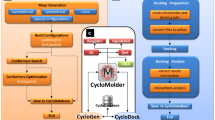
Chart 1 The integrated computation protocol for molecular recognition of host and guest.
Models and methods
The new integrated protocol was proposed as shown in Chart 1 in this work. Specifically, (1) the host and guest molecules are constructed, (2) structures of a host and a guest are optimized by quantum chemistry method, (3) initial inclusion complex of a host and a guest is predicted by molecular docking, (4) favorable conformation of inclusion complexes is fully optimized by different methods, (5) detailed data and visual analyses on interactions between a host and a guest result in conclusions, and (6) proposed desirable hosts for certain guests. In the present work, the initial structures of CDP were constructed using in-home software, which is the same from the previous work [50], in which the E-type backbone CDP was selected due to its favorable conformation in the larger peptide rings [38, 48]. In order to build the original conformation of modified cyclic decapeptide (M-CDP including CDP), the hydrogen atom of one glycine (Gly) was mutated to l-configuration of isoleucine (Ile), glutaminic acid (Glu), and histidine (His) forming I-CDP, E-CDP, and H-CDP, respectively. The components of CDP, I-CDP, E-CDP, H-CDP, AP, and IP enantiomers are presented in Fig. 1. The geometries of the four modified cyclodecapeptides and four guest molecules are fully optimized using three theory methods of B3LYP [52, 53], CAM-B3LYP [54], and M06-2X [55, 56] with the 6-31G(d,p) basis set as implemented in the Gaussian09 program [57]. Furthermore, molecular docking was firstly to select the possible initial conformation of inclusion complex. Namely, the optimized four guest molecules were docked into the optimized M-CDP molecules using AutoDock Vina program [58, 59]. The grid map consists of 28 × 18 × 18 points with a spacing of 1.0. The best-scoring representative conformation for each inclusion was selected as the initial structure for the following quantum mechanics calculation.
All geometries and energies reported in the present study are calculated using three density functional theories of B3LYP, CAM-B3LYP, and M06-2X as implemented in Gaussian09 [57]. Geometry optimizations were performed using the 6-31G(d,p) basis set. Frequency calculations were conducted at the same theory level to check the imaginary frequency. The solvent effects of water were taken into account for all calculations (optimization and frequency) by the self-consistent reaction field (SCRF) method, which is based on the polarizable continuum model using the integral equation formalism variant (IEFPCM) [60]. Based on these optimized geometries, to refine the energy of each studied system, the larger basis set of 6–311++G(d,p) with IEFPCM model was adopted for zero-point energy calculations in aqueous solvent [61]. Further, the electrostatic potential map (shown as in the supporting information of Fig. S1) analyses for the studied hosts of I-CDP, E-CDP, and H-CDP were obtained with the same levels of theory [62]. Moreover, basis set superposition error (BSSE) corrections [63] were carried out to correct the interaction energies. And, Grimme’s DFT-D3 dispersion corrections [64] were employed to account for the long-range weak dispersion interactions. The energy corrections of BSSE and DFT-D3 dispersion mentioned above were obtained at the same theory level as that of zero-point energy calculations.
In order to investigate the driving forces to generate the inclusion complexes, the binding energy (BE) was referenced in our previous work [50] as follows:
where E(inclusion_complex), E(host), and E(guest) represent the energies of inclusion complex, M-CDP, and guest, respectively. According to the equation mentioned above, a negative value of BE represents that the corresponding complex is energetically stable. The more negative the BE value is, the more stable the inclusion complex is. In other words, the greater the absolute value of the BE is, the stronger interaction between host and guest is. On the contrary, the distortion energy (DE) of the modified cyclic decapeptide is the opposing force for the generation of inclusion complex. The DE values of M-CDP are evaluated by the following formula:
where E(inclusion_ring) and E(single_ring) represent the energy of the M-CDP molecule as a inclusion geometry and separate one, respectively. In the light of the equation mentioned above, a positive value of DE represents that the distorted geometry of M-CDP in the inclusion complex is unfavorable for the inclusion of M-CDP with PP. Namely, the larger the absolute value of the DE is, the higher the distortion degree between inclusion geometry and separate geometry one is.
Additionally, charge transfers between host and guest molecules have been studied by natural bond orbital (NBO) population analyses. And, the molecular interactions of hydrogen bond interactions, steric interactions, and coulomb interactions in the inclusion complexes of M-CDP/PPs can be identified by the cooperated programs of VMD [65] and Multiwfn [66]. All molecular dynamics (MD) simulations were carried out using the AMBER14 package [67] with the AMBER force fields of parm99 and gaff [68, 69]. The specific protocol for all MD simulations is the same to that of our previous published work [70].
Results and discussion
Binding energies of the inclusion complexes
Generally, the noncovalent interaction mainly contributes to the inclusion of a host to a guest. To gain the reliable results, DFT-D dispersion correction involving the long-range interaction is necessarily adopted in this work [71]. The BE values of four M-CDP molecules and the AP/IP enantiomers associated with thermodynamic parameters obtained by B3LYP, CAM-B3LYP, and M06-2X are presented in Tables 1, 2, and 3, respectively. Obviously, the data demonstrate that the formations of the inclusion complexes of M-CDP with AP/IP are energetically favorable processes. And, the negative enthalpy changes suggest that the inclusion processes of the M-CDP with the AP/IP enantiomers are exothermic. These negative data also indicate that enthalpy change is the driving force of the formation of the inclusion complex. The negative free energy changes imply the inclusion processes to be spontaneous, and vice versa. Particularly, all negative ΔG values for the formation of complexes of M-CDPs with IP enantiomers clearly signify spontaneous processes. Most of positive ΔG values for the complexes of M-CDPs with AP enantiomers hint that the inclusion phenomena might happen in certain conditions. The entropy changes in Tables 1, 2, and 3 tell us that the driving force of the formation of the inclusion complex is not the entropy change.
We now turn attention to the binding energy (BE) and the ΔΔE value (ΔΔE = |BER − BES|); it can be seen from Table 1 obtained by B3LYP that, for the AP enantiomer, the BE values of CDP with AP ones are larger than those of M-CDPs, indicating that CDP can form more stable inclusion complexes with the R/S-AP molecule than the M-CDPs. BE of CDP/R-AP is almost the same to that of CDP/S-AP with little difference of 0.03 kJ mol−1, which indicates that CDP could not separate the AP enantiomers. For M-CDPs with R/S-AP, the ΔΔE value for I-CDP is 3.26 kJ mol−1, that for E-CDP is 4.36 kJ mol−1, and that for H-CDP is 9.25 kJ mol−1, meaning that H-CDP might identify AP enantiomers. For the R/S-IP, the BE values of CDP with R/S-IP are less than those of M-CDPs except for E-CDP and H-CDP with S-IP, indicating that those M-CDPs inclusion complexes are more stable than the corresponding CDP ones besides the two mentioned above. Moreover, BE for CDP/R-IP is larger than that of CDP/S-IP with about 2.87 kJ mol−1 difference, suggesting that CDP is not a good candidate for the IP enantiomer separation. And, for the M-CDPs with R/S-IP, the ΔΔE value for I-CDP is 5.39 kJ mol−1, that for E-CDP is 4.20 kJ mol−1, and that for H-CDP is 16.28 kJ mol−1, indicating that H-CDP could recognize R/S-IP conformation. Furthermore, it is clear from Table 2 obtained by CAM-B3LYP that the BE values of CDP with R/S-PP are larger than those of M-CDPs, which indicates that the complexes of CDP with R/S-PP are more stable than those of M-CDPs. More specifically, BE of CDP/R-AP is less than that of CDP/S-AP with the difference of 8.38 kJ mol−1, and for the M-CDPs with R/S-AP, the ΔΔE value for I-CDP is 7.84 kJ mol−1, that for E-CDP is 2.14 kJ mol−1, and that for H-CDP is 4.75 kJ mol−1, which gives us one possibility that the adopted CDPs in this work might not separate well the AP enantiomers. However, as for R/S-IP, the BE value of CDP/R-IP is larger than that of CDP/S-IP with about 7.48 kJ mol−1 difference, that for E-CDP is 5.29 kJ mol−1, that for I-CDP is 19.51 kJ mol−1, and that for H-CDP is 16.13 kJ mol−1, which demonstrates that I-CDP and H-CDP can identify the R or S conformation of IP enantiomers. Subsequently, it can be found from Table 3 by M06-2X that BE of CDP with R/S-AP are lower than those of M-CDPs except for H-CDP with R-AP, I-CDP, and H-CDP with S-IP, while the BE values of CDP with R/S-IP are larger than those of M-CDPs besides H-CDP with R-IP. Particularly, based on the ΔΔE values (0.06∼4.98 kJ mol−1), we know that R/S-AP cannot be separated well by M-CDPs. For M-CDPs with R/S-IP, the biggest ΔΔE value of 16.68 kJ mol−1 suggests that I-CDP might be a good candidate for differentiating the R/S-IP conformation.
Further analyses help us find some interesting phenomena as shown in Fig. 2. Specifically, (1) it is obvious that the magnitudes of all energy components for AP enantiomers involved complexes are smaller than those for IP enantiomers involved ones in Fig. 2a–d. (2) Figure 2a shows that the BE values of M-CDPs to R/S-AP calculated by three methods follow the order of CAM-B3LYP > M06-2X > B3LYP, while those of the M-CDPs to R/S-IP are M06-2X > CAM-B3LYP > B3LYP. And, the BE values of M-CDP/AP inclusion complexes fluctuate smaller than those of M-CDP/IP ones. As for the range of BE values change, that for AP/IP complexes is the order of M06-2X > B3LYP > CAM-B3LYP and that for IP is larger than that for AP, which might attribute to the different polar substituents. These results indicate that the function group of PP enantiomers influences significantly on the stability of the inclusion complexes. (3) The thermodynamic parameters of the inclusion complexes of PP enantiomers with M-CDPs are presented in Fig. 2b–d. The results are observed from Fig. 2b that the magnitude order of the enthalpy change values is M06-2X > B3LYP > CAM-B3LYP. Specifically, for the M-CDPs to AP enantiomer, the ΔH values computed by the three methods are close, while for the M-CDPs to IP enantiomers, ΔH computed by M062X is much larger than that by the other two methods. As for the entropy change, the trend of the TΔS values is similar to that of ΔH, yet there is a large difference for one complex obtained by each method. As for the Gibbs free energy change, the value range calculated by the B3LYP, CAM-B3LYP, and M06-2X method is −25.45∼11.44, −24.74∼8.24, and −42.44∼14.67 kJ mol−1, respectively, indicating that the range order is M06-2X > B3LYP > CAM-B3LYP. Interestingly, each ΔG value for M-CDPs/IP enantiomer complexes is negative, while most for the M-CDP/AP complexes are positive ones, which suggests that the inclusion process of M-CDPs to IP enantiomer is spontaneous and that for M-CDPs to AP one is nonspontaneous. These above analyses demonstrate that the results obtained by three DFT methods are similar but somewhat different, that is, the data calculated by B3LYP are similar to those by CAM-B3LYP, but those by M06-2X are quite different. This difference attributes to the consideration of the weak interaction in M06-2X.
Interactions of different types of energy changes: a BE, b ΔH, c TΔS, and d ΔG with the codes of inclusion complexes, in which 1, 2, 3, and 4 respectively refers to the modified cyclic decapetides CDP, I-CDP, E-CDP, and H-CDP, while R/SA and R/SI represent, respectively, R/S-AP and R/S-IP. The symbols of R/S1, R/S2, R/S3, R/S4 and R/S1′, R/S2′, R/S3′, and R/S4′ refers to the inclusion complexes of CDP/R/S-AP, I-CDP/R/S-AP, E-CDP/R/S-AP, H-CDP/R/S-AP and CDP/R/S-IP, I-CDP/R/S-IP, E-CDP/R/S-IP, and H-CDP/R/S-IP, respectively
Conformational characteristics of the inclusion complexes
The favorable conformations of the inclusion complexes optimized by three theory methods of B3LYP, CAM-B3LYP, and M06-2X present visible differences. Here, those that can identify the PP enantiomers were considered in the next text. In another word, the conformations of the inclusion complexes associated with large ΔΔE values (≥9 kJ mol−1) were focused as presented in Figs. 3, 4, and 5.
Specifically, Fig. 3 depicts the inclusion complexes of H-CDP/AP and H-CDP/IP obtained by B3LYP. As for the H-CDP/AP complexes, R-AP floats on the plane of the cavity of H-CDP in Fig. 3a, and S-AP inserts little using the aryl group into the cavity of H-CDP in Fig. 3b, thus suggesting the favorable interaction of S-AP with H-CDP might be more than that of R-AP. The orientation of the amino group of R/S-AP is advantageous to form an intermolecular hydrogen bond. In terms of the H-CDP/IP complexes presented in Fig. 3c and d, R/S-IP inserts into the cavity of H-CDP via the isobutyl or carboxylic acid group, respectively, while the phenyl groups of IP enantiomers float above the plane of the main cycle of H-CDP. Additionally, the orientation of the carboxylic acid group in R-IP/S-IP favors to form hydrogen bond with H-CDP. Figure 4a–d depicts the energy-minimized conformations of I-CDP/IP and H-CDP/IP complexes computed by CAM-B3LYP method. It is observed from Fig. 4a, b that R-IP only adopts the carboxylic acid group inserting into the cavity of I-CDP, while S-IP encapsulates in the cavity of I-CDP with phenyl group, indicating that I-CDP/R-IP is less stable than I-CDP/S-IP. Similarly, it is found in Fig. 4c, d that the optimized conformations of the H-CDP/IP enantiomer complexes are very close to those by the B3LYP as shown in Fig. 3c, d, which further confirms the recognition of H-CDP to R/S-IP enantiomers. Surprisingly, the conformations optimized by M06-2X as presented in Fig. 5a, b are distinct from those by B3LYP and CAM-B3LYP. Namely, the conformation of I-CDP presents obviously distortion and the IP enantiomer molecules insert into the cavity of I-CDP by the carboxylic acid groups. The modified cyclic decapeptide of I-CDP to R-IP distorted more highly than that to S-IP, suggesting that I-CDP/R-IP might be more stable than I-CDP/S-IP. Comparison of these conformations calculated by the three different methods, we found that the configurations by B3LYP and CAM-B3LYP are similar and greatly different from those by M06-2X. These results hint us a fact that the favorable conformation of one complex depends on the adopted theory method. To obtain the trustworthy results, it is necessary to adopt different theory methods to optimize the molecular stable structure.
Furthermore, in order to assess quantitatively the deformation of the cavity of M-CDP, Fig. 6 defines the structural diameters of the cavity of M-CDP and Table 4 compares the five diameters of M-CDP by three methods. The deformation extent of the cavity of M-CDPs by B3LYP and CAM-B3LYP is less than those by M06-2X. And, the most values of distortion energy of the M-CDPs by B3LYP and CAM-B3LYP are positive except for those of H-CDP/R-AP (B3LYP) and I-CDP/S-IP (CAM-B3LYP), which demonstrates that the deformation of M-CDP is unfavorable to stabilize the inclusion complex. While those by M06-2X are negative, indicating that the deformation of M-CDP favors to the formation of inclusion complex. The known conformational flexibility of cyclopeptide hints a fact that, for the studied systems in this work, the conformations obtained by M06-2X are to be more reasonable than those by the other two methods.
Schematic representation for the conformation of M-CDP and the distance of each face-to-face αC atom in a straight line. Specifically, “d1” represents the distance between αC1 and αC6, “d2” represents the distance between αC2 and αC7, “d3” represents the distance between αC3 and αC8, “d4” represents the distance between αC4 and αC9, and “d5” represents the distance between αC5 and αC10
Hydrogen bond interaction and NBO analysis
To address the differences among the geometries of inclusion complexes of the R/S-PP with the M-CDPs, the corresponding hydrogen bonds and NBO analyses are further performed. The detailed intermolecular hydrogen bond interactions are shown in Figs. 7, 8, and 9, and NBO analyses information for the inclusion complexes are listed in Table 5.
It is seen from Figs. 7, 8, and 9 that the significant differences for hydrogen bond interactions occur in the different inclusion complexes. Apparently, it is known from Table 5 that there are six, five, and two hydrogen bond formations as shown in Figs. 7, 8, and 9, respectively. Specifically, for H-CDP/R-AP, a weak hydrogen bond occurs between the electron donor of O4 in H-CDP and the electron acceptor of N11 in R-AP with 3.21 Å distance (d H…O = 2.20 Å) presented in Fig. 7a; for H-CDP/S-AP, a 3.12 Å weak hydrogen bond (d H…O = 2.16 Å) happens between the donor of O10 in H-CDP and the acceptor of N11 in S-AP as shown in Fig. 7b. For H-CDP/R-IP, there are two hydrogen bonds in Fig. 7c, that is, one occurs between N10 of H-CDP and O12 of R-IP, a moderate hydrogen bond of N10-H10…O12 (d H…O = 2.03 Å), the other appears between O10 of H-CDP and O11 of R-IP, a strong hydrogen bond of O11-H11…O10 (d H…O = 1.66 Å). Similarly, for H-CDP/S-IP, two hydrogen bonds are exhibited in Fig. 7d, namely, one 2.74 Å strong hydrogen bond of O11-H11…O1 (d H…O = 1.79 Å), the other comes from the C2 atom of H-CDP to donate a hydrogen atom to O12 in S-IP. Additionally, for those data by CAM-B3LYP, it can be observed from Table 4 that there are two new recognizable I-CDP/IP enantiomer complex formations. I-CDP/R-IP and I-CDP/S-IP possess a strong hydrogen bond of O11-H11…O6 and O11-H11…O7 with the distances of 2.69 Å (d H…O = 1.70 Å) and 2.73 Å (d H…O = 1.79 Å), respectively, as shown in Fig. 8a, b. Unexpectedly, for H-CDP/R-IP as shown in Fig. 8c, the two formation hydrogen bonds are similar to those obtained by the B3LYP, while one strong hydrogen bond in H-CDP/S-IP remains as shown in Fig. 8d. However, for data by M06-2X, two strong hydrogen bonds appear in I-CDP/IP as shown in Fig. 9a, b, which is similar to those by CAM-B3LYP. Distinctly, the intermolecular hydrogen bonds play a vital role for the conformational change, demonstrating a contribution to the binding energies of the inclusion complexes.
Visual interaction analysis
The method of noncovalent interaction index developed by Yang and coworkers was demonstrated to be capable of distinguishing strong interaction [72, 73], van der Waals interaction and repulsive steric interactions. Luckily, the detailed intermolecular interactions in the inclusion complexes of M-CDP/PPs now can be recognized by the cooperation of programs. The visible analysis results for the inclusion complexes are plotted in Figs. 10, 11, and 12.
For the complexes of H-CDP/AP and H-CDP/IP computed by B3LYP, it is obtained from Fig. 10a–d that all these complexes possess favorable and unfavorable inclusion interactions, that is, the favorable interactions include van der Waals (Vdw) interactions and hydrogen bonds (sorted into strong, medium, and weak ones, and abbreviated as SHB, MHB, and WHB, respectively), and the unfavorable interactions involve steric and repulsion interactions. Specifically, for the H-CDP/AP complexes, the favorable Vdw interactions contribute to the inclusion of H-CDP with R-AP apparently much less than those of H-CDP/S-AP as presented in Fig. 10a, b, indicating that H-CDP/S-AP is more stable than H-CDP/R-AP. While, in Fig. 10c, d, for the complexes of H-CDP/IP, the interaction differences are that owns one more intermolecular hydrogen bond (namely the SHB) and much Vdw interactions in H-CDP/R-IP are than those in H-CDP/S-IP, suggesting that the stability of H-CDP/IP is larger than that of H-CDP/S-IP. In terms of the complexes of I-CDP/IP and H-CDP/IP calculated by CAM-B3LYP, the detailed intra/intermolecular interactions are presented in Fig. 11a–d. Herein, we only mark the existing intermolecular interactions of hydrogen bonds, Vdw and repulsion interactions in the complexes. For the I-CDP/IP complexes in Fig. 11a, b, compared with those of I-CDP/S-IP, there is much less Vdw interactions in I-CDP/R-IP, which hints a fact that I-CDP/R-IP is less stable than I-CDP/S-IP. For the H-CDP/IP complexes as shown in Fig. 11c, d, there are similar Vdw interactions and repulsions, and one more WHB and SHB in H-CDP/R-IP than that of H-CDP/R-SP, suggesting the greater stability of H-CDP/R-IP. Comparing with those data provided by B3LYP, the intermolecular Vdw interactions of H-CDP with IP enantiomers calculated by CAM-B3LYP are much greater as shown in Fig. 11c, d. However, as for the complexes of I-CDP/IP obtained by M06-2X, the main intermolecular interactions of I-CDP/IP are Vdw interactions associated with large repulsions except for one SHB. Moreover, the interaction area in I-CDP/R-IP is far larger than that in I-CDP/S-IP as shown in Fig. 12a, b, thus suggesting the more stable conformation of I-CDP/R-IP than that of I-CDP/S-IP. Summarily, the hydrogen bond interaction, Vdw interaction, and coulomb interaction work together for the inclusion complex formation. The cooperation effect will obviously facilitate the enhancement of favorable interaction of the inclusion complexes.
Conclusions
In this work, the inclusion complexes of the four cyclic decapeptides (CDP, I-CDP, E-CDP, and H-CDP) with two enantiomers (AP and IP) were carried out using an integrated computation protocol proposed in this work. The obtained results namely the negative values of BE, ∆H, and ∆G for the formations of 16 inclusion complexes of M-CDPs with PP enantiomers indicate the fusion process favorable and spontaneous. Furthermore, the two enantiomers might be identified by different cyclic decapeptides when different DFT methods were adopted to optimize their stable conformations. Specifically, the five pairs of the inclusion complexes, that is, the two of H-CDP/AP and H-CDP/IP by B3LYP, the two of I-CDP/IP and H-CDP/IP by CAM-B3LYP, and the other one of I-CDP/IP via M06-2X, with higher ∆∆E values (≥9 kJ mol−1) can be differentiated from their corresponding enantiomer complexes. The conformational and structural feature analyses of the recognized inclusion complexes confirmed the results mentioned above. The hydrogen bond, NBO, and visual weak interaction analyses on the inclusion complexes elaborated the main contributions of the bindings of M-CDPs to PP and the reasons why the AP and IP enantiomers are recognized by the modified CDPs at such conformations. Further MD-simulated results also support the above conclusions. The present work may give some detailed molecular level information on the recognition of chiral drug molecules by some modified cyclodecapeptides. More importantly, an integrated computation protocol proposed in this work might provide us a feasible way to study molecular recognition and chiral separation between hosts and guests.
References
Yu G, Jie K, Huang F (2015) Supramolecular Amphiphiles based on host-guest molecular recognition motifs. Chem Rev 115:7240–7303
Gubitz G, Schmid MG (2001) Chiral separation by chromatographic and electromigration techniques. A review. Biopharm Drug Dispos 22:291–336
Amly W, Karaman R (2016) Recent updates in utilizing prodrugs in drug delivery (2013–2015). Expert Opinion on Drug Delivery 13:571–591
Chen H, Ogo S, Fish RH (1996) Bioorganometallic chemistry. 8. The molecular recognition of aromatic and aliphatic amino acids and substituted aromatic and aliphatic carboxylic acid guests with Supramolecular (η5-Pentamethylcyclopentadienyl)rhodium−Nucleobase, nucleoside, and nucleotide cyclic Trimer hosts via non-covalent π−π and hydrophobic interactions in water: steric, electronic, and conformational parameters. J Am Chem Soc 118:4993–5001
Connors KA (1997) The stability of cyclodextrin complexes in solution. Chem Rev 97:1325–1358
Gavin JA, Garcia ME, Benesi AJ, Mallouk TE (1998) Chiral molecular recognition in a tripeptide benzylviologen cyclophane host. J Org Chem 63:7663–7669
Aki H, Niiya T, Iwase Y, Kawasaki Y, Kumai K, Kimura T (2004) Multimodal inclusion complexes of ampicillin with β-cyclodextrins in aqueous solution. Thermochim Acta 416:87–92
Bikádi Z, Iványi R, Szente L, Ilisz I (2007) Hazai E. Cyclodextrin complexes: Chiral recognition and complexation behaviour Curr Drug Discov Technol 4:282–294
Cai W, Sun T, Liu P, Chipot C, Shao X (2009) Inclusion mechanism of steroid drugs into β-cyclodextrins. Insights from Free Energy Calculations J Phys Chem B 113:7836–7843
Cai W, Yao X, Shao X, Pan Z (2005) Bimodal complexations of steroids with cyclodextrins by a flexible docking algorithm. J Incl Phenom Macrocycl Chem 51:41–51
Cai W, Yu Y, Shao X (2005) Chiral recognitio of aromatic compounds by β-cyclodextrin based on bimodal complexation. J Mol Model 11:186–193
Geng Q-X, Wang F, Cong H, Tao Z, Wei G (2016) Recognition of silver cations by a cucurbit [8] uril-induced supramolecular crown ether. Org Biomol Chem 14:2556–2562
Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L (2005) The cucurbit[n]uril family. Angew Chem Int Ed 44:4844–4870
Nicolas H, Yuan B, Zhang X, Schönhoff M (2016) Cucurbit[8]uril-containing multilayer films for the Photocontrolled binding and release of a guest molecule. Langmuir 32:2410–2418
Talapaneni SN, Kim D, Barin G, Buyukcakir O, Je SH, Coskun A (2016) Pillar[5]arene based conjugated Microporous polymers for propane/methane separation through host-guest Complexation. Chem Mater 28:4460–4466
Yu G, Wu D, Li Y, Zhang Z, Shao L, Zhou J, Hu Q, Tangb G, Huang F (2016) A pillar[5]arene-based [2]rotaxane lights up mitochondria. Chem Sci 7:3017–3024
Lee M, Gibson HW (2016) Rotaxane-type hyperbranched polymers from a crown ether host and paraquat guests containing blocking groups. J Polym Sci Pol Chem 54:1647–1658
Abraham W (2002) Inclusion of organic Cations by calix[n]arenes. J Incl Phenom Macrocycl Chem 43:159–174
Yu G, Zhou J, Shen J, Tang G, Huang F (2016) Cationic pillar [6] arene/ATP host–guest recognition: selectivity, inhibition of ATP hydrolysis, and application in multidrug resistance treatment. Chem Sci 7:4073–4078
Ogoshi T, T-a Y, Nakamoto Y (2016) Pillar-shaped Macrocyclic hosts pillar[n]arenes: new key players for Supramolecular chemistry. Chem Rev 116:7937–8002
McHugh SM, Rogers JR, Yu H, Lin Y-S (2016) Insights into how cyclic peptides switch conformations. J Chem Theory Comput 12:2480–2488
Arena G, Bonomo RP, Impellizzeri G, Izatt RM, Lamb JD, Rizzarelli E (1987) Coordination properties of cyclopeptides. Formation, stability, and structure of proton and copper(II) complexes of cyclo-(L-histidyl-L-histidyl) in aqueous solution. Inorg Chem 26:795–800
Bitta J (2001) Complexation of arginine with a cyclopeptide in polar solvents and water. J Supramol Chem 1:293–297
Guisado-Barrios G, Muñoz BK, Kamer PCJ, Lastdrager B, van der Marel G, Overhand M, Vega-Vázquez M, Martin-Pastor M (2013) Cyclic decapeptide gramicidin S derivatives containing phosphines: novel ligands for asymmetric catalysis. Dalton Trans 42:1973–1978
Gulavita NK, Gunasekela SP, Pomponi SA, Robinson EV (1992) Polydiscamide a: a new bioactive depsipeptide from the marine sponge discodermia sp. J Org Chem 57:1767–1772
Ishida H, Donowaki K, Suga M, Shimose K, Ohkubo K (1995) Serine proteinases mimics: hydrolytic activity of cyclic peptides which include a non-natural amino acid. Tetrahedron Lett 36:8987–8990
Ishida H, Suga M, Donowaki K, Ohkubo K (1995) Highly effective binding of phosphomonoester with neutral cyclic peptides which include a non-natural amino acid. J Org Chem 60:5374–5375
Kubik S, Goddard R (1999) A new cyclic pseudopeptide composed of (L)-proline and 3-aminobenzoic acid subunits as a ditopic receptor for the simultaneous complexation of cations and anions. J Org Chem 64:9475–9486
Kessler H, Matter H, Gemmecker G, Kottenhahn M, Bats JW (1992) Structure and dynamics of a synthetic O-gycosylated cyclopeptide in solution determined by NMR spectroscopy and MD calculations. J Am Chem Soc 114:4805–4818
Kessler H (1982) Conformation and biological activity of cyclic peptides. Angew Chem Int Ed Engl 21:512–523
Kobayashi J, Tsuda M, Nakamura T, Mikami Y, Shigemori H (1993) Hymenamides a and b, new proline-rich cyclic heptapeptides from the okinawan marine sponge hymeniacidon sp. Tetrahedron 49:2391–2402
Krause MR, Goddard R, Kubik S (2011) Anion-binding properties of a cyclic pseudohexapeptide containing 1,5-disubstituted 1,2,3-triazole subunits. J Org Chem 76:7084–7095
Kubik S, Bitta J, Goddard R, Kubik D, Pohl S (2001) Receptor properties of cyclic peptides composed of alternating natural amino acids and 3-aminobenzoic acid derivatives. Mater Sci Eng C 18:125–133
Clark TD, Buehler LK, Ghadiri MR (1998) Self-assembling cyclic β3-peptide nanotubes as artificial transmembrane ion channels. J Am Chem Soc 120:651–656
Hudecova J, Kapitán J, Baumruk V, Hammer RP, Keiderling TA, Bour P (2010) Side chain and flexibility contributions to the raman optical activity spectra of a model cyclic hexapeptide. J Phys Chem A 114:7642–7651
Chung BKW, White CJ, Scully CCG, Yudin AK (2016) The reactivity and conformational control of cyclic tetrapeptides derived from aziridine-containing amino acids. Chem Sci 7:6662–6668
Armata N, Dyke JM, Ferrante F, Manna GL (2008) Computational study on cesium azide trapped in a cyclopeptidic tubular structure. J Chem Theory Comput 4:542–548
Chen G, Su S, Liu R (2002) Theoretical studies of monomer and dimer of cyclo [(−l-Phe 1-d-Ala 2-) n] and cyclo [(−l-Phe1-d-me N-Ala2-) n](n= 3-6). J Phys Chem B 106:1570–1575
Duca D, Bifulco G, Barone G, Casapullo A, Fontana A (2004) SCSA code: applications on the cyclopeptide renieramide. J Chem Inf Comput Sci 44:1024–1030
Ferrante F, Manna GL (2007) Theoretical study of the interaction between sodium ion and a cyclopeptidic tubular structure. J Comput Chem 28:2085–2090
Garíca-Fandiño R, Castedo L, Granja JR, Vázquez SA (2010) Interaction and dimerization energies in methyl-blocked r,γ-peptide nanotube segments. J Phys Chem B 114:4973–4983
García-Fandiño R, Granja JR, D’Abramo M, Orozco M (2009) Theoretical characterization of the dynamical behavior and transport properties of α,γ-peptide nanotubes in solution. J Am Chem Soc 131:15678–15686
Jishi RA, Flores RM, Valderrama M, Lou L, Bragin J (1998) Equilibrium geometry and properties of cyclo[(Gly-D-Ala)4] and {cyclo[(Gly-D-Ala)4]}2 from density functional theory. J Phys Chem A 102:9858–9862
Kim H, Jeong K, Lee S, Jung S (2002) Molecular dynamics simulation of cyclosophoroheptadecaose (Cys-a). J Comput Aided Mol Des 16:601–610
Lewis JP, Pawley NH, Sankey OF (1997) Theoretical investigation of the cyclic peptide system cyclo[(d-Ala-Glu-d-Ala-Gln)m=1-4]. J Phys Chem B 101:10576–10583
Takeuchi J, Takeda K (2016) Theoretical study on application of peptide nanoring to chiral recognition of amino acid. Japanese J Applied Phys. doi:10.7567/JJAP.55.03DF09/pdf
Poteau R, Trinquier G (2005) All-cis cyclic peptides. J Am Chem Soc 127:13875–13889
Okamoto H, Nakanishi T, Nagai Y, Kasahara M, Takeda K (2003) Variety of the molecular conformation in peptide nanorings and nanotubes. J Am Chem Soc 125:2756–2769
Shahangi F, Chermahini AN, Dabbagh HA, Teimouri A, Farrokhpour H (2013) Enantiomeric separation of d-and l-lactic acid enantiomers by use of nanotubular cyclicpeptides: a DFT study. Comput Theor Chem 1020:163–169
Zhu Y, Zhao H, Liu C, Wei D, Li X, Li S, Tang M (2014) DFT studies on inclusion complexes of 1-phenyl-1-propanol enantiomers with modified cyclic decapeptides. Struct Chem 25:699–705
Zhao H, Zhu Y, Tong M, He J, Liu C, Tang M (2012) Density functional theory studies on the inclusion complexes of cyclic decapeptide with 1-phenyl-1-propanol enantiomers. J Mol Model 18:851–858
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Account 120:215–241
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. Account Chem Res 41:157–167
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi MC, Rega JMM, Klene M, Knox JE, Cross JB, Bakken CA, Jaramillo, Gomperts R, Stratmann OY, Austin R, Cammi CP, Ochterski RLM, Morokuma VGZ, Voth GA, P Salvador JJD, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. (2010) Gaussian 09, Revision C.01. Gaussian, Inc., Wallingford.
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
J T, B M, E C (1999) THEOCHEM J Mol Struct 464:211–226
Georgieva P, Himo F (2008) Density functional theory study of the reaction mechanism of the DNA repairing enzyme alkylguanine alkyltransferase. Chem Phys Lett 463:214–218
Ahmed L, Rhaman MM, Mendy JS, Wang J, Fronczek FR, Powell DR, Leszczynski J, Hossain MA (2015) Experimental and theoretical studies on halide binding with a p-xylyl-based azamacrocycle. J Phys Chem A 119:383–394
Turi L, Dannenberg JJ (1993) Correcting for basis set superposition error in aggregates containing more than two molecules: ambiguities in the calculation of the counterpoise correction. J Phys Chem 97:2488–2490
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Case DA, Babin V, Berryman JT, Betz RM, Cai Q, Cerutti DS, Cheatham TE, Darden TA, Duke RE, Gohlke H, et al (2014) AMBER 14. University of California, San Francisco
Lee MC, Duan Y (2004) Distinguish protein decoys by using a scoring function based on a new AMBER force field, short molecular dynamics simulations, and the generalized born solvent model. Proteins 55:620–634
Wang JM, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 55:1157–1174
Zhu Y, Tong M, Liu C, Song C, Wei D, Zhao Q, Tang M (2014) Molecular dynamics simulations on inclusion complexes for chiral enantiomers with heterocyclic cyclodecapeptide. Comput Theor Chem 1027:46–52
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21001095 and J1210060), University Key Research Programs of Department of Education in Henan Province (No. 15A150082 and 14A150033), and Undergraduate Innovative Training Program of Zhengzhou University (2016xjxm264).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Figure S1
Electrostatic potential maps for the host molecules of CDP, I-CDP, E-CDP and H-CDP calculated at the B3LYP/6–311++G(d,p) level of theory (red = less positive potential, blue = more positive potential). (DOCX 597 kb)
Rights and permissions
About this article
Cite this article
Li, X., Zhu, Y., Liu, C. et al. Molecular recognition of cyclodecapeptides to ibuprofen and naproxen enantiomers: a theoretical study. Struct Chem 28, 1631–1644 (2017). https://doi.org/10.1007/s11224-017-0929-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0929-8