Abstract
Four tridentate ligands L1-L4, namely N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl) pyridin-2-amine: L1, 5-chloro-N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl) pyridin-2-amine:L2, N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)thiazol-2-amine:L3 and N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-6-methylpyridin-2-amine: L4 were synthesized, characterized (by13C NMR, 1H NMR and mass spectroscopy) and employed for the synthesis of copper(II) complexes in situ. These ligands contain three sp2 nitrogen atoms, two pyrazole nitrogen and one pyridine nitrogen, capable of coordinating with copper (II). Then the catalytic properties of certain complexes formed in situ were evaluated to catalyze the oxidation of catechol to o-quinone. Among these complexes, the L1/Cu(CH3COO)2 complex which showed good catalytic activity of the combination 1:1 ligand/metal in THF for this reaction, with a reaction rate of oxidation of catechol to o-quinone equal to 26.37 μmol L−1 min−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The synthesis of multidentate heterocyclic ligands which have donor atoms such as nitrogen and oxygen are of considerable biological interest and their resulting metal complexes. Most of the mulidentate ligands are prepared for the purpose of mimicking the behavior of various metalloproteins, such as copper-containing proteins, hemocyanin, and tyrosinase [1,2,3,4,5]. Great efforts have been made to mimic tyrosinase activity using copper complexes coordinated to multidentate heterocyclic ligands based on pyrazole. Different types of catechol have been used in the literature as models for this type of study [6,7,8,9] (Scheme 1).
Much information regarding the role of transition metals in oxidative and hydrolytic dinuclear metalloenzymes, such as catechol oxidases, has been obtained from comparative studies of metalloenzymes and synthetic model metal complexes [10,11,12,13,14,15,16]. Studies with model complexes have been described, aiming to mimic the structural and/or functional properties of these metalloenzymes with the presence of labile sites essential for the binding of the substrate and/or nucleophiles available to initiate the catalytic process [17,18,19], and role of the electronegative atom present on the ligand backbone and the mode of binding of the substrate on the catecholase type activities of the complexes [20]. Heterocyclic compounds based on pyrazole have also occupied an important place in modern chemistry due to their various applications in many fields such as catalysis [21,22,23,24,25], pharmacology [26, 27], complexation [28,29,30], electronics [31], corrosion inhibitor [32], cytotoxic activities [33], transport of lithium cations [34], extraction of lithium and cesium cations [35, 36] antifungal and antibacterial activities [37, 38]. Considerable studies focus on the synthesis of complexes with nitrogen ligands designed to model catecholase [39,40,41,42,43,44,45,46,47]. Catalytic activity has been studied as complexes formed in situ [48, 49] or as isolated complexes [50, 51]. This study reveals that the complex formed from the L2/CuCl2 combination in THF shows a higher activity towards the other ligand/copper(II) combinations, with a rate Vmax equal to 16.03 μmol L−1 min−1 and a low value of Km equal to 0.018 mol L−1. In the continuity of laboratory work in this field [21, 48, 49, 52,53,54,55], the ligands L1–L4 based on pyrazole and pyridine were examined for the catecholasic activities formed with different copper(II) salts Cu(CH3COO)2, CuSO4, Cu(NO3)2 and CuCl2, and the effect of ligand concentration, the nature of the ligand, the nature of the counter-anion and the nature of the solvent were studied (MeOH and THF).
In this work, we report the synthesis and caracterisation of the ligands L1-L4, then, we study the catalytic activity of the ligand/copper(II) complex formed in situ of the oxidation of catechol to o-quinone.
Materials and methods
Materials
The (3,5-dimethyl-1H-pyrazol-1-yl) methanol has been prepared according to the literature [56, 57]. Solvents (methanol and THF,) and all other reagents (catechol, anhydrous copper salts Cu(CH3COO)2, CuSO4, Cu(NO3)2 and CuCl2) were purchased from Aldrich and were used as received without further purification.
Physical measurements
Nuclear magnetic resonance (NMR) spectra were recorded using a Bruker-400 instruments operating at 400 MHz for 1H spectra and 101 MHz for 13C spectra. Mass spectra were obtained using the electrospray ionization (ESI) technique and UV–Vis measurements were spectrophotometrically made using a Shimadzu UV-1800 spectrophotometer at 25 °C.
Synthesis of the ligands
The heterocyclic ligands L1–L4 [58, 59] (Scheme 2) where prepared by reaction between two equivalents of 1-hydroxymethyl-3,5-dimethylpyrazole or 1-hydroxymethylpyrazole and one equivalents of corresponding primary amine under reflux (60 °C) for 6 h under magnetic stirring in acetonitrile, then the solution is dried over MgSO4, filtered and then concentrated in a rotary evaporator. The product obtained was extracted with CH2Cl2/H2O.
Catecholase activity measurements
Kinetic measurements were spectrophotometrically made using à Shimadzu UV-1800 PC spectrophotometer, following the appearance of o-quinone over time at 25 °C (390 nm absorbance maximum, ε = 1600 L mol−1 cm−1 in methanol, ε = 1900 L mol−1 cm−1 in THF). The complexes are formed in situ, by mixing successively 0.15 mL of a solution (2 × 10−3 mol L−1) of metals salts with 0.15 mL of ligand solution (2 × 10−3 mol L−1), complexes formed in situ were treated with 2 mL (0.10 mol L−1) of catechol in methanol (MeOH) or tetrahydrofuran (THF) under aerobic conditions.
Results and discussion
Characterisation of ligands
The ligands L1-L4 used in this study are obtained with good yields and characterized by different identification methods such as 1H NMR, 13C NMR and mass spectroscopy.
N,N-Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)pyridin-2-amine: L1
White solid, Yield: 72%, Melting point: 100–104 °C. 1H NMR (400 MHz, CDCl3) δ 8.24–6.68 (m, 4H, CHpy), 5.93–5.83 (m, 2H, CHpz), 5.61–5.22 (s, 4H, CH2), 2.54–2.18 (s, 12H, CH3). 13C NMR (101 MHz, CDCl3) δ 155.00 (C=Npy), 148.49 (C-Npy), 147.20 (C=Npz), 139.98 (C-Npz), 137.87 (Cpy,para), 114.99 (Cpy,meta- left), 113.85 (Cpy,meta- right), 106.21 (CHpz), 70.31 (CH2), 13.10 (CH3–C=N), 10.68 (CH3-C-N). m/z: calcd 310.19 found 311.1 [M + 1].
5-chloro-N,N-Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)pyridin-2-amine: L2
White solid, Yield: 72%, Melting point: 176–180 °C. 1H NMR (400 MHz, CDCl3) δ 8.05–7.97 (m, 1H, CH-Npy), 7.42 (dt, J = 8.8, 2.2 Hz, 1H, CH–CCl), 6.69 (d, J = 8.8 Hz, 1H, CH–C=Npy), 6.09–5.91 (m, 2H, CHpz), 5.83–5.17 (s, 4H, CH2), 3.01–1.58 (s, 12H, CH3). 13C NMR (101 MHz, CDCl3) δ 153.94 (C=Npy), 146.27 (CH-Npy), 145.15(C=Npz), 143.23 (Cpz–N), 137.91 (CH–CCl), 122.07 (C–Cl), 111.09 (CH–C=Npy), 106.17 (CHpz), 54.55 (CH2), 12.09 (CH3–C=N), 11.24 (CH3-C-N). m/z: calcd 344.15 found 345.1 [M + 1].
N,N-Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)thiazol-2-amine [58]: L3
Yellow solid, Yield: 72%, Melting point: 120–124 °C. 1H NMR (400 MHz, CDCl3) δ: 7.22 (d, J = 3.6 Hz, 1H, CH–S), 6.69–6.54 (m, 1H, CHC–S), 6.00–5.56 (s, 4H, CH2), 5.32 (d, J = 106.1 Hz, 2H, CHpz), 2.50–2.16 (s, 12H, CH3). 13C NMR (101 MHz, CDCl3) δ: 168.16(N=C–S), 144.37(C=Npz), 139.02(Cpz–N), 135.86(CHC–S), 109.01(CH–S), 105.40(CHpz), 70.43 (CH2), 13.08 (CH3–C=N), 11.49 (CH3–C–N). m/z: calcd 316.15 found 317.1 [M + 1].
N,N-Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-6-methylpyridin-2-amine [59]: L4
White solid, Yield: 72%, Melting point: 118–122 °C. 1H NMR (400 MHz, CDCl3) δ 7.49–7.36 (m, 1H, CHpy,para), 6.76 (dd, J = 14.6, 8.4 Hz, 1H, CHpy-meta,right), 6.57 (dd, J = 16.8, 7.6 Hz, 1H, CHpy-meta,left), 5.94–5.77 (m, 2H, CHpz), 5.71–5.24 (s, 4H, CH2), 2.52–2.16 (s, 15H, CH3). 13C NMR (101 MHz, CDCl3) δ 155.49 CpyCH3, 153.02 C=Npy, 148.16 (C=Npz), 140.04 (Cpz–N), 138.14 (CHpy,para), 114.39 (CHpy-meta,right), 113.39 (CHpy-meta,left), 105.74 (CHpz), 54.57 (CH2), 22.54 CH3py, 13.46 (CH3–C=N), 11.41 (CH3–C–N). m/z: calcd 324.21 found 325.1 [M + 1].
Catecholase studies
In this work, we have carried out the study of the oxidation activity of the catechol to o-quinone by ligands L1-L4 with different copper(II) salts Cu(CH3COO)2, CuSO4, Cu(NO3)2 and CuCl2 in the two solvents MeOH and THF.
Catecholase studies of in situ complexes formation of ligands L 1 -L 4 in methanol
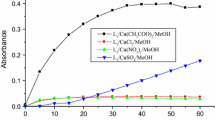
The results are summarized in Table 1 and in Figs. 1 and 2, all the complexes catalyze the oxidation reaction of catechol to o-quinone in methanol with an oxidation rate varying from a maximum of 3.52 and 2.21 μmol of substrate per liter of catalyst per min (µmol L−1 min−1) for L1/CuSO4) and L1/Cu(CH3COO)2) complexes at a low catalytic activity of 0.46 µmol L−1 min−1 for the L2/Cu(NO3)2 complex.
Fig. 1 and 2 show the change in absorbance as a function of time for the four anions CH3COO−, Cl−, NO3− and SO42−. Note that the absorbance in the presence of CH3COO− and NO3− anions is very high, however in the presence of Cl− and SO42− anions, the absorbance remains very low. And in the case of the L2–L4 ligands (Figs. 1 and 2), we note that the absorbance in the presence of the four anions Cl− and NO3−, SO42− and CH3COO− remains low, which does not exceed 0.1. The oxidation rates in the presence of the complexes obtained are collated in Table 1:
Catecholase studies of in situ complexes formation of ligands L 1 -L 4 in THF
We examined the catecholase activity of L1–L4 ligands with different metal salts, (Cu(CH3COO)2, CuCl2 and Cu(NO3)2 in THF on the production of o-quinone in the presence of oxygen. The oxidation rates of catechol to o-quinone and the absorbance of o-quinone as a function of time obtained are presented in Table 2 and Figs. 3 and 4.
As can be seen in Table 2 and Figs. 3 and 4, all the complexes catalyze the oxidation reaction of catechol to o-quinone with an oxidation rate varying from 26.37, 12.26, 11.38 to 10.21 µmol L−1 min−1 for the L1/Cu(CH3COO)2, L2/CuCl2, L4/Cu(CH3COO)2 and L2/Cu(CH3COO)2 complexes at an average oxidation rate of 4.73 µmol L−1 min−1 for the L4/CuCl2 complex.
Catecholase studies of in situ complexes formation of ligands L 1 -L 4 in methanol (1/2)
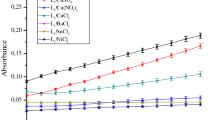
In this study, by mixing 0.1 mL of a 2 × 10–3 mol/L solution of ligand L1-L4 dissolved in methanol for analysis and 0.2 mL of a solution of the copper salt Cu(CH3COO)2, CuSO4, Cu(NO3)2 and CuCl2, and then 2 ml of a solution with a concentration of 0.10 mol/L in catechol are added. After the addition, the evolution of the absorbance at 390 nm is followed as a function of time after adjustment to zero. The evolution of the absorbance of o-quinone as a function of time is given in Figs. 5 and 6 and the rates of oxidation of catechol to o-quinone by complexes formed by ligands L1–L4 and the four salts of copper(II) obtained are collated in Table 3.
From Figs. 5 and 6 and Table 3, it is noted that the oxidation rate of catechol to o-quinone is high when using the copper complex formed by the L4 ligand and the copper(II) salt CuSO4 as a catalyst which reaches 4.24 µmol L−1 min−1 on the other hand, the oxidation rates of catechol to o-quinone remain quite low when using the complexes formed by the other ligands with the various copper(II) salts as catalysts which reach 0.44 and 0.55 µmol L−1 min−1 for combinations formed by L2/CuSO4, L3/CuSO4 and L1/CuSO4. In general, it is noted that each ligand has a special affinity with respect to the anions associated with copper.
All the complexes based on the tridentate ligands with the metal ions Cu(CH3COO)2 represent good rates of oxidation of catechol to o-quinone, on the other hand the complexes formed by the ligands L1–L4 with the other metal salts CuCl2, CuSO4 and Cu(NO3)2 which show low oxidation rates. For the same cation, we note that the oxidation rate varied from ligand to another, for example the combination formed by L1/Cu(CH3COO)2 is the best catalyst since the rate arrives at 26.37 µmol L−1 min−1 than the combinations formed by ligands L2–L4 and the same salt Cu(CH3COO)2 which reach rates of 10.21, 9.03 and 11.38 µmol L−1 min−1. Therefore, it can be concluded that the oxidation rates of catechol to o-quinone strongly depend on the nature of the tripodal ligands and the type of anion used. From Tables 1, 2, 3, it is seen that the nature of the solvent has a tremendous effect on the oxidation rates of catechols. It can be concluded that THF is the right solvent for this oxidation reaction with all the combinations formed such that the oxidation rate reaches 26.37 µmol L−1 min−1 for the L1/(Cu(CH3COO)2 combination.
Vmax and Km study
To determine the equilibrium constant Km and the maximum rate of the reaction Vmax, the Michaelis–Menten curve (Vi = f([catechol]) is used. This is done by studying the absorbance versus time for different substrate concentrations at a fixed catalyst concentration.
For this purpose, the kinetic study of five complexes was carried out: L1/Cu(CH3COO)2 (THF), L2/Cu(CH3COO)2 (THF), L3/Cu(CH3COO)2 (THF), L2/CuCl2 (THF) and L4/Cu(CH3COO)2 (THF).
This study is carried out by mixing 0.15 ml of a copper(II) salt solution with a concentration of 2 10–3 mol/L and 0.15 ml of a solution of corresponding ligands with a concentration of 2 10–3 mol/L, with 2 ml of a solution of catechol at a concentration varying from 10–3 to 0.6 mol/L in a spectrophotometric cell at room temperature and the absorbance of the o-quinone formed in the first five minutes is measured.
The initial rate of formation of o-quinone increases with the concentration of catechol, but from the concentration almost equal to 0.1 mol/L the increase The rate increase becomes low then we reach the maximum rate. The different parameters: the equilibrium constant Km and the rate Vmax of oxidation of catechol to o-quinone for the five combinations, are given in the Table 4.
According to Fig. 7, it is observed that the kinetic study for all combinations studied, that there is a good correlation between the curves found with that of the Michaelis–Menten model. The Michaelis–Menten model is applied to determine the kinetic parameters of the best catalysts to study. The rate Vmax for the different combinations in THF are high and with low Km values, which explains why the affinity is strong in THF. We give in Table 4 the initial rate found and calculated (V0 and V0calc).
The different parameters of the values of the rate Vmax, the equilibrium constant Km and standard deviation of the value of Vmax and Km, the coefficient of determination (R2), sum of squared deviations and the parameter correlation for the different combinations in THF are presented in the Table 5.
These different parameters were determined according to the method described by Gabar Lente [60].
UV–vis spectrophotometric study
To validate the significant catalytic activity of different combinations L1/Cu(CH3COO)2 in the THF and L1/CuSO4 in the MeOH) among the best combinations of our complexes for example, the kinetics of o-quinone and the change in absorbance was recorded every 5 min. Kinetic experiments were performed at room temperature (Fig. 8). The appearance and evolution of the intensity of a band concentrated at 390 nm, explains that the combinations are good catalysts for the oxidation of catechol to o-quinone.
combination a L1/Cu(CH3COO)2 in the THF and b L1/CuSO4 in the MeOH
Conclusion
In this work, we synthesized four new tridentats ligands L1-L4, namely N,N-Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)pyridin-2-amine:L1, 5-chloro-N,N-Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)pyridin-2-amine: L2, N,N-Bis((3,5-dimethyl-1H-pyrazol-1-yl) methyl) thiazol-2-amine:L3 and N,N-Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-6-methylpyridin-2-amine: L4. Moreover, we have characterized these ligands by 13C NMR, 1H NMR and mass spectroscopy. Next, we have carried out the oxidation of catechol to o-quinone by complexes formed in situ, in the presence of oxygen at room temperature; by mixing tridentate ligands based on pyrazole and pyridine with copper(II) salts. The results obtained show that all the complexes catalyze the oxidation of catechol with different oxidation rates. Finally, we studied the parameters influencing the catalytic activity of the complexes studied such as the nature and the concentration of the ligand, the nature of the metal and the anion bound to the metal, the nature of the solvent and the concentration of the catechol substrate. We also studied the kinetics of the oxidation reaction of catechol to o-quinone using the Michaelis–Menten model, to find the kinetic parameters such as Vmax and Km.
Data Availability
Not applicable.
References
Morioka C, Tachi Y, Suzuki S, Itoh S (2006) Significant enhancement of monooxygenase activity of oxygen carrier protein hemocyanin by urea. J Am Chem Soc 128(21):6788–6789. https://doi.org/10.1021/ja061631h
Zal F, Chausson F, Leize E, van Dorsselaer A, Lallier FH, Green BN (2002) Quadrupole time-of-flight mass spectrometry of the native hemocyanin of the deep-sea crab Bythograea thermydron. Biomacromol 3(2):229–231. https://doi.org/10.1021/bm0101668
Wilcox DE, Porras AG, Hwang YT, Lerch K, Winkler ME, Salomon EI (1985) Substrate analog binding to the coupled binuclear copper active site in tyrosinase. J Am Chem Soc 107(13):4015–4027. https://doi.org/10.1021/ja00299a043
Yu L (2003) Inhibitory effects of (S)- and (R)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acids on tyrosinase activity. J Agric Food Chem 51(8):2344–2347. https://doi.org/10.1021/jf0208379
Itoh S, Kumei H, Taki M, Nagatomo S, Kitagawa T, Fukuzumi S (2001) Oxygenation of phenols to catechols by A (μ-η2:η2-Peroxo)dicopper(II) complex: mechanistic insight into the phenolase activity of tyrosinase. J Am Chem Soc 123(27):6708–6709. https://doi.org/10.1021/ja015702i
Malachowski MR, Davidson MG, Hoffman JN (1989) Synthesis, characterization and catecholase activity of a series of novel mononuclear Cu(II) complexes derived from a tripodal ligand. Inorg Chim Acta 157:91–94. https://doi.org/10.1016/S0020-1693(00)83428-3
Iryna AK, Mieke H, Arno FS, Partick G, Olivier R, Catherine B, Jean-Louis P, Eric SA, Matthias L, Bernt K, Martin L, Anthony LS, Reedijk J (2004) Dinuclear CuII complexes with a new phenol-based ligand bearing pyridine and thiophene substituents: synthesis, characterization and interaction with catechol substrates. Eur J Inorg Chem 20:4036–4045. https://doi.org/10.1002/ejic.200400092
Tippu SS, Pamela C, Brian P (2002) Manganese catalysed reduction of dioxygen to hydrogen peroxide: structural studies on a manganese(III)–catecholate complex. Inorg Chim Acta 348:115–122. https://doi.org/10.1016/S0020-1693(02)01511-6
Calancea S, Reis SG, Guedes GP, AllaoCassaro RA, Semaan F, Lopez-Ortiz F, Var MGF (2016) A new family of multinuclear mixed-ligand copper(II) clusters: crystal structures, magnetic properties and catecholase-like activity. Inorg Chim Acta 453:104–114. https://doi.org/10.1016/j.ica.2016.07.057
Mitic N, Smith JS, Neves A, Guddat LW, Gahan LR, Schenk G (2006) The catalytic mechanisms of binuclear metallohydrolases. Chem Rev 106(8):3338–3363. https://doi.org/10.1021/cr050318f
Gichinga MG, Striegler S (2008) Effect of water on the catalytic oxidation of catechols. J Am Chem Soc 130(15):5150–5156. https://doi.org/10.1021/ja078057+
Rey NA, Neves A, Bortoluzzi AJ, Pich CT, Terenzi H (2007) Catalytic promiscuity in biomimetic systems: catecholase-like activity, phosphatase-like activity, and hydrolytic DNA cleavage promoted by a new dicopper(II) hydroxo-bridged complex. Inorg Chem 46(2):348–350. https://doi.org/10.1021/ic0613107
Mondal M, Guha PM, Giri S, Ghosh A (2016) Deactivation of catecholase-like activity of a dinuclear Ni(II) complex by incorporation of an additional Ni(II). J Mol Catal A: Chem 424:54–64. https://doi.org/10.1016/j.molcata.2016.08.012
Osorio REHMB, Peralta RA, Bortoluzzi AJ, de Almeida VR, Szpoganicz B, Fischer FL, Terenzi H, Mangrich AS, Mantovani KM, Ferreira DEC, Rocha WR, Haase W, Tomkowicz Z, Dos Anjos A, Neves A (2012) Synthesis, magnetostructural correlation, and catalytic promiscuity of unsymmetric dinuclear copper(II) complexes: models for catechol oxidases and hydrolases. Inorg Chem 51(3):1569–1589. https://doi.org/10.1021/ic201876k
Gahan LR, Smith S, Neves A, Schenk G (2009) Phosphate ester hydrolysis: metal complexes as purple acid phosphatase and hosphotriesterase analogues. Eur J Inorg Chem 19:2745–2758. https://doi.org/10.1002/ejic.200900231
Neves A, Lanznaster M, Bortoluzzi A, Peralta RA, Casellato A, Castellano EE, Herrald P, Riley MJ, Schenk G (2007) An unprecedented FeIII(μ-OH)ZnII complex that mimics the structural and functional properties of purple acid phosphatases. J Am Chem Soc 129(4):7486–7487. https://doi.org/10.1021/ja071184l
Adhikary J, Chakraborty A, Dasgupta S, Chattopadhyay SK, Kruszynski R, Trzesowska-Kruszynska A, Stepanovic´ S, Gruden-Pavlovic´ M, Swart M, Das D (2016) Unique mononuclear MnII complexes of an end-off compartmental Schiff base ligand: experimental and theoretical studies on their bio-relevant catalytic promiscuity. Dalton Trans 45:12409–12422. https://doi.org/10.1039/C6DT00625F
Gajewska MJ, Ching W, Wen Y, Hung C (2014) Synthesis, structure, and catecholase activity of bispyrazolylacetate copper(ii) complexes. Dalton Trans 43:14726–14736. https://doi.org/10.1039/C4DT01467G
Shyamal M, Mandal TK, Panja A, Saha A (2014) Influence of anionic co-ligands on the structural diversity and catecholase activity of copper(ii) complexes with 2-methoxy-6-(8-iminoquinolinylmethyl)phenol. RSC Adv 4:53520–53530. https://doi.org/10.1039/C4RA08025D
Adhikary J, Majumdar I, Kundu P, Kornweitz H, Kara H, Das D (2018) Role of electronegative atom present on ligand backbone and substrate binding mode on catecholase- and phosphatase-like activities of dinuclear niii complexes: a theoretical support. ChemistrySelect 3(5):1445–1454. https://doi.org/10.1002/slct.201702861
El Kodadi M, Malek F, Touzani R, Ramdani A (2008) Synthesis of new tripodal ligand 5-(Bis(3,5-dimethyl-1H-pyrazol-1-ylmethyl)amino)pentan-1-ol, catecholase activities studies of three functional tripodal pyrazolyl N-donor ligands, with different copper(II) salts. Catal Commun 9(5):966–969. https://doi.org/10.1016/j.catcom.2007.09.038
Titi A, Al-Noaimi M, Kaddouri Y, El Ati R, Yousfi EB, El Kodadi M, Touzani R (2019) Study of the catecholase catalytic properties of copper(II) complexes prepared in-situ with monodentate ligands. Mater Today: Proc 13:1134–1142. https://doi.org/10.1016/j.matpr.2019.04.081
Boyaala R, El Ati R, Khoutoul M, El Kodadi M, Touzani R, Hammouti B (2018) Biomimetic oxidation of catechol employing complexes formed in situ with heterocyclic ligands and different copper(II) salts. J Iran Chem Soc 15(1):85–92. https://doi.org/10.1007/s13738-017-1211-0
Ding HY, Cheng HJ, Wang F, Liu DX, Li HX, Fang YY, Zhao W, Lang JP (2013) [(bmppy)Cu(μ-I)]2 (bmppy = 2,6-Bis(1-methyl-1H-pyrazol-3-yl)pyridine): synthesis, crystal structure and its catalytic performance for MMA polymerization. J Org Chem 741–742:1–6. https://doi.org/10.1016/j.jorganchem.2013.05.012
El Ati R, Takfaoui A, El Kodadi M, Touzani R, Yousfi EB, Almalki FA, Ben Hadda T (2019) Catechol oxidase and copper(I/II) complexes derived from bipyrazol ligand: synthesis, molecular structure investigation of new biomimetic functional model and mechanistic study. Mater Today 13:1229–1237. https://doi.org/10.1016/j.matpr.2019.04.092
Malek F, Draoui N, Feron O, Radi S (2014) Tridentate bipyrazole compounds with a side-arm as a new class of antitumor agents. Res Chem Intermed 40:681–687. https://doi.org/10.1007/s11164-012-0993-z
Harit T, Malek F, El Bali B, Khan A, Dalvandi K, Marasini BP, Noreen S, Malik R, Khan S, Choudhary MI (2012) Synthesis and enzyme inhibitory activities of some new pyrazole-based heterocyclic compounds. Chem Res 21(10):2772–2778. https://doi.org/10.1007/s00044-011-9804-0
Lamsayah M, Khoutoul M, Takfaoui A, Abrigach F, Oussaid A, Touzani R (2015) Selective liquid-liquid extraction of Fe(II) and Cd(II) using N, N’-pyrazole bidentate ligands with theoretical study investigations. Sep Sci Technol 50(14):2170–2176. https://doi.org/10.1080/01496395.2015.1015685
Gamez P, Steensma RH, Driessen WL, Reedijk J (2002) Copper(II) compounds of the planar-tridentate ligand 2,6-Bis(pyrazol-3-yl)pyridine. Inorg Chim Acta 333:51–56. https://doi.org/10.1016/S0020-1693(02)00754-5
Mukherjee R (2000) Coordination chemistry with pyrazole-based chelating ligands: molecular structural aspects. Coord Chem Rev 203:151–218. https://doi.org/10.1016/S0010-8545(99)00144-7
Zavozin AG, Ignat’ev NV, Schulte M, Zlotin SG (2015) Synthesis of novel tridentate pyrazole–bipyridine ligands for Co-complexes as redox-couples in dye-sensitized solar cells. Tetrahedron 71(45):8551–8556. https://doi.org/10.1016/j.tet.2015.09.032
Elmsellem H, Harit T, Aouniti A, Malek F, Riahi A, Chetouani A, Hammouti B (2015) Adsorption properties and inhibition of mild steel corrosion in 1 M HCl solution by some bipyrazolic derivatives: Experimental and theoretical investigations. Metals Phys Chem Surfaces 51(5):873–884. https://doi.org/10.1134/S207020511505007X
El Kodadi M, Benamar M, Bouabdallah I, Zyad A, Malek F, Touzani R, Ramdani A, Melhaoui A (2007) New synthesis of two tridentate bipyrazolic compounds and their cytotoxic activity tumor cell lines. Product Res 21(11):947–952. https://doi.org/10.1080/14786410701371314
Harit T, Malek F (2017) Elaboration of new thin solid membrane bearing a tetrapyrazolic macrocycle for the selective transport of lithium cation. Sep Purif Technol 188:394–398. https://doi.org/10.1016/j.seppur.2017.07.060
Harit T, Malek F, El Bali B, Dusek M, Kucerakova M (2016) Synthesis and characterization of two new tetrapyrazolic macrocycles for the selective extraction of cesium cation. Tetrahedron 72(27–28):3966–3973. https://doi.org/10.1016/j.tet.2016.05.026
Harit T, Isaad J, Malek F (2016) Novel efficient functionalized tetrapyrazolic macrocycle for the selective extraction of lithium cations. Tetrahedron 72(18):2227–2232. https://doi.org/10.1016/j.tet.2016.03.006
Harit T, Bellaouchi R, Mokhtari C, El Bali B, Asehraou A, Malek F (2017) New generation of tetrapyrazolic macrocycles: synthesis and examination of their complexation properties and antibacterial activity. Tetrahedron 73(34):5138–5143. https://doi.org/10.1016/j.tet.2017.07.006
Abrigach F, Bouchal B, Riant O, Macé Y, Takfaoui A, Radi S, Oussaid A, Bellaoui M, Touzani R (2016) New N, N, N’, N’-tetradentate pyrazoly agents: synthesis and evaluation of their antifungal and antibacterial activities. Med Chem 12:83–89. https://doi.org/10.2174/1573406411666150519111800
Merkel M, Möller N, Piacenza M, Grimme S, Rompel A, Krebs B (2005) Less symmetrical dicopper(II) complexes as catechol oxidase models-an adjacent thioether group increases catecholase activity. Chem Eur J 11:1201–1209. https://doi.org/10.1002/chem.200400768
Koval IA, Gamez P, Belle C, Selmeczi K, Reedijk J (2006) Synthetic models of the active site of catechol oxidase: mechanistic studies. Chem Soc Rev 35:814–840. https://doi.org/10.1039/b516250p
Thio Y, Yang X, Vittal JJ (2014) Influence of inductive effects and steric encumbrance on the catecholase activities of copper(II) complexes of reduced Schiff base ligands. Dalton Trans 43:3545–3556. https://doi.org/10.1039/C3DT52829D
Biswas A, Das LK, Drew MGB, Diaz C, Ghosh A (2012) Insertion of a Hydroxido bridge into a diphenoxido dinuclear copper(II) complex: drastic change of the magnetic property from strong antiferromagnetic to ferromagnetic and enhancement in the catecholase activity. Inorg Chem 51:10111–10121. https://doi.org/10.1021/ic300319s
Banu KS, Chattopadhyay T, Banerjee A, Bhattacharya S, Zangrando E, Das D (2009) Catechol oxidase activity of dinuclear copper(II) complexes of robson type macrocyclic ligands: syntheses, X-ray crystal structure, spectroscopic characterization of the adducts and kinetic studies. J Mol Catal A: Chem 310:34–41. https://doi.org/10.1016/j.molcata.2009.05.016
Maiti M, Sadhukhan S, Thakurta S, Zangrando E, Pilet G, Bauzá A, Frontera A, Dede B, Mitra S (2014) Synthesis, structural characterization, theoretical calculations and catecholase mimetic activity of manganese-Schiff base complexes. Polyhedron 75:40–49. https://doi.org/10.1016/j.poly.2014.03.005
Malachowski MR, Davidson MG (1989) Novel mono- and binuclear Cu(II) complexes: synthesis, characterization and catecholase activity. Inorg Chim Acta 162:199–204. https://doi.org/10.1016/S0020-1693(00)83147-3
Bhardwaj VK, Aliaga-Alcalde N, Corbella M, Hundal G (2010) Synthesis, crystal structure, spectral and magnetic studies and catecholase activity of copper(II) complexes with di-and tri-podal ligands. Chim Acta 363:97–106. https://doi.org/10.1016/j.ica.2009.09.041
Boulemche H, Anak B, Djedouani A, Touzani R, François M, Fleutot S, Rabilloud F (2019) Synthesis. X-ray crystallography, computational studies and catecholase activity of new zwitterionic Schiff base derivatives. J Mol Struct 1178:606–616. https://doi.org/10.1016/j.molstruc.2018.10.078
Mouadili A, Attayibat A, El Kadiri S, Radi S, Touzani R (2013) Catecholase activity investigations using in situ copper complexes with pyrazole and pyridine based ligands. Appl Catal A-Gen 454:93–99. https://doi.org/10.1016/j.apcata.2013.01.011
Bouabdallah I, Touzani R, Zidane I, Ramdani A (2007) Synthesis of new tripodal ligand: N, N-Bis[(1,5-dimethylpyrazol-3-yl)methyl]benzylamine.: catecholase activity of two series of tripodal ligands with some copper(II) salts. Catal Commun 8:707–712. https://doi.org/10.1016/j.catcom.2006.08.034
Marion R, Saleh NM, Le Poul N, Floner D, Lavastre O, Geneste F (2012) Rate enhancement of the catechol oxidase activity of a series of biomimetic monocopper(II) complexes by introduction of non-coordinating groups in N-tripodal ligands. New J Chem 36:1828–1835. https://doi.org/10.1039/C2NJ40265C
Mendoza-Quijano MR, Ferrer-Sueta G, Flores-A´lamo M, Aliaga-Alcalde N, Gomez-Vidales V, Ugalde-Saldivara VM, Gasque L (2012) Mechanistic insight on the catecholase activity of dinuclear copper complexes with distant metal centers. Dalton Trans 41:4985–4997. https://doi.org/10.1039/c2dt12155g
Zerrouki A, Touzani R, El Kadiri S (2011) Synthesis of new derivatized pyrazole based ligands and their catecholase activity studies. Arab J Chem 4:459–464. https://doi.org/10.1016/j.arabjc.2010.07.013
Mouadili A, Zerrouki A, Herrag L, Hammouti B, El Kadiri S, Touzani R (2012) Catechol oxidation: activity studies using electron-rich nitrogen-based ligands. Res Chem Intermed 38:2427–2433. https://doi.org/10.1007/s11164-012-0558-1
Saddik R, Khoutoul M, Benchat N, Hammouti B, El Kadiri S, Touzani R (2012) Evaluation of catalytic activity of imidazolo[1,2-a]pyridine derivatives: oxidation of catechol. Res Chem Intermed 38:2457–2470. https://doi.org/10.1007/s11164-012-0561-6
Saddik R, Abrigach F, Benchat N, El Kadiri S, Hammouti B, Touzani R (2012) Catecholase activity investigation for pyridazinone- and thiopyridazinone-based ligands. Res Chem Intermed 38:1987–1998. https://doi.org/10.1007/s11164-012-0520-2
Dvoretzky I, Richter GH (1950) Formaldehyde condensation in the pyrazole series. J Org Chem 15:1285–1288. https://doi.org/10.1021/jo01152a026
Touzani R, Ramdani A, Ben Hadda T, El Kadiri S, Maury O, Le Bozec H, Dixneuf PH (2001) Efficient synthesis of new nitrogen donor containing tripods under microwave irradiation and without solvants. Synth Commun 31:1315–1321. https://doi.org/10.1081/SCC-100104040
Kalanithi M, Rajarajan M, Tharmaraj P, Johnson Raja S (2015) Synthesis, spectroscopic characterization, analgesic, and antimicrobial activities of Co(II), Ni(II), and Cu(II) complexes of 2-[N, N-Bis-(3,5-dimethyl-pyrazolyl-1- methyl)] aminothiazole. Med Chem Res 24:1578–1585. https://doi.org/10.1007/s00044-014-1224-5
Kaddouri Y, Abrigach F, Ouahhoud S, Benabbes R, El Kodadi M, Alsalme A, Al-Zaqri N, Touzani R (2021) Synthesis, characterization, reaction mechanism prediction and biological study of mono, bis and tetrakis pyrazole derivatives against Fusarium oxysporum f. sp. Albedinis with conceptual DFT and ligand-protein docking studies. Bioorganic Chem 110:104696. https://doi.org/10.1016/j.bioorg.2021.104696
Gábor Lente: Deterministic Kinetics in Chemistry and Systems Biology. Springer, 2015, ISBN 978–3–319–15481–7, pp. 52–58. DOI https://doi.org/10.1007/978-3-319-15482-4.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under grant number RGP.2/226/43.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouroumane, N., El Boutaybi, M., El Kodadi, M. et al. Synthesis of new heterocyclic ligands and study of the catecholase activity of catalysts based on copper(II). Reac Kinet Mech Cat 136, 1545–1562 (2023). https://doi.org/10.1007/s11144-023-02370-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02370-7