Abstract
A class of novel multi-tripodal ligands has been prepared via condensation reaction between pyrazole or triazole moieties and N′,N′-bis(2-aminoethyl)ethane-1,2-diamine. Spectroscopic techniques (1H NMR, 13C NMR, IR and mass spectroscopy) have been used to characterize the compounds. The ability of the in situ ligand complexes to catalyze the oxidation reaction of catechol to o-quinone at ambient conditions has been studied. The study demonstrated that the concentration of the metal salt has a pronounced influence on the catalytic activity. Thus, the best result has been obtained with the in situ complex [2 + CuSO4] with an oxidation rate of 21.60 µmol l−1 min−1. Kinetic parameters of the in situ complexes have been also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The catechol oxidase (catecholase) is one of the most metalloproteins investigated due to its interesting biological properties. In fact, it is a dinuclear copper protein well known as an exclusive catalyst of the oxidation reaction of a wide range of catechols to the corresponding o-quinones with no action on monophenols [1]. Like hemocyanin and tyrosinase, catecholase, also known as type-3 copper(II) protein, active site consisting of two closely spaced copper ions each surrounded by three imidazole nitrogen donor atoms of histidine residues [2]. For these reasons, several mono, di- and polynuclear copper complexes were reported and used to mimic the function of catechol oxidase enzyme [3, 4]. In this context, multi-tripodal systems containing nitrogen donor atoms and embedded triazole or pyrazole cores have been the subject of many studies in recent years. Actually, these skeletons have been shown to possess a wide range of potential biological and environmental properties. In addition, they present a very high importance in organometallic chemistry area, especially that concerning the coordination with the transition metals. Indeed, pyrazole and triazole containing complexes show a high structural stability and catalytic ability which is due probably to the strong efficiency capacity of the ligands, in part, to coordinate metals through different binding modes (monodentate, bidentate, tridentate and tetradentate modes) and to the flexible N-donor bridging which allowing the ligands to bend or rotate freely when interacting with the metals in other part [5,6,7].
To this date, a large number and different types of multidentate ligands are reported in the literature. Among these compounds, many mono-tripodal ligands based on pyrazole and triazole (Fig. 1A) were synthesized and investigated for their different biological, catalytic and anticorrosion properties by many reach groups [8,9,10,11,12,13,14,15,16,17]. These structures are characterized in general by the presence of an electron-rich cavity due to the three N-donor atoms, which facilitate the complexation with metals and the binding with enzyme receptors in microorganisms. The studies demonstrated that some of these derivatives are found to exhibit considerable antitumor, antimicrobial, anticorrosion and catalytic activities. In contrast, a few numbers of bis-tripodal pyrazole and triazole-based chelating ligands (Fig. 1B) were prepared. Actually, since the first synthesis reported by Driessen [18], very limited works were directed toward the preparation and the evaluation of different applications of this kind of compounds [16, 19,20,21,22]. Among the tested derivatives, some of them showed a remarkable antifungal activity against budding yeast Saccharomyces cerevisiae. Besides, these molecules present an interesting template in the field of the coordination chemistry due to the presence of two electron-rich cavities which can complex two metal ions.
With the aim of developing new template of multi-tripodal compounds, we reported here our contribution in preparation of some new ligands possessing three tripodal unites based on pyrazole or triazole moieties. Indeed, to the best of our knowledge, this is the first report of this kind of compounds in the literature. In reality, the purpose and the main objective of this part is to design novel dendrimer architectures in the future with multiple tripodal units which can be acting as an efficient template in the field of the coordination chemistry, nanoscience and other sectors.
Experimental section
Materials
Solvents (acetonitrile, diethyl ether, dichloromethane and methanol) and all other chemicals (N′,N′-bis(2-aminoethyl)ethane-1,2-diamine, Na2SO4, catechol and metallic salts) were of reagent grade and used as received from MERCK without further purification. (1H-1,2,4-triazol-1-yl)methanol (a), 3,5-dimethyl-1H-pyrazol-1-yl)methanol (b) and ethyl 1-(hydroxymethyl)-5-methyl-1H-pyrazole-3-carboxylate (c) were synthesized according to the methods described in the literature after some modifications [4, 23, 24].
Physical measurements
Nuclear magnetic resonance (NMR) spectra were recorded using a Bruker-300 instrument (300 MHz for 1H, 75 MHz for 13C). Chemical shifts are reported in parts per million (ppm) downfield from an internal trimethylsilane (s: singlet, t: triplet and m: multiplet). Fourier-transform infrared (FT-IR) spectra were recorded on pressed KBr pellets (4500–500 cm−1) using a Shimadzu infrared spectrophotometer (al: aliphatic and ar: aromatic). Mass spectrometry (MS) spectra were obtained using electrospray ionization method. UV visible (UV–vis) absorption spectra were recorded using a UV 1650 PC Shimadzu spectrophotometer using quartz cuvettes of 1 cm path length.
Compounds synthesis
N′,N′-bis((1H-1,2,4-triazol-1-yl)methyl)-N″,N″-bis(2-(bis((1H-1,2,4-triazol-1-yl)methyl) amino)ethyl)ethane-1,2-diamine (1)

0.36 g (2.5 mmol) of N′,N′-bis(2-aminoethyl)ethane-1,2-diamine was added to an acetonitrile solution (25 ml) of (1H-1,2,4-triazol-1-yl)methanol (0.99 g, 10 mmol). The mixture was stirred and heated under reflux for 4 h, then dried over Na2SO4 and filtered. After evaporation of the solvent under reduced pressure, the resulting product was dissolved in dichloromethane. The addition of diethyl ether yields 1 as a yellow oil. Yield: 60%. 1H NMR (300 MHz, CDCl3, δ, ppm): 8.54 (s, 6H, H14,20,27,33,40,46); 7.94 (s, 6H, H12,18,25,31,38,44); 5.25 (s, 12H, H9,15,22,28,35,41); 2.77 (t, 6H, J = 6.75 Hz, H5,6,7); 2.44 (t, 6H, J = 6.75 Hz, H 2,3,4). 13C NMR (75 MHz, CDCl3, δ, ppm): 151.95 (C12,18,25,31,38,44); 144.65 (C14,20,27,33,40,46); 66.50 (C9,15,22,28,35,41); 52.17 (C2,3,4); 48.07 (C5,6,7). FT-IR (KBr, cm−1): 3099 (νC–Har); 2997; 2955 (νC–Hal); 2912; 1506 (νC=N); 1373; 1354; 1338; 1273; 1192; 1136; 1012 (νC–N); 958; 877; 761 (δC–Har); 678; 644.
N′,N′-bis(2-(bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)ethyl)-N″,N″-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)ethane-1,2-diamine (2)

A solution of 3,5-dimethyl-1H-pyrazol-1-yl)methanol (1.26 g; 10 mmol) and N′,N′-bis(2-aminoethyl)ethane-1,2-diamine (0.24 g; 1.6 mmol) in acetonitrile (25 ml) was refluxed and stirred for 4 h. The mixture was dried over Na2SO4, filtered and evaporated at reduced pressure. The obtained product was extracted three times with diethyl ether. The organic layer was dried again over Na2SO4, and the solvent was removed. The final product was recuperated as a white oil. Yield: 71%. 1H NMR (300 MHz, CDCl3, δ, ppm): 5.75 (s, 6H, H13,19,30,36,47,53); 4.78 (s, 12H, H9,15,26,32,43,49); 2.59 (t, J = 7.5 Hz, 6H, H5,6,7); 2.19 (t, J = 7.5 Hz, 6H, H2,3,4); 2.13(s, 18H, H21,22,38,39,55,56); 2.10 (s, 18H, H23,24,40,41,57,58). 13C NMR (75 MHz, DMSO, δ, ppm): 147.32 (C12,18,29,35,46,52); 139.57 (C14,20,31,37,48,54); 105.77 (C13,19,30,36,47,53); 65.48 (C9,15,26,32,43,49); 51.89 (C2,3,4); 47.25 (C5,6,7); 13.41 (C23,24,40,41,57,58); 10.85 (C21,22,38,39,55,56). FT-IR (KBr, cm−1): 3132 (νC–Har); 2922 (νC-Hal); 1556 (νC=N); 1458 (νC=C); 1421; 1375; 1317; 1247; 1099; 1030 (νC–N); 977; 781 (δC–Har). MS (ESI): m/z calcd: [M + H]+ = 795.5, [M + Na]+ = 817.5; found: [M + H]+ = 795.5, [M + Na]+ = 817.3.
Hexaethyl-1,1′,1′′,1′′′,1′′′′,1′′′′′-(((nitrilotris(ethane-2,1-diyl))tris(azanetriyl))hexakis (methylene))hexakis(5-methyl-1H-pyrazole-3-carboxylate) (3)

Ethyl 1-(hydroxymethyl)-5-methyl-1H-pyrazole-3-carboxylate (1.84 g; 10 mmol) dissolved in 25 ml of acetonitrile was mixed with 0.24 g (1.6 mmol) of N′,N′-bis(2-aminoethyl)ethane-1,2-diamine. After stirring and refluxing for 5 h, the solvent was evaporated in vacuo and the residue was extracted three times with diethyl ether. The organic phase was dried over Na2SO4, and the solvent was removed to obtain a yellow oil. Yield: 63%. 1H NMR (300 MHz, CDCl3, δ, ppm): 6.50 (s, 6H, H13,19,30,36,47,53); 5.01 (s, 12H, H9,15,26,32,43,49); 4.34–4.22 (m, 12H, H61,69,78,80,72,75); 2.80–2.51 (m, 6H, H5,6,7); 2.28–2.19 (m, 6H2,3,4); 2.20–2.04 (m, 18H, H21,22,38,39,55,56); 1.35–1.26 (m, 18H, H62,70,73,76,81,82). 13C NMR (75 MHz, CDCl3, δ, ppm): 162.38 (C23,24,40,41,57,58); 142.59 (C12,18,29,35,46,52); 140.66 (C14,20,31,37,48,54); 108.75(C13,19,30,36,47,53); 66.48 (C9,15,26,32,43,49); 60.89 (C61,69,72,75,78,80); 51.81 (C2,3,4); 47.51 (C5,6,7); 14.31 (C62,70,73,76,81,82); 10.86 (C21,22,38,39,55,56). FT-IR (KBr, cm−1): 3134; 2982 (νC-Hal); 2935; 1716 (νC=O); 1683; 1653 (νC=N); 1541 (νC=C); 1446; 1217 (νC-O); 1107; 1030 (νC-N); 842; 779 (δC–Har). MS (ESI): m/z calcd: [M + H]+ =1143.6, [M + Na]+ = 1165.6; found: [M + H]+ = 1143.3, [M + Na]+ = 1166.1.
Catalysis study
The catechol oxidase activity of the in situ complexes was investigated spectrophotometrically under aerobic conditions at room temperature monitoring the increase in the o-quinone absorbance rate, using the experimental process described previously after some simple modifications [17]. Briefly, the metal complexes were prepared in situ in a quartz cuvette of 1 cm path length mixing 0.15 ml of a 2 × 10−3 mol l−1 solution of manganese (II) acetate salt or copper (II) salts CuX2, nH2O (with X = CH3COO−, Cl−, NO3−, SO42−) with 0.15 ml of ligand solution at 2 × 10−3 mol l−1, then 2 ml of a 10−1 mol l−1 fresh catechol solution were added in the cuvette. All solutions were prepared in methanol 99.99%, and the absorbance evolution of the formed o-quinone was followed at 390 nm for one hour.
Results and discussion
Compounds chemistry
The target ligands 1, 2 and 3 were prepared in one pot 6:1 condensation reaction of different 1-hydroxypyrazoles or triazole on N′,N′-bis(2-aminoethyl)ethane-1,2-diamine in acetonitrile and under reflux as outlined in Scheme 1. The primary precursors a, b and c were previously prepared via a Mannich reaction from pyrazole or triazole derivatives and formaldehyde. The structures of the new ligands were established by different spectroscopic techniques such as 1H and 13C NMR, IR and mass spectroscopy.
Generally, we were able to assign all the corresponding signals to the different types of protons of these macromolecular compounds. The proportionality between the signal’s integrality and the number of chemically equivalent protons was respected. According to 1H NMR analyses, all the spectra of the final products showed the presence of the characteristic signal of the –N–CH2–N– bridge which remained the mean index indicating the formation of our ligands. For all compounds, the signal was appeared as a singlet integrate 12 protons and with chemical shifts of 5.25, 4.78 and 5.01 ppm for 1, 2 and 3, respectively. In general, the observed differences in chemical shifts of the signal under study can be explained by the shielding and deshielding effect of CH2 protons which is due to the different electronic effects introduced by the substituent groups on the pyrazole and triazole rings. In fact, the presence of electron donating groups (R2 = CH3) on the positions 3 of pyrazole rings leads to an increase of the electron density on –N–CH2–N– protons which make them more shielded than the protons in the case of compound 3, where the same positions of the pyrazole moieties are substituted by electron withdrawing groups (R2 = CO2Et). As a simple comparison with chemical shift values of –N–CH2–N– protons of similar compounds in the literature, our results were found to be in good accordance with the described findings in these works, especially in the case of compound 2 which showed a chemical shift of 4.78 ppm very close to that shown by compound 4a (4.74 ppm) in our previous work [19] and compound 6b (4.76 ppm) as described by Driessen [18]. 13C NMR chemical shifts, IR and mass spectra were also in good agreement with the proposed structures.
Catalytic and kinetic studies
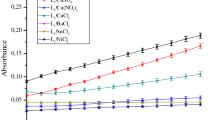
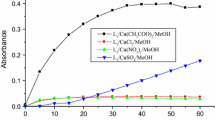
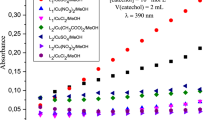
In the latest years, many in situ copper complexes have been investigated for their catalysis power toward different catechol derivatives in order to develop new model systems for mimicking catecholase enzyme activity [17, 25,26,27]. In our case, a series of manganese(II) and copper(II) complexes was prepared in situ from several metallic salts and the two ligands 2 and 3, and investigated toward the aerobic oxidation of catechol at room temperature. Evaluation of the catecholase activity of these complexes was made spectrophotometrically by following the appearance of the maximum absorbance of the corresponding o-quinone at 390 nm over time. The results are illustrated in Fig. 2, showing the change in absorbance at 390 nm versus time for 60 min.
The absorbance plots vs time for the aerobic oxidation of catechol to the corresponding o-quinone performed at 25 °C in MeOH and catalyzed by the complexes prepared in situ with 2 and 3 and different transition metallic salts. A and B present the absorbance plots of complexes prepared, respectively, with 2 and 3 at the molar ratio 1:3 (ligand/Metal), while C and D present the absorbance plots of the same complexes in the same order at the molar ratio 1:6 (ligand/Metal)
In addition, catechol oxidizes very slowly in contact with air oxygen under standard conditions of pressure and temperature (25 °C, 1 bar). Under the experimental conditions used, the spectrum obtained for catechol alone showed practically no absorbance as a function of time, the same result is observed with ligands alone, and also with salts of metals alone in the presence of catechol, which means the absence of o-quinone formation and any significant catalytic activity.
In general, tripods based on triazole units lead to a precipitation in the cuvette with the transition metals; for this reason, the catecholase activity of compound 1 has not been studied.
The plots A and B (Fig. 2) recorded at the concentration of one equivalent of ligand and three equivalents of metallic salts reveal clearly that absorbance of the o-quinone formed after the catalysis reaction by the complexes [2 + Cu(CH3COO)2] and [2 + CuSO4] is higher than the absorbances found by all other complexes including that of compound 3. Comparing these results to that found at the concentration of one equivalent of ligand and six equivalents of metallic salts (Fig. 2C, D), the absorbance resulted by the complex [2 + CuSO4] is increased by double after 60 min. While the complex [3 + Cu(CH3COO)2] showed a final absorbance seven times higher than one found in the first case. The other complexes showed slight change in the absorbance except complex [2 + Cu(CH3COO)2] which did not show any absorbance.
As previously shown in Scheme 1, the studied ligands are characterized by the presence of three chelation centers which made them capable of establishing coordination bonds with three equivalents of metal salts. These tricentric ligands differ from each other by the substituents borne by the pyrazole nucleus. The ligand 2 has methyl electron donating inductive effect groups, while the ligand 3 has electron withdrawing mesomeric effect groups (CO2Et). The nature of the pyrazole substituents explains the difference in catalytic activity exhibited by these complexes. In fact, the presence of electron donating groups like CH3 increases the electronic richness of the coordination sites, which promotes the complexation of the ligand with the metal salts. In contrast, the electron withdrawing groups such as CO2Et make the opposite effect. In addition to the electronic character, the steric hindrance effect of the groups on the pyrazole rings is another important factor which can be affecting both the formation of the metal complex and the accessibility of the substrate (catechol) to the metal ion [4]. More particularly, the complex of 2 with CuSO4 shows the high catalytic activity with a rate reaches 15.86 µmol l−1 min−1 and catalytic activity concentration of about 121.61 µmol l−1 min−1 at the molar ratio 1:3 (Ligand/Metal) (Table 1). This result reflects that the complex–catechol interactions are stronger, and the power of transformation of catechol into o-quinone is very efficient and fast. In addition, it has been shown that dinuclear complexes are generally more active than mononuclear complexes [28].
Furthermore, the effect of the concentration of the metal salts allows a considerable increase in the reaction rate. The oxidation rate of the complex [2 + CuSO4] was increased from 15.86 to 21.60 µmol l−1 min−1, whereas that of complex [3 + CuSO4] was augmented by twice from 4.58 to 11.36 µmol l−1 min−1. These findings can be explained essentially by the increase in the concentration of complexes formed in situ. On the other hand, the oxidation rates remain low for the complexes containing the nitrate or chloride anions, which can be due to the high affinity of these counter anions in solution toward the metal center, and thereafter disfavor the complex–substrate interactions. Additionally, many previous studies mentioned that the catalytic activity can also be influenced by the nature of the solvent used [29, 30].
Moreover, to understand more the extent of the catalytic efficiency of the complexes prepared in situ of 2 and 3 ligands with the metal salts Cu(CH3CO2)2, Mn(CH3CO2)2 and CuSO4 in methanol, kinetic studies determined by the initial rate method were performed. The experiments were carried out in methanol, by maintaining the concentration of each in situ catalyst to a specific value of 1 × 10−3 M and varying the concentration of the substrate 1,2-dihydroxybenzene (catechol) from 1 × 10−2 to 2 × 10−1 M. The initial rates were determined by linear regression from the slope of the tangent to the absorbance vs time curve after the induction period (t = 3 min). Figure 3A and B represents the dependence of the initial rate on the concentration of catechol. The appropriate catalytic rate constant VM and the Michaelis–Menten constant KM were determined from the reciprocal Lineweaver–Burk plots (Fig. 3a, b). Kinetic data are given in Table 2.
Kinetic studies of air oxidation of catechol catalyzed by the in situ complexes showed curves of hyperbolic nature which finally tends toward stabilization. From the obtained results, in situ complexes of the tricentric ligand 2 demonstrate a significant affinity toward catechol compared to the in situ complexes of the tricentric ligand 3; nevertheless, they exhibit good maximum reaction velocities. The best affinity was obtained by the in situ complex [2 + Cu(CH3COO)2] with a Michaelis constant KM of 0.005 M and a maximum reaction velocity VM equal to 138.37 mM min−1, which confirms that this in situ complex presents a strong introductory activity and gave a better result for the oxidation of catechol. Considering this finding, the factor acting on the catalytic activity seems rather to be the transformation of the intermediate (complex-catecholate) into the final product than its dissociation. This reflects the good affinity of the in situ complex, as well as its high catechol oxidation power in terms of initial velocity.
Perspectives: dendrimers and dendrimers complexes design
As previously mentioned, one of the main purposes of this work is to launch an idea concerning the preparation of novel class of dendrimers with pyrazole, pyridine or triazole tripod units on their surface. As it is well known, dendrimers are globular macromolecules or monodisperse polymers with a cauliflower shape. They can be prepared by iterative reactions using different pathways but mainly two, the divergent and the convergent methods [31]. Their unique structure and special chemical and biological properties such as the number and the type of surface functional groups, the high solubility and the high molecular weight make them of significant scientific interest [32,33,34]. These properties and others allow the application of dendrimers in several scientific fields such as biology, medicine, biosensors, catalysis, environmental protection, electronic devices and nanotechnology [35,36,37,38,39].
The possibility to modify dendrimers periphery can greatly expand their applications; in this way, the complexation of the proposed dendrimers with metallic ions is one of the most suitable tasks that we propose due principally to the presence of many chelating sites on the surface. Actually, many works have been studied the complexation of dendrimers [40,41,42,43,44], but the particularity of our proposed dendrimers is their capacity to coordinate many metallic ions as displayed in Fig. 4.
Conclusion
In summary, a class of novel multi-tripodal ligands has been synthesized by the condensation of pyrazole or triazole moieties with N′,N′-bis(2-aminoethyl) ethane-1,2-diamine. 1H NMR, 13C NMR, IR and mass spectroscopy techniques have been used to confirm the structures of the new compounds. 1H NMR analysis revealed that chemical shift of the N–CH2–N bonds is very depending on the nature of the substituents on pyrazole cores. The prepared compounds can be useful in the design of new dendrimers of high interest. The catalytic activities of the complexes formed in situ of studied ligands, and several metallic salts were found to be very influenced by the nature of the ligand, the counter anion and the concentration of metallic salts used. The in situ [2 + CuSO4] complex showed the best catecholase activity for the oxidation reactions rate, which attain 21.60 µmol l−1 min−1. However, the kinetic investigation results determined by the initial rate method showed that the highest activity, in the reactions of the catechol oxidase, is obtained by the in situ complex [2 + Cu(CH3COO)2].
References
J. Kaizer, R. Csonka, G. Speier, M. Giorgi, M. Réglier, J. Mol. Catal. A Chem. 235(1), 81 (2005)
R. Marion, N.M. Saleh, N. Le Poul, D. Floner, O. Lavastre, F. Geneste, New J. Chem. 36(9), 1828 (2012)
A. Santra, G. Mondal, M. Acharjya, P. Bera, A. Panja, T.K. Mandal, P. Mitra, P. Bera, Polyhedron 113, 5 (2016)
R. Marion, M. Zaarour, N.A. Qachachi, N.M. Saleh, F. Justaud, D. Floner, O. Lavastre, F. Geneste, J. Inorg. Biochem. 105(11), 1391 (2011)
S. Choi, S. Kim, H.-J. Lee, H. Lee, Inorg. Chem. Commun. 44, 164 (2014)
F. Abrigach, R. Touzani, Med. Chem. (Los Angeles) 6(5), 292 (2016)
Y.-H. Luo, G.-G. Wu, S.-L. Mao, B.-W. Sun, Inorg. Chim. Acta 397, 1 (2013)
F. Malek, N. Draoui, O. Feron, S. Radi, Res. Chem. Intermed. 40(2), 681 (2013)
R. Touzani, A. Ramdani, T. Ben Hadda, S. El Kadiri, O. Maury, H.L. Bozec, P.H. Dixneuf, Synth. Commun. 31(9), 1315 (2001)
H. Bendaha, L. Yu, R. Touzani, R. Souane, G. Giaever, C. Nislow, C. Boone, S. El Kadiri, G.W. Brown, M. Bellaoui, Eur. J. Med. Chem. 46(9), 4117 (2011)
S. Radi, Y. Toubi, N. Draoui, O. Feron, O. Riant, Lett. Drug Des. Discov. 9(3), 305 (2012)
S. Radi, Y. Toubi, I. Hamdani, A. Hakkou, F. Souna, I. Himri, M. Bouakka, Res. J. Chem. Sci. 2(4), 40 (2012)
T. Harit, M. Cherfi, J. Isaad, A. Riahi, F. Malek, Tetrahedron 68(21), 4037 (2012)
S. Garbacia, C. Hillairet, R. Touzani, O. Lavastre, Collect. Czechoslov. Chem. Commun. 70(1), 34 (2005)
N. Boussalah, R. Touzani, F. Souna, I. Himri, M. Bouakka, A. Hakkou, S. Ghalem, S.E. Kadiri, J. Saudi Chem. Soc. 17(1), 17 (2013)
H. Al Bay, B. Quaddouri, A. Guaadaoui, R. Touzani, N.-E. Benchat, A. Hamal, M. Taleb, M. Bellaoui, S. El Kadiri, Lett. Drug Des. Discov. 7(1), 41 (2010)
A. Zerrouki, K. Karrouchi, F. Abrigach, N. Benchat, M. Taleb, S. El Kadiri, J. Mater. Environ. Sci. 8(1), 90 (2017)
W.L. Driessen, Recl. Trav. Chim. Pays-Bas 101(12), 441 (1982)
F. Abrigach, B. Bouchal, O. Riant, Y. Mace, A. Takfaoui, S. Radi, A. Oussaid, M. Bellaoui, R. Touzani, Med. Chem. 12(1), 83 (2016)
M.R. Malachowski, M.G. Davidson, Inorg. Chim. Acta 162(2), 199 (1989)
S. Kim, D. Kim, H.-J. Lee, H. Lee, J. Mol. Struct. 1063, 70 (2014)
T. Ben Hadda, A. Kotchevar, M. Daoudi, B. Bennani, N. Larbi, A. Kerbal, Lett. Drug Des. Discov. 2(8), 584 (2005)
I. Dvoretzky, G.H. Richter, J. Org. Chem. 15(6), 1285 (1950)
M.S. Pevzner, P.A. Ivanov, N.V. Gladkova, O.N. Sushchenko, V.P. Tverdokhlebov, Z.S. Myasnikova, Chem. Heterocycl. Compd. 16(2), 189 (1980)
R. Boyaala, R. El Ati, F. Abrigach, M. El Kodadi, R. Touzani, B. Hammouti, RJPBCS 8(3), 751 (2017)
A. Mouadili, F. Abrigach, M. Khoutoul, A. Zarrouk, N. Benchat, R. Touzani, J. Chem. Pharm. Res. 7(1), 968 (2015)
A. Takfaoui, I. Bouabdallah, F. Abrigach, M. Khoutoul, N. Benchat, R. Touzani, Moroc. J. Chem. 1, 11 (2013)
C. Eicken, B. Krebs, J.C. Sacchettini, Curr. Opin. Struct. Biol. 9(6), 677 (1999)
A. Mouadili, A. Attayibat, S. El Kadiri, S. Radi, R. Touzani, Appl. Catal. A 454, 93 (2013)
A. Djedouani, F. Abrigach, M. Khoutoul, A. Mohamadou, A. Bendaas, A. Oussaid, R. Touzani, Orient. J. Chem. 31(1), 97 (2015)
U. Boas, J.B. Christensen, P.M.H. Heegaard, J. Mater. Chem. 16(38), 3785 (2006)
R. Esfand, D.A. Tomalia, Drug Discov. Today 6(8), 427 (2001)
A.-M. Caminade, J.-P. Majoral, Prog. Polym. Sci. 30(3–4), 491 (2005)
D. Astruc, E. Boisselier, C. Ornelas, Chem. Rev. 110(4), 1857 (2010)
R. Touzani, J. Mater. Environ. Sci. 2(3), 201 (2011)
J.-H. Kim, K. Park, H.Y. Nam, S. Lee, K. Kim, I.C. Kwon, Prog. Polym. Sci. 32(8), 1031 (2007)
P. Ceroni, G. Bergamini, F. Marchioni, V. Balzani, Prog. Polym. Sci. 30(3–4), 453 (2005)
R. Duncan, L. Izzo, Adv. Drug Deliv. Rev. 57(15), 2215 (2005)
G.R. Newkome, C.D. Shreiner, Polymer 49(1), 1 (2008)
D. Staneva, E. Vasileva-Tonkova, M.S.I. Makki, T.R. Sobahi, R.M. Abdel-Rahman, I.H. Boyaci, A.M. Asiri, I. Grabchev, Tetrahedron 71(7), 1080 (2015)
I. Grabchev, S. Yordanova, E. Vasileva-Tonkova, P. Bosch, S. Stoyanov, Inorg. Chim. Acta 438, 179 (2015)
I.V. Lijanova, R.G. Monter, N.V. Likhanova, F.V. Garibay, X.O.C. Olivares, Supramol. Chem. 24(1), 56 (2012)
M.R. Mankbadi, M.A. Barakat, M.H. Ramadan, H.L. Woodcock, J.N. Kuhn, J. Phys. Chem. B 115(46), 13534 (2011)
M.B. Camarada, M. Zúñiga, J. Alzate-Morales, L.S. Santos, Chem. Phys. Lett. 616–617, 171 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zerrouki, A., Abrigach, F., Taleb, M. et al. Design, synthesis, characterization and catechol oxidase activity of novel class of multi-tripodal pyrazole and triazole-based derivatives. Res Chem Intermed 46, 1453–1467 (2020). https://doi.org/10.1007/s11164-019-04044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04044-3