Abstract
A novel series of α-aminophosphonates containing the trifluoromethyl aniline moiety were obtained in high yields by condensation of 2-methyl-3-trifluoromethyl aniline, aryl/heteroaryl aldehydes and dimethylphosphite in the presence of chitosan as a catalyst. The molecular modeling studies revealed their important structural features of binding affinities towards the target enzyme. The cytotoxicity of these compounds was evaluated against PC-3(prostate cancer), MCF-7 (breast cancer), HeLa(cCervix Cancer), U973, K562 and HL60 human lLeukemia cell lines. Compound 4k with a pyrene moiety showed high potency against a breast cancer cell line, while compounds 4g and 4k exhibited more promising cytotoxicity against U973, K562 and HL60 cell lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trifluoromethyl anilines are important constituents of many bio-active synthetic organic compounds, and this background triggered increasing interest in the chemistry of fluorine-containing compounds (Fig. 1) which have unique properties such as high thermal stability and enhanced lipophilicity compared to non-fluorinated counterparts. The significance of the fluorine substitution in organic compounds is well illustrated by way of their use as antimalarial (1), anti-depressant (2), COX-2 inhibitor (3), HIV protease inhibitors (4) and other important bio-activities, including analgesic and anti-inflammatory (5) [1,2,3,4,5].
On the other hand, the role of α-aminophosphonates in the biological system stimulated the researchers to develop various methods to synthesize novel bioactive α-aminophosphonates [6]. Many natural and synthetic α-aminophosphonates and their derivatives have potential applications as anticancer and antibiotic agents [7, 8]. Furthermore, they are used in agriculture as fungicides, herbicides, and plant growth regulators [9,10,11]. They have been synthesized by using acid catalysts, such as, Lewis (like, SnCl4 [12], InCl3 [13], In(OTf)3 [14], Yb(PfO)3 [15], SmI2 [16] and TaCl5-SiO2 [17]) and Bronsted (like, sulfamic acid [18] and oxalic acid [19]) acids, solid acids H3PMo12O40 [20], silica sulfuric acid [21], and base catalysts (like, CaCl2 [22], PPh3 [23]) and Amberlyst-15 [24]. Other catalysts such as NbCl5 [25], Nano-TiO2 [26], β-cyclodextrine [27] and quaternary ammonium salts [28] have also promoted this reaction. However, these methods have many disadvantages. They require long reaction times, moisture sensitive toxic catalysts, require stoichiometric amounts of catalysts, offer poor product yields and generate large amounts of waste.
In recent years, green chemical synthesis has received extensive attention [29,30,31]. In this context, heterogeneous catalysis has emerged as a useful tool. Since it drives the reaction in eco-friendly conditions, it produces relatively pure products without waste. In this connection, chitosan, which is a biodegradable, optically active polymer with a strong affinity for transition metals has been used as a solid support in the form of colloids, flakes, gel beads, fibers or as an immobilized form on inorganic material supports (like, alumina, silica, or other metal oxides) [32]. The ease with which it can be modified physically and chemically, opens up avenues for manufacturing a wide range of catalysts from it for applications in the fields of hydrogenation, oxidation, and fine chemical synthesis.
Therefore, in the present work we report green one-pot synthesis of a new series of α-aminophosphonates (4a-o) containing a trifluoromethyl aniline moiety with the hope of developing new anticancer agents. Their synthesis was accomplished by reacting different aldehydes, dialkyl phosphites under microwave irradiation conditions using chitosan as an efficient catalyst. The newly synthesized compounds were screened for their in vitro anticancer activities against PC-3(prostate cancer), MCF-7(breast cancer), HeLa(cervix cancer), U-973, K-562 and HL-60 (leukemia) cell lines.
Results and discussion
Chemistry
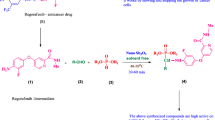
We reinvestigated the one-pot Kabachnik–Fields reaction of 2-Methyl-3-(trifluoromethyl) aniline (1), Aryl/heteroaryl aldehydes (2a–o), and dimethylphosphite (3a) under different experimental conditions to obtain corresponding phosphonate (4a–o) (Scheme 1). First, the optimum experimental conditions for this reaction such as different heating techniques, with or without different solvents, catalysts, optimum time and temperature are determined (Table 1).
We have evaluated different common Lewis acids as catalysts, which promote this three-component Kabachnik–Fields reaction. Lewis acids such as ZnCl2, Tween-20, AlCl3, Incl3, PTSA, PEG-SO3H, TMG, NanoTiO2 and Nano ZnO afforded the desired product but only in moderate yield (Table 1). However, with chitosan as catalyst, the reaction goes to completion rapidly and afforded the desired products in pure form with high yields.
The same reaction under neat conditions with conventional heating resulted in poor yields even after prolonged reaction time (Table 1, entry 1). The reaction in any solvent medium with conventional heating turned out to be ineffective as the product separation and purification was found to be difficult and have lower product yields that resulted even after over 4 h reaction time (Table 1, entries 3 and 4). The same reaction, when run by using Tween 20 and AlCl3 as catalysts at 10 mol% concentration in different solvents and without solvent by both conventional heating and microwave irradiation (MWI), had the product yields improved, but the reaction times were long (Table 1, entries 5–8). The reaction was also tried with other NbCl5 and InCl3 catalysts under solvent-free conditions. The reaction went smoothly, but results were not better (Table 1, entries 9 and 10). The reported advantages of catalysts such as PTSA, PEG-SO3H, TMG, Nano TiO2 and Nano ZnO were not found (Table 1, entries 11–15). But almost quantitative yields were obtained with chitosan as catalyst (Table 1, entry 16). In addition, we found that the MWI of substrates with chitosan catalyst at 60 °C for 2 min without solvent was the optimum condition for the formation of compound 4a in high yield. The quantity of the catalyst required for this reaction was optimized by studying the effect of different amounts of catalyst (Table 1, entries 17–19). We found that 10 mol% of chitosan was sufficient to drive the reaction to completion in 2 min, giving 98% product yield. Less amounts of catalyst gave lower yields even after prolonged reaction time and higher mol% quantities could not increase the product yield and decreased the reaction time. The reusability of the chitosan catalyst was also examined for this reaction. After each run, the catalyst was filtered, washed with petroleum ether and reused. In each subsequent reuse over five cycles of the chitosan catalyst, the yield of α-aminophosphonate (4a) was 98, 97 95, 93, and 92%, respectively, and the results are summarized in Fig. 2. These results indicate that the chitosan catalyst is a reusable one.
It is observed that the Kabachnik–Fields reaction is sensitive to temperature. Even though high wattage of microwaves enhance the reaction rate, they trigges side reactions and decompose the formed products to the corresponding aldehyde and amine. Thus, it was found that irradiation of reaction substrates with 180 W of microwaves for 2 min is sufficient to drive the reaction to completion. The generality and scope of this method were examined. Several substituted aryl/heteroaryl aldehydes, 2-methyl-3-(trifluoromethyl) aniline, and dimethyl phosphite using chitosan under neat conditions offered the desired products (Table 1). In all cases, aromatic aldehydes substituted with either electron-donating or electron-withdrawing groups reacted smoothly and afforded corresponding products in good yields. However, the aldehydes bearing electron-withdrawing groups required shorter reaction time and gave higher yields (Table 2).
2-Methyl-3-(trifluoromethyl)aniline (1) reacted with 4-Nitrobenzaldehydes (2a) and dimethylphosphite (3) in the presence of 10 mol% of chitosan catalyst under microwave irradiation at 180 W for 2 min. The progress of the reaction was monitored by thin layer chromatography. The reaction proceeded smoothly and was completed in 2 min to afford the corresponding α-aminophosphonates in high yield (98%). This showed that chitosan acts as an effective catalyst in this reaction. A probable reaction mechanism for the three-component reaction of thev benzaldehyde, amine and dimethylphosphite scaffold catalyzed by chitosan catalyst is shown in Scheme 2. The free amino group in chitosan distributed on the surface of chitosan activates the carbonyl group of benzaldehyde through nucleophilic attack to produce the corresponding intermediate. Further, amine reacts with this intermediate and produces imine, then chitosan catalyst may be free, which again participates in the mechanism, then imine reacts with dimethylphosphite to afford the target product. An important feature is that the chitosan can be easily recovered from the reaction mixture after its completion and can be reused.
The chemical structures of all the new compounds were confirmed by, IR, 1H-, 13C- and 31P-, 19F-NMR and Mass spectra. Compounds (4a–o) exhibited characteristic IR stretching frequencies in the regions 3320–3490, 1210–1250, 980–1030 cm−1 for N–H, P=O, and C–F, respectively. The aromatic protons of the benzene rings of the α-aminophosphonates (4a–o) showed a complex multiplet at δ 6.81–8.36. The P–C–H proton signal appeared as a doublet at δ 5.04–5.89 due to its coupling with both phosphorus and the N–H proton. The N–H proton signal appeared at δ 5.70–5.99 as a singlet. The methoxy group protons of the dimethylphosphite moiety resonated as two distinct doublets in the range of δ 3.54–3.57 and δ 3.73–3.76, indicating their non-equivalence. The carbon chemical shifts for aliphatic and aromatic carbons in the title compounds were observed at δ 13.0–160.0, respectively. The 31P-NMR chemical shifts appeared in the region δ 20.30–27.80 and 19F-NMR chemical shifts appeared in the region δ −59.13 to −59.93 for these compounds.
Pharmacology
Molecular docking studies
The docking conformations of compounds in topoisomerase-II are shown in Fig. 3. Among the different docking conformations of compounds, the compounds 4a, 4b, 4g, 4k and 4n are shown as the highest docking scores −9.8, −9.0, −9.9, −9.8 and −9.4, respectively, due to the fluorine interaction, and π-Alkyl interactions in addition to conventional hydrogen bonding interactions with active pocket of topoisomerase-II enzyme. These compounds have the highest docking scores when compared to standard anticancer drug adriamycin, which has a docking score of −9.6 only. Adriamycin has only π-Alkyl interactions and conventional hydrogen bonding interactions with an active pocket of topoisomerase-II enzyme. Among the test compounds, 4a, 4g and 4k have higher docking scores than the standard adriamycin because of stronger molecular interactions with topoisomerase-II enzyme and have less docking scores. The types of molecular interactions, interacting atoms of proteins, ligands, and docking scores are shown in Table 3.
Fluorine in α-aminophosphonate compounds (4a–o) was actively binding to topoisomerase protein by the conventional hydrogen bonding and halogen interactions. They were also showing π-alkyl and conventional hydrogen bonding interactions. Because of these molecular interactions of these compounds (4a–o) were strongly inhibiting the topoisomerase-II enzyme of cancer cells.
Cytotoxicity
The cytotoxicity of 4a–o was evaluated in vitro against U-973, K-562 & HL-60 (human leukaemia), PC-3(prostate cancer), MCF-7(breast cancer), HeLa(cervical cancer) cells after 24 h exposure and their GI50 values were determined from a graph of cell capability measured over a range of concentrations between 0.1 mM–0.1 μM. Each data point was the average of four determinations that in all cases differed by 10–20% or less. These results are summarized in Table 4. Initially, the GI50 was determined by a broad range of concentrations, specifically, 0.1, 1, 10 and 0.1 mM of the title compounds against the cancer cell lines. From the data it is revealed that all compounds exhibited a different range of significant cytotoxic activities varying from 0.1 mM–0.1 μM due to structural differences. As evident from the cytotoxicity data, compound 4k with a pyrene moiety at the α-carbon atom showed the highest activity against the MCF-7 breast cancer cell lines at the concentration of 0.1 mM, and it has shown a very low GI50 value (−136.50 ± 5.90) when compared to positive control drug Adriamycin (−121.2 ± 1.68). Similarly, the same compound 4k exhibit high cytotoxicity against HeLa, PC-3, U937 & K562 cell lines at the concentration of 0.1 mM and their GI50 values −119.0 ± 7.34, −96.9 ± 1.03, −52.4 ± 13.63 and 71.5 ± 10.44, respectively. The compounds 4a, 4b, 4g and 4n were shown to have significant cytotoxicity activity against cancer cell lines at the concentration of 0.1 mM and which have low GI50 data in Table 4. There was moderate cytotoxicity at the concentration of 0.1 mM of 4c, 4h and 4i compounds. The cytotoxicity activity of 4d, 4e, 4i, 4j, 4l, 4m and 4o showed a low percentage of inhibition of growth of cancer cell lines at all the concentrations and higher GI50 values when compared to the 4k compound. The results of percentage of growth inhibition (GI50) of compounds when compared with the positive control drug have shown significant cytotoxicity against cancer cell lines. The data of GI50 values of compounds and Adriamycin are shown in Table 4. The percentage inhibition of growth in human leukemia cells (K-562), human prostate cancer (PC-3), human breast cancer cells (MCF-7) and human cervix cancer (HeLa) cells following treatment with the title compounds (4a–o). They were measured at 0.1 mM and 0.1 μM concentrations. They were the mean values determined from three independent experiments run in triplicate. This study thus discovered a new family of dimethyl (2-methyl-3-(trifluoromethyl) phenyl amino) (aryl/heteroaryl) methyl phosphonate (4a–o) that have significant target specific cytotoxicity on some cancer cells (Table 4).
Experimental
Analysis and instruments
Reagents were purchased from common commercial sources. All solvents were purified and dried by standard procedures. All the reactions were monitored by thin-layer chromatography (TLC) on silica gel GF254 plates from Qingdao Haiyang Chemical Co. Ltd (China), visualized in an iodine chamber or with an UV lamp (254 nm). Column chromatography was performed using silica gel (100–200 mesh) purchased from Qingdao Haiyang Chemical Co.Ltd (China). The melting points of the products were determined on a Guna Digital melting point apparatus (China) and are uncorrected. The IR spectra were recorded on a Bruker Alpha ECO-ATR FTIR (Attenuated total reflection–Fourier transform infrared) interferometer with a single reflection sampling module equipped with ZnSe crystal. Elemental analysis was performed on an ElementarVario-III CHN analyzer. NMR spectra were recorded on a Bruker Alpha instrument (400 MHz for 1H, 100 MHz for 13C, 125 MHz for 31P, and 470 MHz for 19F) using CDCl3 and DMSO d6 as solvent. TMS (δ = 0) served as an internal standard for 1H-NMR, CDCl3 (δ = 77.0) was used as an internal standard for 13C-NMR, H3PO4 (δ = 0) was used as an external standard for 31PNMR, CF3COOD was used as an external standard (δ = −76.5) for 19F-NMR. Mass spectra were recorded on a LC–MS/MS-TOF API QSTAR PULSAR spectrometer, samples were introduced by the infusion method using the Electrospray Ionization Technique (ESI). All other chemicals were of analytical grade.
General procedure for synthesis of α-aminophosphonates (4a–o)
An equimolar mixture of 2-methyl-3-(trifluoromethyl)aniline (0.351 g, 0.002 mol), corresponding aldehyde (0.002 mol), dimethyl phosphite (0.18 ml, 0.002 mol) and chitosan catalyst(10 mol%) were taken in a reaction glass tube, degassed for 10 min and microwave irradiated at 180 W for 2 min at 60 °C. The progress of the reaction was monitored by TLC using petroleum ether and ethylacetate (3:7) as solvent. After completion of the reaction, the mixture was diluted with ethyl acetate, washed with water (2 × 15 ml) followed by brine (1 × 10 ml), dried over Na2SO4 and evaporated to dryness. The crude mass was purified by column chromatography on silicagel (100–200 mesh) by using a 7:3 mixture of ethylacetate in hexane to afford the pure α-aminophosphonates.
Dimethyl (2-methyl-3-(trifluoromethyl) phenylamino)(4-nitrophenyl)methylphosphonate ( 4a )
Yellow solid; Yield: 98%. M.p.132–134 °C. IR (cm−1): ν 3420 (NH), 2851 (C–H), 1000 (C–F), 1232 (P=O).1H-NMR (DMSO-d6): δ 8.23(d, J = 8.8 Hz, 2H, Ar–H), 7.86–7.89 (m, 2H, Ar–H), 7.11 (t, J = 16.0 Hz, 1H, Ar–H), 7.00(d, J = 8.0 Hz, 1H, Ar–H), 6.81 (d, J = 8.0 Hz, 1H, Ar–H); 5.92 (s, 1H, NH), 5.58 (d, J = 23.2 Hz, 1H, P–C–H), 3.76 (d, J = 10.8 Hz, 3H, P–OCH3), 3.57 (d, J = 10.4 Hz, 3H, P–OCH3), 2.35 (s, 3H, CH3). 13C-NMR (CDCl3): δ 147.82(C6), 143.90(C15), 143.06(C11), 128.47(C17 & C13), 126.50(C4), 123.61(C14 & C16), 121.36(C18), 116.21(C1), 114.54(C5), 56.44(C9), 54.77(C23), 53.94(C25), 12.32(C7). 31P-NMR (CDCl3): δ 25.24. 19F-NMR (CDCl3): δ −59.86. MS (ESI): m/z 419[M + H]+, 441[M + Na]+ Anal.Calcd.for C17H18F3N2O5P: C, 48.81; H, 4.34; N, 6.70. Found C, 48.03; H, 4.74; N, 6.48.
Dimethyl (4-chlorophenyl)(2-methyl-3-(trifluoromethyl)phenylamino)methylphosphonate ( 4b )
White solid; Yield: 96%. M.p. 125–127 °C. IR (cm−1): ν 3415 (NH), 2845 (C–H), 1007 (C–F), 1228 (P=O).1H-NMR (DMSO-d6): δ 7.69 (d, J = 8.6 Hz, 2H, Ar–H), 7.29–7.31 (m, 2H, Ar–H), 7.09 (t, J = 7.8 Hz, 1H, Ar–H), 6.95 (d, J = 8.0 Hz, 1H, Ar–H), 6.81 (d, J = 8.2 Hz, 1H, Ar–H); 5.90 (s, 1H, NH), 5.53 (d, J = 22.6 Hz, 1H, P–C–H), 3.73(d, 3H, J = 10.2 Hz, OCH3), 3.54 (d, J = 9.8 Hz, 3H, P–O–CH3), 2.35 (s, 3H, CH3). 13C-NMR (CDCl3): δ 147.80(C6), 136.20(C11), 135.40(C15), 129.03(C14 & 16), 128.70(C2), 127.32(C13&17), 124.86(C4), 123.70(C18), 116.05(C1), 114.50(C3), 56.73(C9), 54.93(C23), 53.98(C25), 13.40(C7). 31P-NMR(CDCl3): δ 25.16. 19F-NMR (CDCl3): δ −59.56. MS (ESI): m/z (%) 408[M + H]+, 430[M + Na]+. Anal.Calcd.for C17H18ClF3NO3P: C, 50.08; H, 4.45; N, 3.44. Found C, 50.12; H, 4.41; N, 3.47.
Dimethyl (3-fluorophenyl) (2-methyl-3-(trifluoromethyl) phenylamino) methylphosphonate ( 4c )
Orange solid; Yield: 97%. M.p.128–130 °C. IR (cm−1): 3426 (NH), 2841 (C–H), 1003 (C–F), 1230 (P=O). 1H-NMR (DMSO-d6): δ 7.33(m, 1H, Ar–H), 7.09(d, J = 7.70 Hz, 1H, Ar–H), 6.97 (m, 2H,Ar–H), 6.82 (m, 2H, Ar–H), 6.77 (s, 1H, Ar–H), 6.65 (d, J = 8.0 Hz, 1H, Ar–H); 5.94 (s, 1H, NH), 5.55 (d, J = 23.4 Hz, 1H, P–C–H), 3.77(d, J = 10.6 Hz,3H, OCH3), 3.52 (d, J = 9.6 Hz, 3H, P–O–CH3), 2.37 (s, 3H, CH3). 13C-NMR (CDCl3): δ 163.05(C16), 145.89(C6), 137.20(C11), 129.80(C14), 128.56(C2), 126.78(C4), 123.70(C18), 115.97(C1), 114.45(C5), 113.89(C17), 56.93(C9), 54.39(C23), 53.86(C25), 12.43(C7). 31P-NMR (CDCl3): δ 25.13. 19F-NMR (CDCl3): δ −59.93. MS (ESI): m/z (%) 392[M + H]+, 414[M + Na]+. Anal. Calcd. for C17H18F4NO3P: C, 52.18; H, 4.64; N, 3.58. Found C, 52.14; H, 4.59; N, 3.62.
Dimethyl((2-mercaptophenyl)((2-methyl-3-(trifluoromethyl)phenyl)amino)methyl)phosphonate ( 4d )
Yellow solid: Yield: 95%. M.p.136–138 °C. IR (cm−1): ν 3445 (NH), 2874 (C–H), 1009 (C–F), 1228 (P=O).1H-NMR (DMSO-d6): δ 7.65(d, J = 8.2 Hz, 1H, Ar–H), 7.51 (m, 2H, Ar–H), 7.47(d, J = 7.8 Hz,1H, Ar–H), 7.45(d J = 7.2 Hz, 1H, Ar–H), 7.28 (d, J = 7.0 Hz, 1H, Ar–H), 7.18 (d, J = 7.3 Hz, 1H, Ar–H), 7.01 (d, J = 7.0 Hz, 1H, Ar–H),; 5.90 (s, 1H, NH), 5.15 (d, J = 22.4 Hz, 1H, P–C–H),3.75(d, J = 10.4 Hz, 3H, OCH3), 3.68 (d, J = 10.6 Hz, 3H, P–O–CH3), 3.59 (s, 1H, SH), 3.17 (s, 1H, SH), 2.27 (s, 3H, CH3).13C-NMR (CDCl3): δ 145.06(C6), 144.93(C11), 138.87(C17), 130.06(C16), 129.78(C2), 128.73(C13), 127.41(C15), 126.51(C14), 126.01(C19), 123.28(C1), 121.73 116.30(C5), 77.45(C9), 54.34(C24), 52.63(C26), 12.92(C7).31P-NMR (CDCl3): δ 25.28. 19F-NMR (CDCl3): δ −59.73. MS (ESI): m/z (%) 406[M + H]+, 428[M + Na]+. Anal.Calcd.for C17H19F3NO3PS: C, 50.37; H, 4.72; N, 3.46. Found C, 50.98; H, 4.38; N, 3.06.
Dimethyl ((3,4-dimethoxyphenyl)((2-methyl-3-(trifluoromethyl)phenyl)amino)methyl) phosphonate ( 4e )
Orange solid: Yield: 93%. M.p.133–135 °C. IR (cm−1): ν 3415 (NH), 2834 (C–H), 1003 (C–F), 1220 (P=O). 1H-NMR (DMSO-d6): δ 7.54(s, 1H, Ar–H), 7.22 (d, J = 7.7 Hz, 2H, Ar–H), 7.13(t, J = 6.8 Hz, 1H, Ar–H), 7.09(d, J = 8.2 Hz, 1H, Ar–H), 6.98 (d, J = 8.0 Hz, 1H, Ar–H), 6.92 (d, J = 7.8 Hz, 1H, Ar–H); 5.94 (s, 1H, NH), 5.17 (d, J = 9.6 Hz, 1H, P–C–H),3.75(d, J = 8.6 Hz, 6H, OCH3),3.72(d, J = 9.6 Hz, 3H, OCH3), 3.49 (d, J = 9.1 Hz, 3H, OCH3), 2.32 (s, 3H, CH3).13C-NMR (CDCl3): δ 149.39(C16), 149.09(C15), 145.36(C6), 129.90(C11), 128.74(C2), 127.24(C4), 126.42(C19), 123.30(C13), 121.25(C17), 119.97(C1), 115.72(C3), 114.86(C5), 111.36(C14), 77.46(C9), 56.36(C28), 55.83(C30), 54.84(C26), 53.92(C24), 12.97(C7). 31P-NMR (CDCl3): δ 24.89.19F-NMR (CDCl3): δ −59.31. MS (ESI): m/z (%) 434[M + H]+, 456[M + Na]+. Anal. Calcd.for C19H23F3NO5P: C, 52.66; H, 5.35; N, 3.23. Found C, 52.64; H, 5.43; N, 3.20.
Dimethyl(benzo[d] [ 1, 3 ] dioxol-5-yl((2-methyl-3-(trifluoromethyl)phenyl)amino)methyl)phosphonate ( 4f )
Red solid: Yield: 94%. M.p.110–112 °C. IR (cm−1): ν 3441 (NH), 2878 (C–H), 1003 (C–F), 1232 (P=O); 1H-NMR (DMSO-d6): δ 6.97(t, J = 8.2 Hz, 1H, Ar–H), 6.91 (s, 1H, Ar–H), 6.82(d, J = 7.2 Hz, 1H, Ar–H), 6.76(d, J = 6.8 Hz, 1H, Ar–H), 6.67 (d, J = 7.4 Hz, 1H, Ar–H),6.01 (s, 2H, CH2),; 5.91 (s, 1H, NH), 5.13 (d, J = 22.6 Hz, 1H, P–C–H), 3.71(d, 3H, J = 10.6 Hz, OCH3),3.63(d, J = 10.2 Hz, 3H, OCH3), 2.29 (s, 3H, CH3).13C-NMR (CDCl3): δ 148.60(C14), 146.28(C15&6), 129.94(C11), 128.40(C2), 127.20(C4), 123.78(C21), 120.74(C17), 116.87(C1), 114.30(C3), 113.89(C5), 112.80(C13&16), 102.70(C19), 69.52(C9), 52.98(C26), 52.83(C28), 12.34(C7). 31P-NMR (CDCl3): δ 26.32. 19F-NMR (CDCl3): δ −59.03. MS (ESI): m/z (%) 418[M + H]+, 440[M + Na]+. Anal. Calcd. for C18H19F3NO5P: C, 51.81; H, 4.59; N, 3.36. Found C, 51.36; H, 4.83; N, 3.09.
Dimethyl((2-mercapto-5-nitrophenyl)((2-methyl-3(trifluoromethyl)phenyl)amino)methyl) phosphonate ( 4g )
Orange solid: Yield: 96%. M.p.141–143 °C. IR (cm−1): ν max 3398 (NH), 2842 (C–H), 1002 (C–F), 1226 (P=O). 1H-NMR (DMSO-d6): δ 8.03(s, 1H, Ar–H), 7.98 (d, J = 7.7 Hz, 1H, Ar–H), 7.59(d, J = 7.2 Hz, 1H, Ar–H), 6.97(t, J = 8.0 Hz, 1H, Ar–H), 6.89 (d, J = 7.8 Hz, 1H, Ar–H), 6.68 (d, J = 6.8 Hz, 1H, Ar–H); 5.95 (s, 1H, NH), 5.53(d, J = 23.4 Hz, 1H, P–C–H), 3.73(d, J = 10.6 Hz, 3H, P–OCH3), 3.54(d, J = 9.8 Hz, 3H, P–OCH3), 2.25 (s, 3H, CH3). 13C-NMR (CDCl3): δ 146.78(C6), 144.40(C14), 137.90(C17), 132.70(C16), 127.30(C2), 125.90(C4), 123.40(C18), 121.80(C15), 118.06(C1), 113.60(C5), 63.08(C9), 53.90(C23), 53.80(C25), 12.90(C7). 31P-NMR (CDCl3): δ 25.31. 19F-NMR (CDCl3): δ −59.76. MS (ESI): m/z (%) 451[M + H]+, 473[M + Na]+. Anal.Calcd.for C17H18F3N2O5PS: C, 45.34; H, 4.03; N, 6.22. Found C, 45.81; H, 4.34; N, 6.07.
Dimethyl(((2-methyl-3-(trifluoromethyl)phenyl)amino)(6-methylpyridin-2-yl)methyl)phosphonate ( 4h )
Brown solid: Yield: 91%. M.p. 136–138 °C. IR (cm−1): ν 3423 (NH), 2864 (C–H), 1006 (C–F), 1218 (P=O) 1H-NMR (DMSO-d6): δ 7.68(d, J = 7.8 Hz, 2H, Ar–H), 7.45 (d, J = 8.2 Hz, 2H, Ar–H), 7.67(m, 3H, Ar–H), 7.08(t, J = 7.4 Hz, 1H, Ar–H), 6.82 (d, J = 7.2 Hz, 2H, Ar–H), 6.70 (d, J = 7.6 Hz, 2H, Ar–H); 5.81 (s, 1H, NH), 5.48 (d, J = 22.8 Hz, 1H, P–C–H), 3.73(d, J = 9.8 Hz, 3H, OCH3), 3.52(d, J = 9.6 Hz, 3H, P–O–CH3), 2.61 (s, 3H, CH3), 2.16 (s, 3H, CH3).13C-NMR (CDCl3): δ 160.52(C11), 157.94(C16), 147.80(C6), 137.48(C14), 129.20(C2), 126.82(C4), 123.40(C18), 123.20(C15&13), 115.91(C1), 115.80(C3), 113.89(C5), 57.34(C9), 54.80(C23), 54.45(C25), 24.90(C26), 13.70(C7). 31P-NMR (CDCl3): δ 25.68. 19F-NMR (CDCl3): δ −59.81. MS (ESI): m/z (%) 389[M + H]+, 411[M + Na]+. Anal.Calcd.forC17H20F3N2O3P: C, 52.58; H, 5.19; N, 7.21. Found C, 52.17; H, 5.38; N, 7.29.
Dimethyl(((2-methyl–3-(trifluoromethyl)phenyl)amino)(piperidin-1-yl)methyl)phosphonate ( 4i )
Orange solid: Yield: 90%. M.p. 118–116 °C.IR (cm−1): ν 3436 (NH), 2834 (C–H), 1012 (C–F), 1232 (P=O); 1H-NMR (DMSO-d6): δ 7.12(t, J = 8.0 Hz, 1H, Ar–H), 7.05 (d, J = 7.7 Hz, 1H, Ar–H), 7.68(d, 1H, J = 7.2 Hz, Ar–H); 5.79 (s, 1H, NH), 5.43 (d, J = 21.8 Hz, 1H, P–C–H), 3.72(d, 3H, J = 10.4 Hz, POCH3), 3.52 (d, J = 10.6 Hz, 3H, P–O–CH3), 2.53 (m, 4H, CH2), 2.19 (s, 3H, CH3), 1.65 (m, 2H, CH2), 1.56 (m, 4H, CH2).13C-NMR (CDCl3): δ 146.98(C6), 128.80(C2), 127.08(C4), 124.45(C13), 116.17(C1), 114.30(C3), 94.34(C9), 54.80(C25), 53.84(C21), 53.70(C20&18), 26.40(C24), 25.94(C22), 25.60(C23), 13.70(C7). 31P-NMR (CDCl3): δ 25.02. 19F-NMR (CDCl3): δ −59.43. MS (ESI): m/z (%) 381[M + H]+ 403[M + Na]+. Anal.Calcd.for C16H24F3N2O3P: C, 50.55; H, 6.36; N, 7.37. Found C, 50.46; H, 6.38; N, 7.29.
Dimethyl((4-(dimethylamino)phenyl)((2-methyl-3-(trifluoromethyl)phenyl)amino)methyl) phosphonate ( 4j )
Orange solid: Yield: 95%. M.p. 126–128 °C. IR (cm−1): ν 3438 (NH), 2854 (C–H), 1004 (C–F), 1227 (P=O); 1H-NMR (DMSO-d6): δ 7.36(d, J = 7.6 Hz, 2H, Ar–H), 7.10 (d, J = 8.7 Hz, 2H, Ar–H), 6.94 (t, J = 7.4 Hz, 1H, Ar–H), 6.89(d, J = 7.2 Hz, 1H, Ar–H), 6.69 (d, J = 6.8 Hz, 1H, Ar–H); 5.84 (s, 1H, NH), 5.10 (d, J = 22.8 Hz, 1H, P–C–H), 3.77 (d, J = 9.8 Hz, 3H, P–OCH3), 3.69 (d, J = 9.6 Hz, 3H, P–OCH3), 2.90 (s, 6H, CH3), 2.29 (s, 3H, CH3).13C-NMR (CDCl3): δ 147.02(C22), 143.78(C6), 128.43(C2), 126.05(C4), 123.56(C25), 121.26(C12), 116.12(C1), 114.43(C3), 56.34(C9), 54.76(C17&19), 53.92(C27&28), 41.33(C29&30), 12.33(C7). 31P-NMR (CDCl3): δ 25.34. 19F-NMR (CDCl3): δ −59.78. MS (ESI): m/z (%) 417[M + H]+, 439[M + Na]+. Anal.Calcd.for C19H24F3N2O3P: C, 54.81; H, 5.81; N, 6.73. Found C, 54.98; H, 5.83; N, 6.40.
Dimethyl(2-methyl-3-(trifluoromethyl)phenylamino)(pyren-1-yl)methyl phosphonate ( 4k)
Brown solid: Yield: 97%. M.p. 138–140 °C.IR (cm−1): ν 3445 (NH), 2874 (C–H), 1009 (C–F), 1228 (P=O) 1H-NMR (DMSO-d6): δ 8.12(d, J = 7.8 Hz, 1H, Ar–H), 7.94 (d, J = 7.0 Hz, 1H, Ar–H), 7.89(m, 1H, Ar–H),7.82 (d, J = 6.7 Hz, 1H, Ar–H), 7.87 (m, J = 7.2 Hz, 4H, Ar–H), 7.78 (d, J = 7.6 Hz, 1H, Ar–H), 6.98(d, J = 7.0 Hz, 1H, Ar–H), 6.89 (d, J = 6.6 Hz, 1H, Ar–H), 6.73(d, J = 6.8 Hz, 1H, Ar–H),; 5.87 (s, 1H, NH), 5.57 (d, J = 23.1 Hz, 1H, P–C–H), 3.78(d, J = 10.2 Hz, 3H, OCH3), 3.59 (d, J = 10.4 Hz, 3H, P–O–CH3), 2.19 (t, 3H, CH3). 13C-NMR (CDCl3): δ 147.28(C6), 140.52(C29), 133.90(C30), 134.45(C26), 128.83(C2&35), 126.84(C4), 126.48(C21), 126.28(C31,32&34), 125.92(C23&27), 124.94(C28), 123.70(C24), 123.48(C25), 118.74(C1), 115.87(C3), 57.34(C9), 53.48(C17), 53.26(C19), 12.84(C7). 31P-NMR (CDCl3): δ 25.28. 19F-NMR (CDCl3): δ −59.73. MS (ESI): m/z (%) 498[M + H]+, 520[M + Na]+. Anal.Calcd.for C27H23F3NO3P: C, 65.19; H, 4.66; N, 2.82. Found C, 64.98; H, 4.38; N, 2.90.
Dimethyl((4-hydroxyphenyl)((2-methyl-3-(trifluoromethyl)phenyl)amino)methyl)phosphonate ( 4l )
White solid; Yield: 94%. M.p. 113–115 °C. IR (cm−1): ν 3418 (NH), 2839 (C–H), 1000 (C–F), 1223 (P=O).1H-NMR (DMSO-d6): δ 9.46 (s, 1H, Ar–H), 7.13 (d, J = 7.70 Hz, 2H, Ar–H), 7.09 (m, 1H, Ar–H), 6.89 (d, J = 8.0 Hz, 1H, Ar–H), 6.78 (d, J = 7.8 Hz, 1H, Ar–H), 6.63 (d, J = 6.6 Hz, 2H, Ar–H); 5.84 (s, 1H, NH), 5.53 (d, J = 22.0 Hz, 1H, P–C–H), 3.67 (d, J = 10.2 Hz, 3H, OCH3), 3.54 (d, J = 10.0 Hz, 3H, P–O–CH3), 2.31 (s, 3H, CH3). 13C-NMR (CDCl3): δ 157.45(C22), 144.85(C6), 130.40(C24), 129.40(C21), 126.10(C4), 123.90(C2), 122.83(C12), 116.80(C1), 115.80(C23), 115.70(C20), 113.50(C5), 61.24(C9), 53.80(C17), 53.70(C19), 12.90(C7). 31P-NMR (CDCl3): δ 26.12. 19F-NMR (CDCl3): δ −59.18. MS (ESI): m/z (%) 390[M + H]+, 412[M + Na]+. Anal.Calcd.for C17H19ClF3NO4P: C, 52.08; H, 4.86; N, 3.64. Found C, 52.12; H, 4.81; N, 3.67.
Dimethyl((1H-indol-3-yl)((2-methyl-3-(trifluoromethyl)phenyl)amino)methyl)phosphonate( 4m )
Red solid: Yield: 95%. M.p.122–124 °C. IR (cm−1): ν 3446 (NH), 2883 (C–H), 1006 (C–F), 1236 (P=O) 1H-NMR (DMSO-d6): δ 10.91 (s, 1H, NH), 7.93(d, J = 8.2 Hz, 1H, Ar–H), 7.39(d, J = 7.8 Hz, 1H, Ar–H), 7.38 (s, 1H, CH), 7.19 (m, 2H, Ar–H), 7.12(t, J = 7.2 Hz,1H, Ar–H), 6.85(d, J = 7.6 Hz, 1H, Ar–H), 6.69 (d, J = 7.4 Hz, 1H, Ar–H), 5.89 (s, 1H, NH), 5.18 (d, J = 21.4 Hz, 1H, P–C–H), 3.74(d, J = 10.3 Hz, 3H, OCH3), 3.64(d, J = 10.6 Hz, 3H, OCH3), 2.31 (s, 3H, CH3).13C-NMR (CDCl3): δ 146.82(C6), 136.70(C29),136(C25), 128.52(C2), 126.68(C21&24), 125.70(C20&23), 124.48(C30), 122.83(C22), 121.34(C32&34), 119.98(C33), 118.74(C26), 116.42(C1), 115.30(C3), 114.09(C5), 112.80(C31), 68.52(C9), 54.94(C17), 54.60(C19), 13.34(C7). 31P-NMR (CDCl3): δ 25.92. 19F-NMR (CDCl3): δ −59.13. MS (ESI): m/z (%) 413[M + H]+, 435[M + Na]+. Anal.Calcd.for C19H20F3N2O3P: C, 55.34; H, 4.89; N, 6.79. Found C, 55.36; H, 4.83; N, 6.90.
Dimethyl((4-methoxyphenyl)((2-methyl-3-(trifluoromethyl)phenyl)amino)methyl)phosphonate ( 4n )
Orange solid: Yield: 92% M.p. 130–132 °C.IR (cm−1): ν 3412 (NH), 2823 (C–H), 1005 (C–F), 1213 (P=O). 1H-NMR (DMSO-d6): δ 7.22 (d, J = 7.4 Hz, 2H, Ar–H), 7.03(t, J = 7.2 Hz, 1H, Ar–H), 6.94(d, J = 8.0 Hz, 2H, Ar–H), 6.88 (d, J = 7.8 Hz, 1H, Ar–H), 6.76 (d, J = 7.4 Hz, 1H, Ar–H); 5.94 (s, 1H, NH), 5.19 (d, J = 22.5 Hz, 1H, P–C–H),3.85(s, 3H, OCH3),3.72(d, J = 9.6 Hz, 3H, OCH3), 3.64 (d, J = 9.1 Hz, 3H, OCH3), 2.32 (s, 3H, CH3).13C-NMR (CDCl3): δ 158.39(C22), 149.36(C6), 129.60(C2), 128.40(C25), 126.82(C4), 123.30(C12), 118.72(C1), 114.86(C3), 113.56(C20&23) 111.36(C5), 61.82(C9), 56.32(C27), 5484(C17), 53.92(C19), 12.79(C7). 31P-NMR (CDCl3): δ 24.78.19F-NMR (CDCl3): δ −59.23. MS (ESI): m/z (%) 404[M + H]+, 426[M + Na]+. Anal. Calcd. for C18H21F3NO4P: C, 53.60; H, 5.25; N, 3.47. Found C, 53.24; H, 5.34; N, 3.35.
Dimethyl (2-methyl-3-(trifluoromethyl) phenylamino) (3-nitrophenyl) methylphosphonate ( 4o )
Yellow solid; Yield: 96%. M.p. 128–130 °C. IR (cm−1): ν max 3412 (NH), 2814 (C–H), 1000 (C–F), 1224 (P=O). 1H-NMR (DMSO-d6): δ 8.18(s, 1H, Ar–H), 8.09(d, J = 8.2 Hz, 2H, Ar–H), 7.75 (d, J = 7.7 Hz, 1H, Ar–H), 7.61(m, 1H, Ar–H), 7.05(t, J = 7.8 Hz, 1H, Ar–H), 6.83 (d, J = 8.0 Hz, 1H, Ar–H), 6.75(d, J = 7.4 Hz, 1H, Ar–H); 5.90 (s, 1H, NH), 5.35 (d, J = 22.4 Hz, 1H, P–C–H), 3.76(d, J = 2.6 Hz, 3H, P–OCH3), 3.57 (d, J = 2.6 Hz, 3H, P–OCH3), 2.35 (s, 3H, CH3). 13C-NMR (CDCl3): δ 148.02(C20), 146.90(C6), 138.06(C25), 135.47(C24), 129.43(C23), 128.50(C2), 126.61(C4), 121.36(C12), 121.08(C21), 116.21(C1), 114.54(C3), 56.44(C9), 54.77(C17), 53.94(C19), 12.36(C7). 19F-NMR (CDCl3): δ −59.68. MS (ESI): m/z (%) 419[M + H]+, 441[M + Na]+. Anal.Calcd.for C17H18F3N2O5P: C, 48.81; H, 4.34; N, 6.70. Found C, 48.68; H, 4.51; N, 6.64.
Conclusion
An efficient and environmentally benign method for the synthesis of new α-aminophosphonates from 2-methyl-3-(trifluoromethyl)aniline, aryl/heteroaryl aldehydes, and dimethyl phosphite using 10 mol% chitosan by microwave irradiation under neat conditions has been developed. The advantages of this protocol are the use of inexpensive, reusable catalysts, short reaction times at low temperature, easy work-up and high product yields. The efficacy of all synthesized α-aminophosphonate derivatives was assayed for their in vitro cytotoxic activities against a panel of six human cancer cell lines including PC-3(prostate cancer), MCF-7(breast cancer), HeLa(cervical cancer), U973, K562, HL60 (human leukemia). Compound 4k with a pyrene moiety showed higher cytotoxic potency against a breast cancer cell line. But compounds 4g and 4k have exhibited promising cytotoxicity against U973, K562 and HL60 cancer cell lines.
References
R.W. Steketee, J.J. Wirima, L. Slutsker, C.O. Khoromana, D.L. Heymann, J.G. Breman, Am. J. Trop. Med. Hyg. 55, 50 (1996)
D.T. Wong, F.P. Bymaster, E.A. Engleman, Life Sci. 57, 411 (1995)
S. Hayashi, N. Ueno, A. Murase, Y. Nakagawa, J. Takada, Eur. J. Med. Chem. 50, 179 (2012)
B. Stowasser, K.H. Budt, L. Jain-Qi, A. Peyman, D. Ruppert, Tetrahedron Lett. 33, 6625 (1992)
C. Isanbor, D.O. Hagan, J. Fluor. Chem. 127, 303 (2006)
M.S. Wu, Q.Q. Feng, G.N. Lia, D.H. Wana, Acta Cryst. C69, 1070–1072 (2013)
A.K. Bhattacharya, S.R. Dnyaneshwar, C.R. Kalpeshkumar, K.P. Innaiah, M. Sajid Khan, I. Sana, Eur. J. Med. Chem. 66, 146 (2013)
F.R. Atherton, C.H. Hassall, R.W. Lambert, J. Med. Chem. 29, 29 (1986)
L. Maier, H. Sporri, Phosphorus, Sulfur Silicon Relat. Elem. 61, 69 (1991)
L. Maier, Phosphorus, Sulfur Silicon Relat. Elem. 47, 43 (1990)
P. Kafarski, B. Lejczak, R. Tyka, L. Koba, E. Pliszczak, P. Wiecczorek, J. Plant Growth Regul. 14, 199 (1995)
S. Laschat, H. Kunz, Synthesis 1992, 90 (1992)
B.C. Ranu, A. Hajra, U. Jana, Org. Lett. 1, 1141 (1999)
R. Ghosh, S. Maiti, A. Chakraborty, D.K. Maiti, J. Mol. Catal. A: Chem. 210, 53 (2004)
J. Tang, L. Wanga, W. Wang, L. Zhang, S. Wu, D. Mao, J. Fluor. Chem. 132, 102 (2011)
F. Xu, Y.Q. Luo, M.Y. Deng, Q. Shen, Eur. J. Org. Chem. 2003, 4728 (2003)
S.C. Sekhar, S.J. Prakash, V. Jagadeshwar, C. Narsihmulu, Tetrahedron Lett. 42, 5561 (2001)
S.D. Mitragotri, D.M. Pore, U.V. Desai, P.P. Wadgaonkar, Catal. Commun. 9, 1822 (2008)
S.M. Vahdat, R. Baharfar, M. Tajbakhsh, A. Heydari, S.M. Baghbanian, S. Haksar, Tetrahedron Lett. 49, 6501 (2008)
P. Sreekanth Reddy, P. VasuGovardhana Reddy, S. Mallikarjun Reddy, Tetrahedron Lett. 55, 3336 (2014)
J.J. Yang, N. Dang, Y.W. Chang, Lett. Org. Chem. 6, 470 (2009)
S.S. Sudha, C.S. Sundar, N.B. Reddy, K.U.M. Rao, S.H.J. Prakash, C.S. Reddy, Phosphorus, Sulfur Silicon Relat. Elem. 188, 1402 (2013)
B. Kaboudin, H. Zahedi, Chem. Lett. 37, 540 (2008)
Y.P. Tian, F. Xu, Y. Wang, J.J. Tang, H. Li, J. Chem. Res. 2009, 78 (2009)
J.T. Hou, J.W. Gao, Z.H. Zhang, Appl. Organomet. Chem. 25, 47 (2011)
C. Syama Sundar, N. Bakthavatchala Reddy, S. Sivaprasad, K. Uma Maheswara Rao, S.H. Jaya Prakash, C. Suresh Reddy, Phosphorus, Sulfur Silicon Relat. Elem. 189, 551 (2014)
B. Kaboudin, M. Sorbiun, Tetrahedron Lett. 48, 9015 (2007)
Y.Q. Yu, D.Z. Xu, Synthesis 47, 1869–1876 (2015)
P. Kafarski, M.G. Gorniak, I. Andrasiak, Curr. Green Chem. 2, 218 (2015)
G. Keglevich, A. Szekrenyi, Lett. Org. Chem. 5, 616 (2008)
G. Keglevich, E. Balint, Molecules 17, 12821 (2012)
D. Pradip Kumar, D. Joydeep, V.S. Tripathi, JSIR 63, 20 (2004)
Acknowledgements
The authors express thanks to Prof. C. Devendranath Reddy, Department of Chemistry, Sri Venkateswara University, Tirupati, for his helpful discussions, acknowledge the DST-SERB, New Delhi, India for providing financial support through the Project File No: SB/S1/OC-96/2013, dated: 05-11-2014) and the Anti-Cancer Drug screening facility in ACTREC, Tata Memorial Centre, Navi Mumbai for the in vitro SRB assay of compounds for anti-cancer activity.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reddy, K.M.K., Sadik, S.M., Saichaithanya, N. et al. One-pot green synthesis and cytotoxicity of new α-aminophosphonates. Res Chem Intermed 43, 7087–7103 (2017). https://doi.org/10.1007/s11164-017-3060-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3060-y