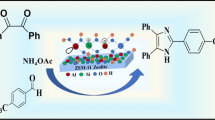
Abstract
Efficient and reusable ZS-1 zeolite as a novel catalyst was successfully synthesized by the hydrothermal method. Characterizations of catalysts were carried out using diverse analysis techniques such as XRD, FT-IR, FESEM, EDAX, HRTEM, BET, and NH3TPD. Promoted as an environment-friendly protocol for the facile synthesis of various substituted 1-amidoalkyl-2-naphthols through the reaction of β-naphthol, aldehyde, and amides under solvent-free conditions. This catalyst provides many advantages such as shorter reaction times, operational simplicity, reusability, an excellent yield of the product, facile work-up, and easily recoverable. Moreover, the recovered catalyst can be recycled and reused for the next five runs without significant loss of catalytic activity.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The production of pesticides, pharmaceuticals, and petrochemicals on large scale is responsible for chemical pollution in the development of green chemistry [1]. Nowadays, the development of eco-friendly technologies is the most laborious task in the contemporary chemistry and chemical industry. With these objectives, reducing the wastes together with the use of renewable feedstocks, environmentally harmless solvents, reagents, and effectively recoverable catalysts are an important guideline to attain more sustainable approaches according to the green chemistry principles [2,3,4].
In this aspect, heterogenous catalysts have been of great importance, they can be reused, cheap and one of the great advantages is that they avoid the environmental contamination related to commercial catalysts, such as H2SO4, HF, AlCl3, BF3, and H3PO4. Heterogeneous catalysis has emerged as a useful parameter to reduce waste, lower contamination of the products with the active catalytic species, avoiding toxic solvents, efficient separations, and recycling of the catalysts [5,6,7,8]. Zeolite is a heterogeneous catalyst; current zeolite catalysis research has focused on the synthesis of SBA and MCM types of mesoporous silica. Unfortunately, these mesoporous materials have low thermal stability, poor surface acidity, and restrictive application as a catalyst or support. Literature study reports that, the impregnation of transition metals, such as Ti, Zr, V, Fe, Cu, Nb, and Mo inside the structure of some mesoporous silicates for enhancing the catalytic performance in epoxidation, transesterification, and alcohol dehydration reactions [9, 10]. Zeolites establish a family of crystalline microporous aluminosilicates will promote many applications in a wide variety of fields in science and technology [11,12,13,14]. However, broadened the application of zeolite as a heterogeneous catalyst, isomorphic substitution of isolated zirconium, niobium, and tantalum metals species in tetrahedral coordination into the framework of silicate confers lewis acid character to these metal sites [15]. The compositions of Zirconium silicate are also constructed to remove toxins, e.g., ammonium ions or potassium ions from the gastrointestinal tract at an elevated rate without causing unwanted side effects and also useful in the therapeutic reagent for the treatment of hyperkalemia [16]. Impregnated zirconia-containing material with high surface area and acidity was used as a catalyst in several catalytic organic transformation reactions [17]. Among various techniques of green chemistry, multicomponent reactions (MCRs) have been very classic and efficient methods in modern synthetic chemistry. The Discovery and development of novel and known MCRs are highly compatible with the aims of sustainable and green chemistry. One-pot multicomponent reaction (MCRs) under solvent-free condition attracted as a new approach for the synthesis of biologically active molecules, without the isolation of any intermediates and thus reducing reaction time with energy resulting in atom economy and high selectivity [18, 19].
The synthesis of amidoalkyl naphthols derivatives is an attractive example of these MCRs. 1-Amidoalkyl-2-naphthols derivatives bearing 1,3-amino oxygenated functional groups have gained remarkable attention due to their immanent role as essential building blocks for a variety of biologically active natural products, potent drugs, and synthetic pharmaceuticals including several nucleoside antibiotics and HIV protease inhibitors [20]. In literature survey reveals that the established method for the preparation of amidoalkyl naphthols has been carried out by a three-component condensation of diverse aldehydes, 2-naphthols and amides in presence of a variety of catalyst such as Ba3(PO4)2 [21], graphene oxide [22], sulfonated poly naphthalene [23], supported heteropolyacid [24], b-CD-BSA [25], MSNs-HPZ-SO3H [26], phosphoric acid [27], NanoAl2O3 [28], p-TSA [29], NaHSO4.SiO2 [30], and SiO2-HClO4 [31]. However, some of these reported procedures are not eco-friendly and suffer from one or more disadvantages yet, for example, prolonged reaction time, use of volatile organic solvent, additional ultrasonic irradiation, tedious work-up, by-products, and use of toxic, highly acidic, expensive catalysts. Therefore, it is desirable to introduce clean procedures, and utilizing eco-benign reusable heterogeneous catalyst for the synthesis of 1-amidoalkyl-2-naphthols derivatives is an alternative procedure to construct these valuable organic compounds is still in high demand.
Herein, to achieve these objectives and continuation our effort in the synthesis of novel catalysts and their efficiency in organic synthesis, we have developed a synthetic strategy to design zirconium silicate and demonstrated its effectiveness as an efficient catalyst for the synthesis of 1-amidoalkyl-2-naphthols via a three-component reaction of b-naphthol, various aldehydes, and amides under solvent-free conditions. This novel highly reusable and efficient catalyst exhibited excellent catalytic activity towards the synthesis of 1-amidoalkyl-2-naphthols (Scheme 1) and it possesses several promising features such as high surface area, good thermal stability, easy handling, easy work-up, shorter reaction times, and easy purification. The catalyst can be reused without any significant loss in catalytic efficiency for five consecutive runs of model reaction.
2 Experimental Section
2.1 Materials and Instruments
All chemicals included Tetraethylorthosilicate (TEOS, wt.%, 99.9), Tetra propyl ammonium hydroxide (TPAOH, ~ 40 wt.% in water), anhydrous alcohol (EtOH, wt.%, 99%), Zirconium nitrate (Zr (NO)3), Acetone (marked as ACT, wt.%, 99.5%), The used deionized water (DI water, 18.2 MΩ) was Lab made and all required organic regent were purchased from Merck and Sigma-Aldrich and used without further purification. All the yield refers to an isolated product after purification. The NMR spectra were recorded on a Bruker Advance DPX 400 MHz instrument, the spectra were measured in DMSO-d6 relative to TMS (0.00 ppm), The chemical shifts are indicated in parts per million (ppm) and tetramethyl silane (TMS) was used as an internal reference. The powder X-ray diffraction patterns were measured with a Bruker D8 Advance diffractometer using Cu-Ka irradiation. FE-SEM was taken by a Hitachi S-4160 photograph to examine the shape and size of Zirconium silicate zeolite, Elemental composition was recorded on Energy Dispersive spectroscopy (EDS), High-resolution transmission electron microscopy (HRTEM) were recorded on JEOL JEM 2100 Plus, Fourier Transform Infrared (FTIR) Spectroscopy were recorded on Shimadzu, the specific surface area of catalyst was determined according to the Brunauer–Emmett–Teller method while average pore diameters, acidic sites of catalyst calculated by NH3-TPD and total surface area were measured using the BJH method.
2.2 Catalyst Preparation
ZS-1 (zirconium silicate) zeolites were synthesized by using a hydrothermal method, which is shown in scheme 2. Initially, mixed hydroxy precipitates were obtained by hydrolysis of tetraethylorthosilicate (TEOS) and zirconium nitrate salt solutions using tetra propyl ammonium hydroxide (TPAOH) as a mild hydrolysing agent as well as a structure-directing agent., In a typical procedure, required amount of 0.57 g zirconyl nitrate as a zirconia source, 50 ml water and 27 ml of TPAOH as a structure-directing regent were mixed. The resulting solution was stirred until a clear solution was obtained at room temperature. Subsequently, 26 ml of TEOS as a silica source and 20 ml of ethanol were stirred for 30 min. Finally, the TEOS solution and zirconia solution was mixed dropwise with vigorous stirring. After that desired volume of TPAOH was added until the pH value became 10–12. The suspension was stirred until the mixture was completely clarified. After aging, the above suspension was transferred in a Teflon-lined autoclave for 24 h. at 175 °C for hydrothermal treatment. After the crystallization was completed, the crystals were cooled. The obtained white solid product was filtered, subsequently, washed several times with a large amount of DI water until the pH was 8. The obtained solid product was then dried in a hot air oven for 2 h at 110 °C and calcined at 550 °C for 5 h, suspension was prepared for later use.
2.3 General Procedure for the Synthesis of 1-amidoalkyl-2-naphthols
The reaction was carried out at 1 mmol level for the aryl aldehyde, benzamide\acetamide (1.3 mmol), b-naphthol (1 mmol) in the presence of zirconium silicate catalyst was stirred at the appropriate time in a 25 ml round bottom flask (R.B) in an oil bath at 110 °C under solvent-free condition. Progress of the reaction was monitored by thin-layer chromatography (TLC) with ethyl acetate: ether (4: 6) as a solvent system. Complete disappearance of starting material within 30 min was observed by TLC spots. After the completion, the reaction mixture was cooled to room temperature. 10 ml acetone was added to the reaction mixture and the catalyst was separated by filtration, the contents were poured onto the crusted ice and the product was precipitated with ice-cold water. The precipitate was collected by vacuum filtration and washed with ice-cold ethanol under vacuum to remove residual impurities. The crude product was recrystallized in hot ethanol for affording the desired 1-amidoalkyl-2-naphthols derivative with a good yield. Later on, the separated catalyst was washed several times with acetone and transferred in an oven for 2 h at 200 °C for activation. The further activated catalyst was used for the next four cycles for Scheme 1.
3 Catalyst Characterizations
The FTIR spectra of the as-synthesized hierarchical ZS-1 zeolite samples were obtained in the range of 1300–400 cm−1 which is shown in Fig. 1. All the peaks exhibited the typical MFI framework topology of zeolite. The IR spectra of the synthesized sample clearly showed the characteristic absorption bands at 1662, 1226, 1068, 798, 626, 547, 428, and 401 cm−1 which were assigned to different vibrations of tetrahedral and framework atoms in the ZS-1 zeolite. The well-defined IR bands at 798 and 1226 cm−1 region are characteristic of symmetric and asymmetric stretching vibration of T–O respectively, (where T is Si or Zr) while the vibrational band at 547 cm−1confirms the presence of a double five-ring member of pentasil structure [32, 33]. The sharp absorption band appearing at 798 cm−1 is ascribed to Si–O–Si symmetric stretching vibration band. The bands at 1226 cm−1 have been assigned to the internal asymmetric stretching vibration of Si–O–T linkage respectively and band at 1068 cm−1 was attributed to the asymmetric stretching vibration or formation of Si–O–Zr bonds. [32, 34]. The band around 1068 cm−1 is assigned to the SiO4 asymmetric vibrations of internal tetrahedra and the band found around 820–780 cm−1 corresponds to the symmetric stretching of a T-O. The bands appearing at 626, 563, and 428 cm−1 may be due to the vibrations of the Zr-O bending mode. [35, 36]
The powder X-ray diffraction patterns of ZS-1 zeolite synthesized by a hydrothermal method are shown in Fig. 2. The synthesized materials exhibit the characteristic X-ray diffraction pattern of the ZSM-5 type and MFI topology with the peaks at 2θ = 7.9°, 8.88°, 14.83°, 23.15°, 23.96°, 24.62°, and 29.95° that are associated with (001), (010), (200), (220), (022), (221) and (400) planes, respectively [32, 33, 37]. According to international zeolite association data (IZA), ZSM-5 type zeolite falls under MFI topological structure. The intense peak of ZS-1 observed at 2θ = 7.9° is very close to the IZA standard data value [38] in Fig. 2. the ZS-1 sample showed characteristic two diffraction peaks between 6 and 10° (2θ) and three diffraction peaks between the range of 22–25° (2θ) indicating the MFI topological structure [39, 40]
Figure 3 provides the N2 adsorption–desorption isotherm of as-synthesized ZS-1 zeolite sample is very close to type IV with an H3 p/po hysteresis loop at a relative pressure (p/po) of 0.01–1.0. Adsorption isotherm at low relative pressure region (p/po < 0.2) corresponds to the N2 filling in the micropores and the hysteresis loop is slightly broad at relative pressure p/po of 0.45–1.0 demonstrate the presence of mesopores, which can be attributed to the intercrystalline voids created by the accumulated materials shown in Fig. 4 HR-TEM image [33, 39]. which was further proved by the as-synthesized ZS-1 zeolite has a BET surface area (355.670 m2/g), micropores pore volume 0.061 cc/g, and average pore diameter (3.36511e+00 nm) respectively.
The morphological characteristic of the ZS-1 zeolite has been studied using FE-SEM and HR-TEM techniques. Figure 4 shows that the crystals are well-dispersed in the ZS-1 zeolite sample. Most large crystals have pseudo-hexagonally prismatic shapes with crystal sizes of 1–5 µm. The high magnification of FE-SEM images (Fig. 4a, b) clearly show that the average length of a large ZS-1 zeolite crystal is about 3.58 µm. and thickness is around 592.9 nm.
To get more details, the ZS-1 sample was studied by High-resolution transmission electron microscopy (HR-TEM). ZS-1 zeolite shows lattice fringes (micropores) with consistent regular orientations over the entire crystal region and some irregular orientations of lattice fringes observed are due to the broken mesopores structure (Marked by red cycle). We can observe that the broad size distribution of mesopores 2.2 nm was precisely formed due to the decomposition of several micropores of 0–1 nm. Based on HR-TEM images Fig. 4c and d results, it can be concluded that the micropores and mesopores were dispersed inside a crystal of ZS-1 zeolite [41,42,43].
EDAX mapping data represent the homogeneous dispersion of Zirconium (red), Silicon (green), and Oxygen (blue) in the framework of synthesized ZS-1 zeolite material which is shown in Fig. 5. The EDAX spectra reveal that the percentage weight of zirconium, silicon, and oxygen elements are 27, 32, and 41 % respectively introduced into the ZS-1 zeolite structure (Fig. 6).
Figure 7 shows the temperature-programmed desorption of ammonia (NH3-TPD) curve of ZS-1 zeolite material. Two peaks in the range between 200 and 600 °C were presented in the NH3-TPD curves. It is postulated that the two different types of acidic sites exist in the framework of ZS-1 zeolite i.e., weak acidic sites and strong acidic sites. The peak range of temperature approximately 200–350 K is due to the presence of weak acidic sites (Bronsted acidic sites), while the peak in between temperature range 420–600 °C is due to the strong acidic sites (Lewis acidic sites) which are present in the ZS-1 zeolite framework.
4 Result and Discussion
4.1 Catalytic Activity of ZS-1 in Synthesis of 1-amidoalkyl-2-naphthols
After characterization of ZS-1, catalytic efficiency was investigated in the exclusive synthesis of the 1-amidoalkyl-2-naphthols analogues, it is a classic example of a three-component reaction. (Scheme 1) Initially, our study was aimed to determine the appropriateness of ZS-1 catalyst for the synthesis of 1-amidoalkyl-2-naphthols by using aldehyde, b-naphthol and benzamide/acetamide preferred as the model reaction. We examined the different rection conditions like effects of diverse catalyst amount, temperature and solvent on model reaction. In order to calculate the appropriate amount of catalyst, we increase the catalyst amount from 0.01 to 0.05 gm and observed that increase the amount of catalyst; available active site of catalyst will be increase and product yield also increase. There is no further increase in percentage yield of product (Table 1) while increase in amounts of catalyst above 0.05 gm. In model reaction we found that 0.05 gm of catalyst amount was sufficient for 95% yield in a 30 min. The reaction does not sufficiently proceed in the absence of a catalyst after 180 min at 110 °C. The temperature effect was studied at various temperatures such as 80, 100, 110 and 120 °C with ZS-1 as a catalyst in a solvent free condition. Model reaction at 80 °C proceeded but with low yield. An enhancement of temperature from 80 to 110 °C showed increase in activity of catalyst which in enhancement of resulted the product yield with significant reduction in reaction time, further increase in temperature did not affect the percentage yield. Therefore, best result was achieved at 110 °C, which was chosen as the optimum reaction temperature (Table 2).
To compare the effects of various solvents concerning reaction time and percentages yield with the solvent-free conditions we have carried out the model reaction in the presence of solvents such as Water, EtOH, PEG, Chloroform, and DMF, almost all the reactions with solvent were less advantageous than the absence of solvent. Hence, solvent-free was regarded as the best condition for model reaction. it is cost-effective and environmentally benign. In all the reactions, pure products were obtained by aqueous quenching followed by filtration, washing with the water, and recrystallization in ethanol as shown in Table 3.
After optimizing the reaction conditions, we examined structurally aromatic aldehydes bearing either electron-withdrawing and electron-donating groups. It is observed that the substrate with electron-withdrawing group took short time with high yield as compared to the electron-donating group.
The efficiency of the present ZS-1 catalyst was compared with some other reported catalysts for the synthesis of 1-amidoalkyl-2-naphthols as summarized in Table 4. ZS-1 showed a much higher catalytic activity concerning the reaction times and product yield. Therefore, Table 4 result suggests that the present protocol with ZS-1 catalyst is much efficient and promising than the previously reported catalytic methods for the synthesis of 1-amidoalkyl-2-naphthols.
The proposed reaction mechanism for the synthesis of 1-amidoalkyl-2-naphthols mediated by ZS-1 catalyst is depicted in Scheme 3 [10, 14]. Considering the nature of the ZS-1 catalyst, it decreases the electron density of the carbonyl group of aldehydes. Intermediate (II) is formed by the nucleophilic attack of b-naphthol to the carbonyl group of activated aldehydes. Then, the ortho-quinone methide (III) intermediate was formed by the condensation of the water molecule. Afterward, ortho-quinone methide (III) intermediate activated by catalyst ZS-1 and affords the expected 1-amidoalkyl-2-naphthols desired product (IV) through the Michael addition of amide on ortho-quinone methide intermediate (III).
4.2 Reusability and Reproducibility of ZS-1 Zeolite Catalyst
After the reaction was completed, the major drawback for other catalysts in separation and recovery. Investigate the reusability of ZS-1 catalyst for the reaction benzaldehyde, β-naphthol, and benzamide/acetamide as model substrates (Scheme 1). After the completion of the reaction, the catalyst was easily separated by filtration and washed with acetone 3–4 times. Then catalyst was dried and activated in a vacuum oven for 2 h at 200 °C. Finally, the recycled catalyst was reused in mentioned reaction for another successive five cycles without any significant loss of reactivity and yield. Thus, it could be evidence that the ZS-1 catalyst is a suitable catalyst for this reaction with high reactivity and good reusability as shown in Fig. 8.
5 Conclusion
In summary, we have synthesized ZS-1 zeolite as a catalyst by using a hydrothermal pathway. Synthesized catalyst material was characterized by various characterization techniques such as FT-IR, XRD, FESEM, HRTEM, EDS, NH3TPD, and BET. Then, the synthesized catalyst was employed as a novel zeolite catalyst in an efficient, one-pot multicomponent synthesis of 1-aminoalkyl-2-naphthols derivatives of diverse aromatic aldehydes, b-naphthol, and benzamide/acetamide, under solvent-free conditions. It provides several promising advantages such as high yields, easy handling, easy work-up, shorter reaction times, and easy purification. The catalyst can be easily separated from the reaction mixture by filtration and can be reused without any significant loss in catalytic efficiency for five consecutive cycles of model reaction (Scheme 1). Hence, highly reusability and economic applicability, it is facile and environmental benign for the synthesis of biologically active substituted 1-aminoalkyl-2-naphthols derivatives.
Data Availability
Manuscript including all data correct and unpublished.
References
Singh RK, Duvedi R (2018) Environment-friendly green chemistry approaches for an efficient synthesis of 1-amidoalkyl-2-naphthols catalyzed by tannic acid. Arab J Chem 11:91–98. https://doi.org/10.1016/j.arabjc.2014.08.022
Zhang Q, Gao Y-H, Qin S-L, Wei H-X (2017) Facile one-pot synthesis of amidoalkyl naphtholsand benzopyrans using magnetic nanoparticle-supported acidic ionic liquid as a highly efficientand reusable catalyst. Catalysts 7:351. https://doi.org/10.3390/catal7110351
Erfaninia N, Tayebee R, Foletto EL, Amini MM, Dusek M, Zonoz M (2018) Preparation of magnetically recyclable ZnFe2O4 nanoparticles by easy single-step co-precipitation methodand their catalytic performance in the synthesis of 2-aminothiophenes. Appl Organomet Chem 32:1–7. https://doi.org/10.1002/aoc.4047
Li B, Tayebee R, Esmaeili E, Namaghi MS, Maleki B (2020) Selective photocatalytic oxidation of aromatic alcohols to aldehydes with air by magnetic WO3ZnO/Fe3O4. In situ photochemical synthesis of 2-substituted benzimidazoles. RSC Adv 10:40725–40738. https://doi.org/10.1039/D0RA08403D
Rekunge DS, Bendale HS, Chaturbhuj GU (2018) Activated Fuller’s earth: an efficient, inexpensive, environmentallybenign, and reusable catalyst for rapid solvent-free synthesis of1-(amido/amino)alkyl-2-naphthols. Monatsh Chem 149:1991–1997. https://doi.org/10.1007/s00706-018-2247-2
Hashemzadeh A, Amini MM, Tayebee R, Sadeghian A, Durndell LJ, Isaacs MA, Osatiashtiani A, Parlett CMA (2017) Amagnetically separable H3PW12O40@Fe3O4/EN-MIL-101 catalyst for the one-pot solventless synthesis of 2H-indazolo [2,1-b] phthalazine-triones. Mol Catal 440:96–106. https://doi.org/10.1016/j.mcat.2017.07.010
Tayebee R, Pejhan A, Ramshini H et al (2018) Equisetum arvense as an abundant source of silica nanoparticles. SiO2/H3PW12O40 nanohybrid material as an efficient andenvironmental benign catalyst in the synthesis of 2-amino-4H-chromenes under solvent-free conditions. Appl Organomet Chem 32:e3924. https://doi.org/10.1002/aoc.3924
Tayebee R, Mojtaba FA, Nasrin E et al (2019) Phosphotungstic acid grafted zeolite imidazolate framework as an effective heterogeneous nanocatalystfor the one-pot solvent-free synthesis of 3,4-dihydropyrimidinones. Appl Organomet Chem. https://doi.org/10.1002/aoc.4959
Benito HE, Alamilla RG, Enríquez JMH et al (2015) Porous silicates modified with zirconium oxide and sulfate ions for alcohol dehydration Reactions. Adv Mater Sci Eng. https://doi.org/10.1155/2015/325463
Mahdizadeh Ghohe N, Tayebee R, Amini MM (2019) Synthesis and characterization of mesoporous Nb-Zr/KIT-6 as a productive catalyst for the synthesis of benzylpyrazolyl coumarins. Mater Chem Phys 223:268–276. https://doi.org/10.1016/j.matchemphys.2018.10.067
Yang G, Pidko EA, Hensen EJM (2013) Structure, stability, and lewis acidity of mono and double Ti, Zr, and Sn framework substitutions in BEA zeolites: a periodic density functional theory study. J Phys Chem C 117:3976–3986. https://doi.org/10.1021/jp310433r
Dipake SS, Lande MK, Rajbhoj AS, Gaikwad ST (2021) Zeolite ZSM-11 as a reusable and efficient catalyst promoted improved protocol for synthesis of 2,4,5-triarylimidazole derivatives under solvent-free condition. Res Chem Intermed. https://doi.org/10.1007/s11164-021-04423-9
Gadekar SP, Dipake SS, Gaikwad ST, Lande MK (2018) Solid acid TS-1 catalyst: an efficient catalyst in Knoevenagel condensation for the synthesis of 5-arylidene-2,4-thiazolidinediones/Rhodanines in aqueous medium. Res Chem Intermed 44:7509–7518. https://doi.org/10.1007/s11164-018-3570-2
Narenji-Sani F, Tayebee R, Chahkandi M (2020) New task-specific and reusable ZIF-like grafted H6P2W18O62 catalyst for the effective esterification of free fatty acids. ACS Omega 5:9999–10010. https://doi.org/10.1021/acsomega.0c00358
Moliner M (2014) State of the art of Lewis acid-containing zeolites:lessons fromfine chemistry to new biomasstransformation processes. Dalt Trans 43:4197–4208. https://doi.org/10.1039/c3dt52293h
KEYSER DJ, Guillem AF (12) (2015) Patent Application Publication (10) Pub. No.: US 2015/0258769 A1 lifted-off layer Patent Application Publication
Lambert SL, 1994, Patent Number: 5,338,527, US005338527A
Cioc RC, Ruijter E, Orru RVA (2014) Multicomponent reactions: advanced tools forsustainable organic synthesis. Green Chem 16:2958–2975. https://doi.org/10.1039/C4GC00013G
Tayebee R, Fattahi Abdizadeh M, Maleki B, Shahri E (2017) Heteropolyacid-based ionic liquid [Simp] 3PW12O40 nanoparticles as a productive catalyst for the one-pot synthesis of 2H-indazolo [2,1-b] phthalazine-triones under solvent-free conditions. J Mol Liq 241:447–455. https://doi.org/10.1016/j.molliq.2017.06.033
Tayebee R, Amini MM, Rostamian H, Aliakbari A (2014) Preparation and characterization of a novel Wells–Dawson heteropolyacid-based magneticinorganic–organic nanohybrid catalyst H6P2W18O62/pyridino-Fe3O4 for the efficient synthesis of 1-amidoalkyl-2-naphthols under solvent-free conditions. Dalt Trans 43:1550–1563. https://doi.org/10.1039/C3DT51594J
Taghrir H, Ghashang M, Biregan MN (2016) Preparation of 1-amidoalkyl-2-naphthol derivatives using bariumphosphate nano-powders. Chin Chem Lett 27:119–126. https://doi.org/10.1016/j.cclet.2015.08.011
Gupta A, Kour D, Gupta VK, Kapoor KK (2016) Graphene oxide mediated solvent-free three component reaction for the synthesis of 1-amidoalkyl-2-naphthols and 1,2-dihydro-1-arylnaphth [1,2-e][1,3] oxazin-3-on. Tetrahedron Lett 57:4869–4872. https://doi.org/10.1016/j.tetlet.2016.09.067
Pourmousavi SA, Moghimi P, Ghorbani F, Zamani M (2017) Sulfonated polynaphthalene as an effective and reusable catalyst for the one-pot preparation of amidoalkyl naphthols: DFT and spectroscopic studies. J Mol Struct 1144:87–102. https://doi.org/10.1016/j.molstruc.2017.05.010
Tayebee R, Amini MM, Akbari M, Aliakbari A (2015) A novel inorganic–organic nanohybrid materialH4SiW12O40/pyridino-MCM-41 as efficient catalystfor the preparation of 1-amidoalkyl-2-naphtholsunder solvent-free conditions. Dalt Trans 44:9596–9609. https://doi.org/10.1039/c5dt00368g
Gong K, Wang H, Ren X et al (2015) β-Cyclodextrin-butane sulfonic acid: an efficientand reusable catalyst for the multicomponent synthesis of 1-amidoalkyl-2-naphthols under solvent-free conditions. Green Chem 17:3141–3147. https://doi.org/10.1039/c5gc00384a
Nasresfahani Z, Kassaee MZ, Eidi E (2016) Homopiperazine sulfamic acid functionalized mesoporous silica nanoparticles (MSNs-HPZ-SO3H)as an efficient catalyst for one-pot synthesis of1-amidoalkyl-2-naphthols. New J Chem 40:4720–4726. https://doi.org/10.1039/c5nj02974k
Zolfagharinia S, Kolvari E, Salehi M (2017) Highly efficient and recyclable phosphoric acid functionalized zirconia encapsulated-Fe3O4 nanoparticles: clean synthesis of 1,4-dihydropyridineand 1-amidoalkyl-2-naphthol derivatives. Reac Kinet Mech Cat. https://doi.org/10.1007/s11144-017-1186-y
Kiasat AR, Hemat-Alian L, Saghanezhad SJ (2016) Nano Al2O3: an efficient and recyclable nanocatalystfor the one-pot preparation of 1-amidoalkyl-2-naphthols under solvent-free conditions. Res Chem Intermed 42:915–922. https://doi.org/10.1007/s11164-015-2062-x
Khodaei MM, Khosropour AR, Moghanian H (2006) A simple and efficient procedure for the synthesis of amidoalkyl naphthols by p-TSA in solution or under solvent-free conditions. Synlett. https://doi.org/10.1055/s-2006-939034
Shaterian HR, Yarahmadi H, Ghashang M (2009) One-pot synthesis of amidoalkyl naphthols using NaHSO4.SiO2as an efficient and recyclable heterogeneous catalyst. Turkish J Chem 33:449–457. https://doi.org/10.3906/kim-0812-67
Shaterian HR, Yarahmadi H, Ghashang M (2008) Silica supported perchloric acid (HClO4eSiO2): an efficientand recyclable heterogeneous catalyst for the one-potsynthesis of amidoalkyl naphthols. Tetrahedron 64:1263–1269. https://doi.org/10.1016/j.tet.2007.11.070
Jesudoss SK, Vijaya JJ, Kaviyarasu K et al (2017) Anti-cancer activity of hierarchical ZSM-5 zeolitessynthesized from rice-based waste materials. RSC Adv 8:481–490. https://doi.org/10.1039/C7RA11763A
Li Y, Sun H, Feng R et al (2015) Synthesis of ZSM-5 zeolite from diatomite for fluid catalyticcracking (FCC) application. Appl Petrochem Res 5:347–353. https://doi.org/10.1007/s13203-015-0113-2
Gadekar SP, Lande MK (2018) Solid acid catalyst TS-1 zeolite-assisted solvent-free one-pot synthesis of poly-substituted 2,4,6-triaryl-pyridines. Res Chem Intermed 44:3267–3278. https://doi.org/10.1007/s11164-018-3305-4
Bortun AI, Bortun LN, Clearfield A (1997) Hydrothermal synthesis of sodium zirconium silicatesand characterization of their properties. Chem Mater 9:1854–1864
Ko YS, Ahn WS (1998) Synthesis and characterization of zirconium silicalite-1. Korean J Chem Eng 15:423–428
Franklin KR, Lowe BM (1987) Hydrothermal crystallization of piperazine- ZSM-39, vol 7. Butterworth & Co. Ltd, Oxford, pp 0144–2449
Treacy MMJ, Higgins JB (2007) Collection of simulated XRD powder patterns zeolites, 5th edn. Elsevier, Amsterdam
Chen H, Zhang X, Zhang J, Wang Q (2017) Controllable synthesis of hierarchical ZSM-5 for hydro conversion of vegetable oil to aviation fuel-like hydrocarbons. RSC Adv 7:46109–46117. https://doi.org/10.1039/c7ra08867a
Shao J, Cheng S, Li Z, Huang B (2020) Enhanced catalytic performance of hierarchical MnOx/ZSM-5 catalyst for the low-temperature NH3-SCR. Catalysts 10:311
Peng B, Zou H, He L, Wang P, Shi Z, Zhu L, Wang R, Zhang Z (2013) Engineering growth defects: a new route towards hierarchical ZSM-5 zeolite with high-density intracrystalline mesopores. CrystEngComm 00:1–3. https://doi.org/10.1039/x0xx00000x
Na J, Liu G, Ding TZG, Hu S, Wang L et al (2013) Synthesis and catalytic performance of ZSM-5/MCM-41 zeolites with varying mesopore size by surfactant-directed recrystallization. Catal Lett 143:267–275
Subramanian N, Vijaya JJ, Sivasanker S, Arjunan A (2014) Enhanced selectivity to benzaldehyde in the liquid phase oxidationof benzyl alcohol using nanocrystalline ZSM-5 zeolite catalyst. J Porous Mater 21:633–641
Ansari SAMK, Sangshetti JN, Kokare ND (2010) Oxalic acid catalyzed solvent-free synthesis of α-amidoalkyl-β-naphthols. Indian J Chem Technol 17:71–73
Mahdavinia GH, Bigdeli MA (2009) Wet cyanuric chloride promoted efficient synthesis of amidoalkyl naphthols under solvent-free conditions. Chin Chem Lett 20:383–386. https://doi.org/10.1016/j.cclet.2008.12.018
Patil SB, Singh PR, Surpur MP, Samant SD (2007) Ultrasound-promoted synthesis of 1-amidoalkyl-2-naphthols via a three-component condensation of 2-naphthol, ureas/amides, and aldehydes, catalyzed by sulfamic acid under ambient conditions. Ultrason Sonochem 14:515–518. https://doi.org/10.1016/j.ultsonch.2006.09.006
Nandi GC, Samai S, Kumar R, Singh MS (2009) Atom-efficient and environment-friendly multicomponent synthesisof amidoalkyl naphthols catalyzed by P2O5. Tetrahedron Lett 50:7220–7222. https://doi.org/10.1016/j.tetlet.2009.10.055
Sheik Mansoor S, Aswin K, Logaiya K, Sudhan SPN (2016) ZrOCl2Æ8H2O: an efficient and recyclable catalystfor the three-component synthesis of amidoalkylnaphthols under solvent-free conditions. J Saudi Chem Soc 20:138–150. https://doi.org/10.1016/j.jscs.2012.06.003
Kantevari S, Vuppalapati SVN, Nagarapu L (2007) Montmorillonite K10 catalyzed efficient synthesis of amidoalkyl naphthols under solvent free conditions. Catal Commun 8:1857–1862. https://doi.org/10.1016/j.catcom.2007.02.022
Srihari G, Nagaraju M, Murthy MM (2007) Solvent-free one-pot synthesis of amidoalkyl naphthols catalyzed by silicasulfuric acid. Helv Chim Acta 90:1497–1504. https://doi.org/10.1002/hlca.200790156
Nagarapu L, Baseeruddin M, Apuri S, Kantevari S (2007) Potassium dodecatungstocobaltate trihydrate (K5CoW12O40.3H2O): a mild and efficient reusable catalyst for the synthesis of amidoalkyl naphthols in solution and under solvent-free conditions. Catal Commun 8:1729–1734. https://doi.org/10.1016/j.catcom.2007.02.008
Lei M, Ma L, Hu L (2009) Thiamine hydrochloride as a efficient catalyst for the synthesis of amidoalkyl naphthols. Tetrahedron Lett 50:6393–6397. https://doi.org/10.1016/j.tetlet.2009.08.081
Acknowledgements
One of the authors, Mr. Sudarshan S. Dipake, gratefully thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi for the award of fellowship and the Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431004 (M.S.), India. for support and providing the necessary laboratory facility.
Author information
Authors and Affiliations
Contributions
SSD-conduct the whole experiment, writing an original draft. SPG-investigation. PBT-investigation. MKL-review and editing. ASR-review and editing. STG-writing, review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Consent to Participate
All Authors are agreed for submission.
Consent for Publication
Agreed to submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dipake, S.S., Gadekar, S.P., Thombre, P.B. et al. ZS-1 Zeolite as a Highly Efficient and Reusable Catalyst for Facile Synthesis of 1-amidoalkyl-2-naphthols Under Solvent-Free Conditions. Catal Lett 152, 755–770 (2022). https://doi.org/10.1007/s10562-021-03684-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03684-8