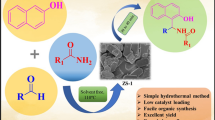
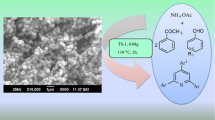
Abstract
Zeolite ZSM-11 catalyst was prepared by hydrothermal method and characterized by FTIR, XRD, SEM, HRTEM, EDS, and BET analysis techniques. The catalyst shows good catalytic activity toward synthesis of 2,4,5-triarylimidazole derivatives which is prepared by using benzil, aldehyde and ammonium acetate in solvent-free condition. The reaction, one pot synthesis is highly adaptable and eco-friendly and has several merits such as short reaction time, mild reaction conditions, and high yield. The ease of reusability and recovery of catalyst for five consecutive reactions makes this protocol highly suitable.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The imidazoles are one of the most important substructures found in natural products (e.g., histidine, biotin, histamine and pilocarpine alkaloids), agrochemical (e.g., herbicide, fungicide, plant growth regulators), pharmacologically active compounds (e.g., analgesic, anti-inflammatory, antibacterial, antitumor activities, anti-thrombotic agents, and therapeutic agents), and many substituted imidazoles are extensively used as glucagon receptor antagonist, inhibitors of interleukin (IL)-1, 5-lipoxygenase [1,2,3,4,5,6]. There are several drug moieties with imidazole core such as Trifenagrel, Eposartan, Olmesartan, Losartan, P38 kinase inhibitor (SB 203,580), and cyclooxygenase-2 (COX-2) inhibitor II (Fig. 1) [5, 7].
The N-heterocyclic imidazole family has expanded its applications in various fields such as cosmetics, polymer chemistry, agrochemicals, materials chemistry (optical electronics, dye sensitized solar cells (OLEDS) [2, 4]. Imidazole derivatives are also used in photography as photosensitive compounds and in industry as a corrosion inhibitor of definite transition metals [8].
Generally, synthesis of 2,4,5-trisubstituted imidazole derivatives via three-component cyclo-condensation of 1,2-diketones, diverse aldehyde, and ammonium acetate. Recently, several methods have been described for the synthesis of 2,4,5-triarylimidazole derivatives by using microwave, ionic liquids [9], InCl3.3H2O [10], AcOH [11] PEG-400 [12], DABCO [13], silicasulfuric acid [14], ytterbium perfluorooctanesulfonate [15], ytterbium trifoliate [16], H2SO4 [17], Oxalic acid [18], and P-toluene sulfonic acid [19]. Most of these synthetic methods suffer from one or more consequential drawback such as long duration, harsh reaction conditions, poor yields, complex workup, prolonged time periods, occurrence of side reactions leading to mixture of products, hazardous or expensive acid, moisture-sensitive catalysts, excessive reagents or catalyst. Eco-friendly and effective approach for the synthesis 2,4,5-triarylimidazole derivatives is still in the demand due to increasing market value of imidazole moieties.
In this sense, ZSM-11 zeolite catalysts have various advantages, like reusability, environmental acceptability, and easy separation of products. It exhibits excellent catalytic properties in some reactions such as higher paraffin hydro-isomerization [20], dehydration of glycerol to acrolein, [21] catalytic pyrolysis of heavy oil aromatization, and isomerization of 1-hexene [22]. ZSM-11 zeolites are also well-known heterogenous catalysts and have been extensively utilized in the production of petrochemicals, they have unique structures, high thermal stability, and shape selectivity [23] to enhance demand in organic transformations. It was prepared by kokotailo et al. [24, 25] in 1978 with tetrabutyl ammonium (TBA) as the structure-directing agent (SDA). ZSM-11 is one type of high-silica zeolite with MEL topology [26].
Herein, in the present work, to overcome all limitations in the synthesis of 2,4,5-triarylimidazole derivatives, we developed safe, efficient, reusable, and eco-friendly ZSM-11 catalyst. We also describe a sustainable, efficient, cost-effective, and excellent protocols for the synthesis of 2,4,5-triarylimidazole derivatives using benzil, diverse aldehyde and ammonium acetate as an ammonia source under solvent-free condition in the presence of ZSM-11 catalyst. It observed that the catalyst shows strong catalytic activity toward synthesis of 2,4,5-triarylimidazole derivatives under solvent-free condition.
Experimental
Chemicals and methods
All the chemicals were purchased by Sigma-Aldrich chemical company with high purity without further purification. Solvents were distilled and purified by standard procedure and used in reaction. Several characterization techniques have been done, using calibrated instruments, including powder X-ray diffraction (XRD) using a Bruker D8-Advanced diffractometer using Cu Kα radiation between 5° and 80° (2θ values). The FTIR spectra (Fourier transform infrared spectra) were revealed using a Shimadzu FTIR 8300 spectrophotometer, and element composition (EDS) was recorded on Objects 8724. The specific surface area and pore volume were calculated on Quantachrome instruments version 3.01 by Brunauer–Emmett–Teller (BET) method. Morphology of the zeolite samples was characterized by FE-SEM on Nova Nano SEM NPEP303 and high-resolution transmission electron microscopy (HRTEM) images were obtained using a JEOL JEM 2100 Plus microscope. Reactions were indicated by thin-layer chromatography (TLC). The 1H NMR (400 MHz) and 13C NMR (101 MHz) were run on a Bruker (400 MHz) spectrometer using tetramethyl silane (TMS) as an internal reference. All reactions were carried out using a laboratory assembly of oil bath with magnetic stirrer. Melting points were measured in open capillary tube.
Catalyst preparation
Synthesis of ZSM-11 catalyst was prepared by using hydrothermal pathway as shown in Scheme 1. In first step, the mixture of 23 ml of TEOS (Tetra ethyl orthosilicate) as a silica source, 20 ml of alcohol, and 20 ml of distilled deionized water were stirred vigorously to form silicate solution. In second step, 1.73 gm of Al(NO3)3 (aluminum nitrate) as an alumina source was taken in 20 ml of distilled deionized water and 27 ml of TPAOH (tetrapropyl ammonium hydroxide) as a structure-directing agent as well as base was added for maintaining pH 12 with vigorous stirring to form alumina solution. The solution was mixed by dropwise addition with vigorous stirring, the mixture was then stirred for 30 min, which resulted in transparent viscous gel and was transferred in a Teflon-lined stainless-steel autoclave and closed tightly for hydrothermal treatment at 175 °C for 48 h. Solid product formed was washed with distilled water for several times and dried in an oven at 120 °C for 2 h and finally the product was calcined at 550 °C for 6 h in a muffle furnace and then cooled. The catalyst was characterized and then utilized in the synthesis of 2,4,5-triarylimidazole derivatives.
Synthesis of 2,4,5-triarylimidazole derivative
The suspension of benzil (1 mmol), benzaldehyde (1 mmol), and ammonium acetate (3 mmol) were stirred in oil bath at 110 °C for 30 min under solvent-free condition using cold water condenser in presence of ZSM-11 (0.05 gm) as a catalyst, progress of reaction was monitored by TLC using petroleum ether/ethyl acetate (6:4) as a solvent system. Completion of reaction was indicated by disappearance of starting materials on the TLC spot. The mixture was cooled, and 5 ml of acetone was added in reaction mixture for the separation of catalyst by filtration. The catalyst was separated and then activated at 110 °C in oven for 30 min, and crude product was poured on ice cold water. The obtained precipitate was washed and recrystallized in ethanol to offer pure product. We applied same procedure for the synthesis of the other derivatives of model reaction, which is shown in Table 1.
Spectral data of represented compound
2-(4-Nitrophenyl)-4,5-diphenylimidazole (4e)
Yellow solid; m.p.: 230–232 °C, Lit.14, 232–33 °C, IR (υmax cm−1): 1331 and 1508 (NO2), 1591 (C=N), 3383 (NH) cm−1. 1H NMR (400 MHz, DMSO-d6, δ ppm) 13.15 (s, 1H), 8.42 – 8.21 (m, 4H), 7.54 (d, 4H), 7.49–7.20 (m, 6H). 13C NMR (101 MHz, DMSO-d6, δ ppm) 146.54, 143.39, 136.11, 128.51, 127.18, 125.74, 124.27; HRMS for C21H15N3O2, M+: 342.11.
2-(4-bromophenyl)-4,5-diphenyl-1H-imidazole (4 k)
White solid; m.p.: 258–260° C, Lit.27 m.p.: 254–256 °C; IR (υmax cm−1): 3023 (Ar–H), 1677 (C=N), 1483, 1593 (C=C, Ar), 609 (C–Br). 1H NMR (400 MHz, DMSO-d6, δ ppm) 12.81 (s, 1H), 8.06 (d, 2H), 7.68 (d, 2H), 7.55 (dd, 4H), 7.47–7.18 (m, 6H). 13C NMR (101 MHz, DMSO-d6, δ ppm) 144.50, 137.36, 135.02, 131.67, 130.94, 129.56, 128.66, 128.43, 128.20, 127.86, 127.11, 126.60, 121.41; HRMS for C21H15N2, M+: 375.04.
2-(4-methylphenyl)-4,5-diphenyl-1H-imidazole (4b)
White solid; m.p 231–232 °C, Lit.14 m.p; 230–232 °C IR (υmax cm−1): 3464 (NH), 3037 (Ar–H), 1596 (C=N), 1H NMR (400 MHz, DMSO-d6, δ ppm) 12.60 (s, 1H), 7.97 (d, 2H), 7.54 (d, 2H), 7.50 (d, 2H), 7.44 (d, 2H), 7.37 (d, 1H), 7.30 (d, 4H), 7.21 (d, 1H), 2.35 (s, 3H). 13C NMR (101 MHz, DMSO-d6, δ ppm) 145.65, 137.68, 136.91, 135.25, 131.15, 129.25, 128.64, 128.41, 128.17, 127.93, 127.68, 127.05, 126.46, 125.16, 20.89; HRMS for C22H18N2, M+: 311.15.
4-(4,5-diphenyl-1H-imidazol-2-yl) phenol (4 g)
Orange solid; m.p. 256–258 °C, Lit. 2, 254–256 °C IR (υmax cm−1): 3461 (NH), 3033 (Ar–H) 1603 (C=N). 1H NMR (400 MHz, DMSO-d6, δ ppm) 12.49 (s, 1H, NH), 9.79 (s, 1H, OH), 7.95 (d, 2H), 7.54 (d, 4H), 7.42–7.22 (m, 6H, Ar–H), 6.90 (d, 2H). 13C NMR (101 MHz, DMSO-d6, δ ppm) 157.87, 146.17, 128.43, 127.72, 126.95, 121.71, 115.50; HRMS for C21H16N2O, M+: 313.13.
2-(4-fluorophenyl)-4,5-diphenyl-1H-imidazole (4j)
White solid; m.p. 190–191 °C, Lit. 14, 188–190 °C IR (υmax cm−1): 3050 (Ar–H), 1603 (C=N), 1540 (C=C). 1H NMR (400 MHz, DMSO-d6, δ ppm) 12.72 (s, 1H), 8.14 (dd, 2H), 7.54 (dd, 4H), 7.44 (d, 2H), 7.34 (m, 5H, Ar–H), 7.25–7.20 (d, 1H). 13C NMR (101 MHz, DMSO-d6, δ ppm) 163.35, 160.91, 144.71, 137.08, 135.12, 131.05, 128.67, 128.41, 128.26, 128.19, 127.79, 127.37, 127.29, 127.07, 127.02, 126.54; HRMS for C21H15 FN2, M+: 315.12.
5-triphenyl-1H-imidazole (4a)
White solid; m.p.: 271–273 °C, Lit.28 m.p.: 270–272 °C; IR (υmax cm−1): 3034 (Ar–H), 1587 (C=N), 1444, 1587 (C=C aromatic). 1H NMR (400 MHz, CHLOROFORM-D/ DMSO-D6, δ ppm) 8.33–7.87 (d, 2H), 7.52 (d, 4H), 7.36 (dd, 2H), 7.26 (m, 5H, Ar–H), 7.20 (d, 2H). 13C NMR (101 MHz, CHLOROFORM-D/ DMSO-D6, δ ppm) 146.37, 130.49, 128.58, 128.37, 128.27, 128.14, 127.08, 127.05, 125.63; HRMS for C21H16N2, M+: 297.14.
Result and discussion
Characterization of catalyst
Synthesis of ZSM-11 catalyst by hydrothermal method was done and then after that the calcination was carried out at 550 °C. The weight of catalyst decreased from the actual weight, due to the loss of surfactant and moisture. Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), scanning electron microscopy (FESEM), Brunauer–Emmett–Teller (BET) method, energy-dispersive spectrometry (EDS), and high-resolution transmission electron microscopy (HRTEM) of samples were collected to investigate the effect of morphology on the framework of product zeolites. As shown in Fig. 2a, the samples revealed characteristic absorption bands of ZSM-11 catalyst at 445, 547, 800, 1066 and 1250 cm−1. The absorption bands at 1250, 1066, 1022 and 800 cm−1 could be assigned to the external asymmetric, internal asymmetric, silanol group [O3Si–OH], and external symmetric stretching of Si–O–T linkage in ZSM-11 framework, respectively. The bending vibration of TO4 (T = Si or Al) in ZSM-11 framework was recorded at around 445 cm−1, while the vibration band at around 547 cm−1 indicated the presence of the double five rings of the characteristic structure of pentasil family zeolite (MEL) [27]. Particularly, due to broad peak from 900 to 1300 the weak absorption band related to [O3Si–OH] units and external asymmetric stretching of Si–O–T linkage showed no defined absorption but showed on intense neighboring band at 1066 cm−1. It was postulated that the close stack of nanocrystals resulted in the disappearance of surface [O3Si–OH] units and particle size of zeolites decreased to some extent (Fig. 2a) [22]. It is interesting that ZSM-11 type zeolite shows intense band at near 550 cm−1 whose position changes with aluminum content. Nevertheless, the absence of the FTIR band around 550 cm−1 definitely indicates that ZSM-11 framework type zeolite is not present in the sample [22, 28].
Crystallinity of catalyst is conformed by X-ray diffraction analysis using Cu Kα radiation (λ = 1.5406 A°). Generally, it is very difficult to prepare pure-phase ZSM-11 without any ZSM-5 phase because of the similarities in topology between both types of zeolites. The appearance of doublet peaks in the range between 23° and 25° and a single reflection peak at 2θ of 45.4° demonstrated the absence of ZSM-5 intergrowth in ZSM-11 particles [22, 27]. International zeolite association data also confirm the synthesized zeolite sample near to the ZSM-11 zeolite type and MEL topology in which the highest intensity double peak was observed in between 2θ° value 23° to 25° and one single peak observed at 45.4° are used to distinguish MEL from that of the MFI topology [29]. The intense peak at 23.30° is near about ZSM-11-type phase with MEL topology [30]. which is shown in Fig. 1b. Peak shows at 2θ° 8.06, 9.01, 14.95, 23.30, 24.01, 45.4 with corresponding plane (100), (011), (112), (221), (031), (522) respectively, which confirm the ordered orthorhombic framework and having ZSM-11 type phase with MEL topology. There is slightly differences observed in the 2θ° value of reported ZSM-11 due to alkyl chains of TPAOH which give rise to an slightly different zeolitic framework [31].
Scanning electron microscopy (FESEM) of material was used to determine surface morphology and crystal size with different magnification and micrograph, which is presented in Fig. 5a and b. FESEM image suggest that aluminum is agglomerated in silicate framework which has irregular sheet type shape. Structure and morphology of catalyst determined by high-resolution transmission electron microscopy (HRTEM) show that most of the particles are irregular spherical and their diameters vary between 5 and 20 nm. Figure 5c reveals that these particles were constructed by many primary nanocrystals of 5–10 nm. HRTEM micrographs and microdiffraction have shown that each grain of sample 100 nm has formed by the aggregation of very small zeolite particles (5–10 nm) [28]. It can also be seen that some mesopores appear on the surface and edge of ZSM-11 crystals. The typical coffin-shape morphology of the ZSM-11 zeolite observed [32]. Figure 5d show that the lattice fringes of several adjacent nanorods in the particles were completely parallel in the same direction but some particles were in slightly different direction, demonstrating their highly oriented and ordered intergrowth structure. The interfringe distances of the representative ZSM-11 nanorods were measured to be between 1 and 2 nm. The crystal morphology shown in HRTEM of 10 and 20 nm are nearly similar to the FESEM images of several parallel single-crystalline-like matrices [31]. We know the elemental composition of material from EDX analysis. Figure 3 shows material having oxygen 49.17%, silicon 46.65%, and aluminum 4.18% and EDX composition elemental mapping of ZSM-11 material shown in Fig. 6. The catalyst was characterized by using BET and BJH methods. Pore size distribution was analyzed by using N2 adsorption–desorption measurement. Total surface area, pore volume, and pore radius observed were 369.791 m2/g, 7.625e-01 cc/g, and 4.12377e + 01 Å, respectively. Furthermore, the micropore and external surface area of catalyst were 288.388 and 349.937 m2/g, respectively. According to the IUPAC classification N2 adsorption–desorption isotherms of the ZSM-11 zeolites, Fig. 4 are type IV in nature with H1-type hysteresis loop [33]. The nitrogen adsorption isotherm shows a steep regular increase at a low relative pressure and a hysteresis loop from P/P0 = 0.45 to about P/P0 = 1 which demonstrate the coexistence of micropores and intercrystal mesopores [22, 32, 34] (Figs. 5, 6).
Optimization of catalyst
Model reaction are monitored at various temperature, amount of catalyst and solvent at 110 °C under solvent-free condition, which shown in Table 2. We have compared our results with results of some other solvent for the synthesis of 2,4,5 triaryl imidazole and concluded that the solvent like water, ethanol, chloroform, and DMF did not give better yield as compared to the solvent-free condition of model reaction. Again, we checked the catalytic activity with diverse temperature and found that best results are obtained at 110 °C in solvent-free condition, further increase in temperature up to 120 °C did not show any improvement in product yield. The model reaction did not proceed in the absence of catalyst even after 240 min, which was shown in Table 2. Also, we optimized the actual amount of catalyst that gives better yield. According to Table 2, as amount of catalyst increased, the percentage of 2,4,5-triarylimidazole derivatives also increased due to the presence of more active sites on the catalyst, and it was found that 0.05 gm catalyst gave better yield within short reaction time in solvent-free condition. There were no further increased in yield after increasing amount of catalyst (0.05 gm). We used different aldehyde as a substrate for this model reaction in presence of ZSM-11 catalyst under solvent-free condition, and the results are summarized in Table 1. ZSM-11 catalyst is more effective for the wide variety of aldehyde with corresponding imidazole derivative with excellent yield, easy workup, and short reaction time.
Again, we have compared our catalyst to other reported catalyst such as acetic acid, Montmorillonite K10, Polymer-ZnCl2, Nano-SnCl4-SiO2, CAN and Yb (OTf)3 in the 2,4,5-triarylimidazole derivatives, which is shown in Table 3. Therefore, after optimization of reaction condition, we clearly demonstrate that the solvent-free synthesis of 2,4,5-triarylimidazole derivatives in presence of ZSM-11 (0.05 gm) catalyst at 110 °C was a best reaction condition.
A mechanism of catalytic activity of ZSM-11 catalyst in synthesis of 2,4,5-triarylimidazole derivatives is shown in Scheme 2. Carboxyl groups of benzil and aldehydes (1) are activated by ZSM-11 catalyst. Then nucleophilic attack of the nitrogen of ammonia, which was formed from ammonium acetate, on the activated carbonyl group of benzil and aldehydes resulted in the formation of α-imino ketone intermediate (3) and aryl aldimine intermediate (4), then followed by the nucleophilic attack of aryl aldimine intermediate (4) to the carbonyl carbon of α-imino ketone (3), give up the intermediate (5). After the cyclization, formation of intermediate (6) turns to 2,4,5-triarylimidazole derivatives by dehydration.
Catalyst activation and reusability
Solid ZSM-11 catalyst is insoluble in most of the solvent, so it can be easily separate out from reaction mixture by filtration with the help of suction pump, recovered catalyst washed with acetone or any other polar solvent for 2–3 times. For activation, transfer washed recovered catalyst under vacuum-oven for 30 min at 110 °C and recovered catalyst was used for five runs without any further treatment in subsequent reaction and it was found that catalyst almost have consistent activity. No observation of any appreciable loss in catalytic activity (Fig. 7).
Conclusion
In this work, we have successfully synthesized ZSM-11 catalyst and developed easy and efficient three-component method to prepare a variety of 2,4,5-triarylimidazole derivatives in the presence of ZSM-11 catalyst in solvent-free condition. We have demonstrated that the ZSM-11 is a potential alternative to the use of other catalyst for efficient synthesis of wide range of 2,4,5-triarylimidazole derivatives. This procedure has many obvious advantages compared to those reported in the previous studies, including the avoidance of discharging harmful catalysts, low reaction temperature, high yield of products, simplicity of the methodology, recovery, and reusability of ZSM-11 catalyst for five consecutive runs. The notable merits offered by this methodology are mild reaction conditions, easy workup, clean reaction profiles, good agreement with the clean protocols, and being solvent-free, and this renders the approach as an interesting alternative to existing methods. Furthermore, products were isolated in excellent yield which significant regarding environmentally benign processes.
References
S. Dehghan Khalili, S.H. Banitaba, J. Safari, Sci. Iran. 20, 1855 (2013)
M. Esmaeilpour, J. Javidi, M. Zandi, New J. Chem. 39, 3388 (2015)
H. Naeimi, D. Aghaseyedkarimi, New J. Chem. 39, 9415 (2015)
J. Jayram, V. Jeena, Green Chem. 19, 5841 (2017)
A. Shaabani, R. Afshari, S.E. Hooshmand, New J. Chem. 41, 8469 (2017)
Y. Chen, R. Wang, F. Ba, J. Hou, A. Ding, M. Zhou, H. Guo, J. Saudi Chem. Soc. 21, 76 (2017)
A. Shaabani, A. Maleki, M. Behnam, Synth. Commun. 39, 102 (2009)
J. Jayram, V. Jeena, RSC Adv. 8, 37557 (2018)
S.A. Siddiqui, U.C. Narkhede, S.S. Palimkar, T. Daniel, R.J. Lahoti, K.V. Srinivasan, Tetrahedron 61, 3539 (2005)
J. Wang, R. Mason, D. VanDerveer, K. Feng, X.R. Bu, J. Org. Chem. 68, 5415 (2003)
S. Sarshar, D. Siev, A.M.M. Mjalli, Tetrahedron Lett. 37, 835 (1996)
X.C. Wang, H.P. Gong, Z.J. Quan, L. Li, H.L. Ye, Chinese Chem. Lett. 20, 44 (2009)
S.N. Murthy, B. Madhav, Y.V.D. Nageswar, Tetrahedron Lett. 51, 5252 (2010)
A. Shaabani, A. Rahmati, J. Mol. Catal. A Chem. 249, 246 (2006)
M.G. Shen, C. Cai, W. Bin Yi, J. Fluor. Chem. 129, 541 (2008)
L.M. Wang, Y.H. Wang, H. Tian, Y.F. Yao, J.H. Shao, B. Liu, J. Fluor. Chem. 127, 1570 (2006)
H. Weinmann, M. Harre, K. Koenig, E. Merten, U. Tilstam, Tetrahedron Lett. 43, 593 (2002)
N.D. Kokare, J.N. Sangshetti, D.B. Shinde, Synthesis (Stuttg). (2007). https://doi.org/10.1055/s-2007-983872
M.M. Khodaei, K. Bahrami, I. Kavianinia, J. Chinese Chem. Soc. 54, 829 (2007)
T.L.M. Maesen, M. Schenk, T.J.H. Vlugt, B. Smit, J. Catal. 203, 281 (2001)
Y. Gu, N. Cui, Q. Yu, C. Li, Q. Cui, Appl. Catal. A Gen. 429–430, 9 (2012)
Q. Yu, C. Cui, Q. Zhang, J. Chen, Y. Li, J. Sun, C. Li, Q. Cui, C. Yang, H. Shan, J. Energy Chem. 22, 761 (2013)
L. Zhang, H. Liu, X. Li, S. Xie, Y. Wang, W. Xin, S. Liu, L. Xu, Fuel Process. Technol. 91, 449 (2010)
P.M. Piccione, M.E. Davis, Microporous Mesoporous Mater. 49, 163 (2001)
G.T. Kokotailo, P. Chu, S.L. Lawton, W.M. Meier, Comptes Rendus Chimie 275, 119 (1978)
X. Wang, F. Meng, H. Chen, F. Gao, Y. Wang, X. Han, C. Fan, C. Sun, S. Wang, L. Wang, Comptes Rendus Chim. 20, 1083 (2017)
K.P. Dey, S. Ghosh, M.K. Naskar, Ceram. Int. 39, 2153 (2013)
G. Coudurier, C. Naccache, J.C. Vedrine, J. Chem. Soc. Chem. Commun. (1982). https://doi.org/10.1039/c39820001413
W. Song, Z. Liu, L. Liu, A.L. Skov, N. Song, G. Xiong, K. Zhu, X. Zhou, RSC Adv. 5, 31195 (2015)
M.M.J. Treacy, J.B. Higgins (eds.), Collection of Simulated XRD Powder Patterns for Zeolites, 5th edn. (Amsterdam, Elsevier, 2007), p. 477. https://doi.org/10.1016/B978-0-444-53067-7.X5470-7
K. Shen, N. Wang, X. Chen, Z. Chen, Y. Li, J. Chen, W. Qian, F. Wei, Catal. Sci. Technol. 7, 5143 (2017)
J. Yang, S. Yu, H. Hu, Y. Zhang, J. Lu, J. Wang, D. Yin, Chem. Eng. J. 166, 1083 (2011)
S. S. Lapari, Z. Ramli, S. Triwahyono, 2015 (2015).
H. Chen, X. Zhang, J. Zhang, Q. Wang, RSC Adv. 7, 46109 (2017)
M. Kidwai, P. Mothsra, V. Bansal, R. Goyal, Monatshefte fur Chemie 137, 1189 (2006)
S. Balalaie, A. Arabanian, M.S. Hashtroudi, Monatshefte fur Chemie 131, 945 (2000)
H.D. Hanoon, S.M. Radhi, S.K. Abbas, A.I.P. Conf, Proc. 2144, 1 (2019)
V.S.V. Satyanarayana, A. Sivakumar, Chem. Pap. 65, 519 (2011)
A.A. Marzouk, V.M. Abbasov, A.H. Talybov, S.K. Mohamed, World. J. Org. Chem. 1, 6 (2013)
J. Safari, Z. Zarnegar, Ultrason. Sonochem. 20, 740 (2013)
G.V.M. Sharma, Y. Jyothi, P.S. Lakshmi, Synth. Commun. 36, 2991 (2006)
A. Teimouri, A.N. Chermahini, J. Mol. Catal. A Chem. 346, 39 (2011)
H.D. Hanoon, E. Kowsari, M. Abdouss, M.H. Ghasemi, H. Zandi, Res. Chem. Intermed. 43, 4023 (2017)
L. Wang, C. Cai, Monatshefte fur Chemie 140, 541 (2009)
B.F. Mirjalili, A. Bamoniri, M.A. Mirhoseini, Sci. Iran. 20, 587 (2013)
Acknowledgements
One of the authors, Mr. Sudarshan S. Dipake, gratefully thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi for the award of fellowship and to the Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431004 (M.S.), India. for support and providing the necessary laboratory facility.
Author information
Authors and Affiliations
Contributions
SSD-Conduct the whole experiment, Writing original draft. MKL-Review and editing. ASR-Review and editing. STG-Principal author.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Consent to participate
All Authors are agreed for submission.
Consent for publication
Agreed to submission.
Data availability
Manuscript including all data correct and unpublished.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dipake, S.S., Lande, M.K., Rajbhoj, A.S. et al. Zeolite ZSM-11 as a reusable and efficient catalyst promoted improved protocol for synthesis of 2,4,5-triarylimidazole derivatives under solvent-free condition. Res Chem Intermed 47, 2245–2261 (2021). https://doi.org/10.1007/s11164-021-04423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04423-9