Abstract
Cerium phosphates have been successfully synthesized under microwave irradiation in the absence of organic solvent. The effects of different molar ratios of phosphoric acid (85 %) to cerium hydroxide on the product phosphates were investigated. A single-phase CeP2O7 compound and a mixture of tetravalent cerium pyrophosphate (CeP2O7 ) and metaphosphate (Ce(PO3)4 ) were synthesized at 800 W for 5 min under microwave irradiation when the mole ratio of H3PO4 to Ce(OH)4 was equal to 2 and 2.5–4, respectively. In the presence of chemometric excess of H3PO4 (mole ratio of H3PO4 to Ce(OH)4 is between 5 and 6), a single-phase Ce(PO3)4 compound can be prepared at 480 W for 10 min under microwave irradiation. Scanning and transmission electron microscope images showed that particle sizes of CeP2O7 and Ce(PO3)4 were 0.1–3 and 1–5 μm, respectively. The adsorption properties of CeP2O7 and Ce(PO3)4 on uranium(VI) were examined. In the same condition, uranium(VI) was removed by Ce(PO3)4 more effectively than CeP2O7, and there are greater advantages for products synthesized under microwave irradiation than those calcination products on adsorption of uranium(VI).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, considerable attention has been paid to rare earth phosphates because of their applications in novel functional materials, such as fluorescence materials [1, 2], proton conductors [3–6], catalysts [7–10], magnetic cooling material [11], sunscreen materials [12–14], negative thermal expansion materials [15, 16], and efficient adsorbents for radwaste [17–19]. Several phosphates such as Zr2O(PO4)2, ZrP2O7 and Th4(PO4)4P2O7 have been studied as potential substitutes for immobilization and long-term storage of uranium [18]. Phosphates, especially rare earth phosphates, have poor solubility, strong irradiation resistance, excellent thermal stability, good binding ability with actinide elements, and high adsorption capability on actinide elements and heavy metals [15–28]. For this reason, rare earth phosphates as adsorbents are expected to play a significant role in the safety of underground radwaste repositories due their potential use for an engineered containment barrier. Therefore, more rare earth phosphates should be applied in adsorption of Uranium(VI) [U(VI)] so as to provide strong support for underground nuclear waste disposal. To the best of our knowledge, there is no literature reported about the adsorption properties of tetravalent cerium pyrophosphate (CeP2O7) and metaphosphate (Ce(PO3)4) on U(VI).
CeP2O7 and Ce(PO3)4 are difficult to obtain, and many resources are wasted because of their required harsh synthesis condition. Only a conventional calcination technique has been used to prepare CeP2O7 and Ce(PO3)4 in previous studies [3, 7, 8, 15, 29–31]. CeP2O7 powder can reportedly be obtained by the dehydration of Ce(HPO4)2·xH2O, which was prepared by heating H3PO4 and Ce(SO4)2·xH2O under reflux conditions, using a heat treatment at 600 °C for 16 h. CeO2 and 85 % H3PO4 have also been calcined at 300 °C for 8 h [3], or at 700 °C for 20 h [29]. Amorphous CeP2O7 powder has been obtained as well using Ce(SO4)2·4H2O and Na4P2O7 as initial materials at 80 °C for 24 h; crystallization from the amorphous phase occurred at around 500 °C to form crystalline cerium pyrophosphate [30]. Regarding Ce(PO3)4, it can reportedly be obtained by calcining CeO2 and 85 % H3PO4 at 700 °C for 20 h [7, 31], or at 500 °C for longer than 6 h [27]. These calcination methods are time-consuming, resource-wasteful, and require high temperatures. Thus, a new and rapid synthesis route for CeP2O7 and Ce(PO3)4 should be required. In the current study, CeP2O7 and Ce(PO3)4 are directly synthesized using only microwave (MW) irradiation in the absence of any organic solvent, and are then applied as new mediums of U(VI)-sorption/fixation for radioactive liquid wastes processing.
Experimental
CeP2O7 and Ce(PO3)4 powders were synthesized using phosphoric acid (H3PO4; 85.0 % by weight) and cerium hydroxide (Ce(OH)4; 99.9 %) at different mole ratios [R = 2, 2.5, 3, 4, 5, 5.5, 6, and 7, where R is the mole ratio of H3PO4 to Ce(OH)4]. Different amounts of 85 % H3PO4 and 0.01-mol Ce(OH)4 were adequately mixed in an agate mortar. The mixture was stored in a porcelain crucible with an insulation layer and directly irradiated at various MW powers for different times. The resultant powders were sufficiently washed with distilled water, and then dried at 60 °C for 1.5 h. For comparison, the reactions were also investigated by a high-temperature calcination method in a temperature-programmed muffle furnace when R was equal to 2, 4, and 5.
The equilibration of CeP2O7 or Ce(PO3)4 with uranyl ions was done by a batchwise operation. 0.02 g of CeP2O7 or Ce(PO3)4 was taken into a sealable Teflon tube ample at 25 °C for a certain time together with 20 mL of aqueous solution containing 2–5 mL of 4-mmol L−1 uranyl nitrate [UO2(NO3)2·6H2O] solution (in 0.1-M HNO3), 2 mL of 0.1-M KNO3 solution, and 0.1-M KOH solution adjusted to pH values of 2–7. After U(VI) solutions were centrifuged at 3000 rpm for 15 min, the U(VI) concentration in the upper layer was determined spectrophotometrically using a arsenazo-III method [32, 33]. The effects of pH value, contact time, and initial concentrations of uranyl ions on U(VI) adsorption were studied.
MW irradiation was carried out using a MW oven (Galanz GB80)with a frequency of 2450 MHz and several power level settings up to a maximum output power of 800 W. The structure of products was confirmed by powder XRD using a D/MAX–RB X-ray diffractometer (Rigaku, Japan) with a graphite monochromator and Cu Kα radiation (40 kV, 100 mA). The step scan mode was used with a step width of 0.02°, at a rate of 4° (2θ) min−1, in the scan range 10–70°. Phase indexing was performed using MDI Jade 6.5 software. Scanning electron microscope (SEM, JSM–7500F–EDS) observations were made using a Hitachi S–4800 microscope operated at 0.5 kV. High-resolution morphology and selected-area electron diffraction (SAED) were performed using transmission electron microscopy (TEM, JEOL–2100). The samples were dispersed on a carbon-coated copper grid for TEM observation. The uranium content was determined using a visible light spectrophotometer (722S Vis) at 652 nm for the U(VI)–arsenazo(III) complex against a reagent blank as the reference.
Results and discussion
Phase analysis
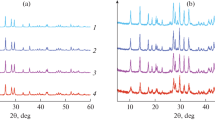
Numerous experiments were performed by changing the parameters molar ratio, reaction time, and MW power. As seen in Fig. 1, the diffraction peaks of CeH2P2O8 were observed in the XRD pattern of the sample which was prepared using H3PO4 and Ce(OH)4 with R = 2 at 800 W for 3 min. H3PO4 and Ce(OH)4 thoroughly reacted, and CeH2P2O8 transformed into CeP2O7 when the reaction time was prolonged to 5 min. All the peaks in Fig. 1a can be indexed to a pure phase, cubic structured CeP2O7 [Joint Committee on Powder Diffraction Standards (JCPDS) no. 30-0164] [34]. Therefore, synthesizing single-phase CeP2O7 at 800 W for 5 min with R = 2 is highly suitable (entries 1 and 2 in Table 1) among other many trials.
To investigate the morphology, grain size, and dispersion status of CeP2O7 under different conditions [35], an excess amount of H3PO4 (R = 2.5) was used at 800 W for 5 min. It was found from Fig. 2a, however, the diffraction peaks of Ce(PO3)4 significantly appeared in the CeP2O7 pattern. The diffraction peaks of Ce(PO3)4 in the XRD pattern of the products (Fig. 2b) became strong when excess H3PO4 (R = 3) was used at 800 W for 5 min. It is expected that Ce(PO3)4 was obtained directly by MW irradiation using H3PO4 and Ce(OH)4 as raw materials at 800 W for 5 min. Therefore, a stoichiometric reaction between H3PO4 and Ce(OH)4 was investigated to obtain a pure Ce(PO3)4 compound. In this case, when R = 4, Ce(PO3)4 was obtained and small amount of CeP2O7 was observed as a side product at a diffraction peak at 20.66°. Moreover, in the same manner, also by keeping R = 4 constant and changing the other MW parameters such as 800 W for 3 or 10 min, and 640 W for 5 or 10 min (entries 7–11), the diffraction peak at 20.66° never disappeared. Therefore, pure Ce(PO3)4 cannot be obtained in the stoichiometric reaction where R = 4 regardless of the changes in the reaction conditions.
To synthesize single-phase Ce(PO3)4 under MW irradiation, experiments in which R = 5 under different powers and times were carried out. The CeP2O7 phase still existed in the products irradiated at 800 W for 5 or 10 min. However, the CeP2O7 diffraction peak at 20.66° disappeared when the reactants were irradiated at 640, 560, and 480 W for 10 min (Fig. 3). By comparing the obtained data (Fig. 3c, d and e) with the JCPDS no. 25-0188 [36], pure-phase orthorhombic structured Ce(PO3)4 with a PbCn space group was successfully synthesized by MW irradiation at a low power (640, 560, and 480 W) for 10 min, in which R = 5. Additionally, single-phase Ce(PO3)4 powders were also obtained when R = 5.5 and 6. Contrarily, when R = 7, single-phase Ce(PO3)4 powder cannot be synthesized under MW irradiation. As can be seen in Table 1 (entries 15, 16, and 25), a high power is unsuitable for the synthesis of pure Ce(PO3)4 powders.
For comparison, the reactions were also performed by a conventional heating method. The CeP2O7 powder was obtained by calcinating H3PO4 and Ce(OH)4 at 300 °C for 60 min when R = 2 and 4 (entries 4 and 12 in Table 1). When R = 5, a higher temperature (500 °C) and longer time (180 min) were used to obtain Ce(PO3)4 powders (entry 21).
Both CeP2O7 and Ce(PO3)4 were synthesized using H3PO4 as a MW susceptor agent. The possible reaction path of H3PO4 under MW irradiation was investigated. Free water was firstly removed from phosphoric acid 85 % under MW irradiation. Subsequently, anhydrous H3PO4 was dehydrated to form H4P2O7, and then further dehydrated to form HPO3. The chemical change evidently resulted from the MW effects. H3PO4 reacted with Ce(OH)4 to produce the intermediate CeH2P2O8 in an active state, and then H2O was eliminated to form CeP2O7. With an increased amount of H3PO4, fresh CeP2O7 further reacted with H3PO4 to form Ce(PO3)4. This process agreed with the action of H3PO4 under MW irradiation. This reaction process can be explained by the following Eq. (1), and the hypothesis is demonstrated in Figs. 1 and 2.
SEM, TEM and SAED patterns
The SEM, TEM and SAED images of CeP2O7 (entry 2) and Ce(PO3)4 (entry 18) are shown in Figs. 4 and 5. It is suggested that the particles of CeP2O7 and Ce(PO3)4 are irregular in shape and 0.1–3 and 1–5 μm in size, respectively. The insets in Figs. 4b and 5b represent the SAED patterns of as-synthesized CeP2O7 and Ce(PO3)4, indicating that both samples had a monocrystalline structure.
SEM (a) and TEM (b) images of CeP2O7 synthesized at 800 W for 5 min (entry 2) before U(VI) adsorption, SEM (c) image of CeP2O7 (entry 2) after U(VI) adsorption, and SEM (d) image of CeP2O7 synthesized at 300 °C for 60 min (entry 12) before U(VI) adsorption. The inset in the TEM image is the SAED patterns of CeP2O7 (entry 2)
SEM (a) and TEM (b) images of Ce(PO3)4 synthesized at 560 W for 10 min (entry 18) before U(VI) adsorption, SEM (c) image of Ce(PO3)4 (entry 18) after U(VI) adsorption, and SEM (d) image of Ce(PO3)4 synthesized at 500 °C for 180 min (entry 21) before U(VI) adsorption. The inset in the TEM image is the SAED patterns of Ce(PO3)4 (entry 18)
Figures 4c and 5c shows SEM images of CeP2O7 (entry 2) and Ce(PO3)4 (entry 18) after U(VI) adsorption. By contrast with Figs. 4a and 5a, there are comparatively obvious differences between the SEM images before and after U(VI) adsorption. The aggregation of CeP2O7 particles (Fig. 4c), the decreasing space between Ce(PO3)4 particles (Fig. 5c) and rougher surface for both samples are observed. The possible reason is that U(VI) was adsorbed on the surface of both samples. The compact surface of CeP2O7 particles prepared at 300 °C for 60 min (entry 12) and angular particles of Ce(PO3)4 prepared at 500 °C for 180 min (entry 21), which are completely different from CeP2O7 and Ce(PO3)4 gained by MW irradiation (Figs. 4a, 5a), can be observed in Figs. 4d and 5d, respectively.
Adsorption properties of U(VI)
Figure 6 shows the relationship curves of the U(VI) removal rate by CeP2O7 (entry 2) and by Ce(PO3)4 (entry 18) and pH value, contact time, initial concentrations of uranyl ions. It can be seen from Fig. 6a that U(VI) adsorption by both CeP2O7 and Ce(PO3)4 is very sensitive to the pH value and the optimum pH range is 5–7. The removal rate of U(VI) increased sharply within the early 30 min, and then gradually approached equilibrium (Fig. 6b). Figure 6c suggests that the initial concentration had a slight effect on the removal rate of U(VI). To enhance the adsorption capacity of CeP2O7 and Ce(PO3)4 on U(VI), the large initial concentration (1.0 mmol L−1) was used in the current experiment. Therefore, the following discussed adsorption of U(VI) on a series of cerium phosphates listed in Table 1 was achieved at a pH of 5, within 30 min and at an initial concentration of 1.0 mmol L−1.
Compared to entries 5, 6, 8 and 15 in Table 1, it can be discerned that the removal rate of U(VI) increases with the increasing of Ce(PO3)4 content in the obtained mixtures of CeP2O7 and Ce(PO3)4. This can be proved from Fig. 6 and by comparing entries 2–18 in Table 1. It is implied that U(VI) is adsorbed on Ce(PO3)4 more effectively than CeP2O7. It might be mainly attributable to different sorption sites probably formed by the oxygens of the PO3 and P2O7 surface groups, and a difference in the number of ≡ P–O sites resulted from distinct P/Ce molar ratios between Ce(PO3)4 and CeP2O7. By contrast, the removal rate of CeP2O7 and Ce(PO3)4 obtained by calcinations on U(VI) are significantly lower than those synthesized under MW irradiation. The possible reason is that far more time was required to obtained products at high temperatures by the calcination method than MW irradiation, which resulted in the agglomeration and sintering of particles in the calcinated products.
In order to evaluate the controlling mechanism of the adsorption process, three kinetic models, namely the pseudo-first-order model, pseudo-second-order model and the intraparticle diffusion model were tested to interpret the data obtained from the batch experiments. These three models can be expressed by the following Eqs. (2)–(4), respectively [37].
where q e and q t (mg g−1) are the amount of UO2 2+ adsorbed at the equilibrium state and time t. k 1 (min−1), k 2 (g mg−1 min−1) and k 3 (mg g−1 min−1/2) are the pseudo-first-order, the pseudo-second-order and the intraparticle diffusion rate constants, respectively. I (mg g−1) is the constant.
k 1, k 2, k 3, q e , and the correlative coefficient R 2 of three kinetic models were obtained from plots of log(q e − q t ) versus t, t/q t versus t and q t versus t 1/2, via linear regression of experimental data. As presented in Table 2, the higher correlative coefficient R 2 and correlation value with the q e ,cal closer to the experimental adsorption capacity q e ,exp suggested that the pseudo-second order kinetic model could be fit to descript the adsorption process. It is implied that this adsorption process might be considered as chemisorption which is the rate-controlling step [37].
Conclusions
Different products can be obtained through a green (i.e., environmentally friendly) route using different mole ratios of H3PO4 to Ce(OH)4 as raw materials under MW irradiation. When the mole ratio of H3PO4 to Ce(OH)4 is equal to 2 and 2.5–4, monophase CeP2O7 and the mixture including CeP2O7 and Ce(PO3)4 could be synthesized at 800 W for 5 min under MW irradiation, respectively. Under MW irradiation condition, monophase Ce(PO3)4 can be obtained at a low power of 480 W for 10 min in the presence of a chemometric excess of H3PO4 (R = 5–6). The particle sizes of the as-synthesized CeP2O7 ranged from 0.1 to 3 μm, whereas those of Ce(PO3)4 ranged from 1 to 5 μm, as observed in SEM and TEM images. The kinetics analysis suggested that chemisorption was the rate-controlling step in the adsorption process of CeP2O7 and Ce(PO3)4 for U(VI). Differences of adsorption site and the P/Ce molar ratio between CeP2O7 and Ce(PO3)4 are possible reasons for their different adsorption abilities on U(VI). Both CeP2O7 and Ce(PO3)4 synthesized under MW irradiation are promising adsorbents for the efficient removal of U(VI) from aqueous solutions, and their removal rates are markedly better than those of CeP2O7 and Ce(PO3)4 obtained by calcination of H3PO4 (85 %) and Ce(OH)4. Therefore, the proposed technique was effective and environmentally friendly for the synthesis of CeP2O7 and Ce(PO3)4, and has potential future applications.
References
S. Chall, S.S. Mati, S. Rakshit, S.C. Bhattacharya, J. Phys. Chem. C 117, 25146 (2013)
A. Phuruangrat, N. Ekthammathat, S. Thongtem, T. Thongtem, Res. Chem. Intermed. 39, 1363 (2013)
M.V. Le, D.S. Tsai, C.Y. Yang, W.H. Chung, H.Y. Lee, Electrochim. Acta 56, 6654 (2011)
T. Parangi, B. Wani, U. Chudasama, Electrochim. Acta 148, 79 (2014)
B. Singh, J.H. Kim, S.Y. Jeon, J.Y. Park, S.J. Song, J. Electrochem. Soc. 161, F133 (2014)
C. Chatzichristodoulou, J. Hallinder, A. Lapina, P. Holtapples, M. Mogensen, J. Electrochem. Soc. 160, F798 (2013)
H. Onoda, H. Nariai, A. Moriwaki, H. Maki, I. Motooka, J. Mater. Chem. 12, 1754 (2002)
I.C. Marcu, I. Sandulescu, J.M.M. Millet, Appl. Catal. A Gen. 227, 309 (2002)
J. Kang, S. Byun, S. Nam, S. Kang, T. Moon, B. Park, Int. J. Hydrogen Energy 39, 10921 (2014)
T. Parangi, B. Wani, U. Chudasama, Appl. Catal. A Gen. 467, 430 (2013)
S.L. Zhong, L.F. Luo, L. Wang, L.F. Zhang, Powder Technol. 230, 151 (2012)
N. Imanaka, T. Masui, H. Hirai, G. Adachi, Chem. Mater. 15, 2289 (2003)
V.C. Seixas, O.A. Serra, Molecules 19, 9907 (2014)
J.F. de Lima, O.A. Serra, Dyes Pigments 97, 291 (2013)
K.M. White, P.L. Lee, P.J. Chupas, K.W. Chapman, E.A. Payzant, A.C. Jupe, W.A. Bassett, C.S. Zha, A.P. Wilkinson, Chem. Mater. 20, 3728 (2008)
R. Asuvathraman, K.V.G. Kutty, Thermochim. Acta 581, 54 (2014)
E. Ordoñez-Regil, R. Drot, E. Simoni, J. Colloid Interface Sci. 263, 391 (2003)
R. Drot, E. Simoni, M. Alnot, J.J. Ehrhardt, J. Colloid Interface Sci. 205, 410 (1998)
P. Zeng, Y.C. Teng, Y. Huang, L. Wu, X.H. Wang, J. Nucl. Mater. 452, 407 (2014)
L.A. Boatner, B.C. Sales, in Radioactive Waste Forms for the Future, ed. by W. Lutze, R.C. Ewing (Elsevier, Amsterdam, 1988), p. 495
N. Clavier, E.D.F. de Kerdaniel, N. Dacheux, P.L. Coustumer, R. Drot, J. Ravaux, E. Simoni, J. Nucl. Mater. 349, 304 (2006)
R. Drot, C. Lindecker, B. Fourest, E. Simoni, New J. Chem. 22, 1105 (1998)
V. Brandel, N. Dacheux, E. Pichot, M. Genet, Chem. Mater. 10, 345 (1998)
N. Dacheux, B. Chassigneux, V. Brandel, P.L. Coustumer, M. Genet, G. Cizeron, Chem. Mater. 14, 2953 (2002)
P. Bénard, V. Brandel, N. Dacheux, S. Jaulmes, S. Launay, C. Lindecker, M. Genet, D. Louër, M. Quarton, Chem. Mater. 8, 181 (1996)
P. Bénard, D. Louër, N. Dacheux, V. Brandel, M. Genet, Chem. Mater. 6, 1049 (1994)
H.A. Höppe, M. Daub, Z. Kristallogr. 227, 535 (2012)
Y.H. Lai, Y.C. Chang, T.F. Wong, W.J. Tai, W.J. Chang, K.H. Lii, Inorg. Chem. 52, 13639 (2013)
H. Onoda, Y. Inagaki, A. Kuwabara, N. Kitamura, K. Amezawa, A. Nakahira, I. Tanaka, J. Ceram. Process. Res. 11, 344 (2010)
T. Masui, H. Hirai, N. Imanaka, G. Adachi, Phys. Stat. Sol. A 198, 364 (2003)
M. Tsuhako, S. Ikeuchi, T. Matsuo, I. Motooka, M. Kobayashi, Chem. Lett. 6, 195 (1977)
P. Michard, E. Guibal, T. Vincent, P. Le Cloirec, Microporous Mater. 5, 309 (1996)
E. Guibal, I. Saucedo, J. Roussy, P. Le Cloirec, React. Polym. 23, 147 (1994)
I.L. Von Botto, E.J. Baran, Kristallographische daten Z. Anorg. Allg. Chem. 430, 283 (1977)
R. Wang, J.W. Ye, G.L. Ning, H. Jiang, W.L. Zhou, F.H. Sun, Mater. Lett. 83, 130 (2012)
R. Masse, J.C. Grenier, Bull. Soc. Fr. Minéral. Cristallogr. 95, 136 (1972)
S.A. Sadeek, M.A. El-Sayed, M.M. Amine, M.O. Abd. El-Magied, J. Radioanal. Nucl. Chem. 299, 1299 (2014)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21276046 and 21406045), the Natural Science Foundation of Liaoning Province (2015020199), the Fundamental Research Funds for the Central Universities of China (DUT14LK32), and the Scientific Research Project of the Education Department of Liaoning Province (L2012134).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, R., Ye, J., Ning, G. et al. Microwave-assisted rapid synthesis of cerium phosphates and their adsorption on uranium(VI). Res Chem Intermed 42, 5013–5025 (2016). https://doi.org/10.1007/s11164-015-2342-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2342-5