Abstract
A novel glycidyl methacrylate chelating resin has been synthesized through copolymerization of glycidyl methacrylate (GMA) in the presence of divinylbenzene (DVB), the resulting resin was immobilized with 3,4,5-trihydroxybenzoic acid (THBA) to give GMA/DVB/THBA chelating resin. The adsorption of Th(IV) and U(VI) on GMA/DVB/THBA adsorbent was studied as a function of initial concentration, pH, shaking time and temperature. The novel chelating resin shows a high capacity for Th(IV) and U(VI), maximum adsorption of Th(IV) and U(VI) were 56 and 83.6 mg/g, respectively. Kinetic studies showed that the adsorption follows the pseudo second order model referring to the influence of the textural properties of the resin on the rate of adsorption. Thermodynamic parameters such as ∆H° and ∆S° were studied and indicated an endothermic process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nuclear technology is being utilized for significant benefits in number of fields including power sector, defense, agriculture and medicine while many other areas are being explored [1–4]. Uranium and Thorium are very essential elements in nuclear and atomic energy program. Their recovery is essential to minimize their discharge into the environment from the point of view of safety and economy. So their extraction and separation from geological samples, waste sources becomes a very significant task [2, 5, 6].
A lot of efforts are being made to develop the processes for extracting uranium and thorium such ion exchange and chelating resins [5–8]. Solid sorbents are considered to be the most effective and convenient technique for enrichment, purification, environmental and radioactive waste disposal point of view, because of its simplicity, reliability, selectivity, high capacity, ease of handling and environmental safety…etc. [9].
Phosphonate polystyrene based resins have been prepared [10]. Both the free phosphonic acid as well as the diethyl, dibutyl and bis(2-ethylhexyl)-phosphonate-derivatized sorbents were investigated for U(VI) and Th(IV) extraction. A polystyrene-divinylbenzene resin containing 1-(2-thiazolylazo)-2-naphthol functional group has been synthesized [11]. The total capacities of Th(IV) and U(VI) were 176 and 98 mg/g, respectively. Amberlite XAD-4 functionalized with succinic acid for separation of U(VI) has been synthesized [12]. The adsorption capacity of functionalized resin is 12.3 mg/g. Desorption of U(VI) from resin can be achieved by using HCl. A chelating resin has been developed using Merrifield chloromethylated resin anchored with calixarene-o-vanillinsemicarbazone for preconcentration of U(VI) and Th(IV). The optimum pH range for U(VI) and Th(IV) were 6.0–7.0 and 3.5–4.5. The validity of the proposed method was checked by analyzing these metal ions in natural water samples, monazite sand and standard geological materials [13]. The separation of Th(IV) and U(VI) by Duolite XAD761 resin have been achieved [14]. Th(IV) and U(VI) are adsorbed on Duolite XAD761 where Th(IV) and U(VI) are quantitatively eluted by HCl.
Glycidyl methacrylate based copolymer are receiving great attention due to the presence of reactive epoxide ring, which offer it on opportunity to enter into wide range of chemical reactions. Epoxide ring can be cured through the reaction of epoxide groups with a suitable curing agent. The present work was directed to the separation of U(VI) and Th(IV) from their aqueous solutions using Glycidyl methacrylate chelating resin and the different factors affecting on the adsorption behaviour such as pH, time and temperature will be studied.
Experimental
Chemicals
All chemicals were analytical grade and purchased from Merck or Sigma-Aldrich and were used as received without further purification. All of the solutions were prepared with fresh double distilled water. A uranium stock solution containing 1,000 mg/L of U(VI) was prepared by dissolving 1.782 g of uranyl acetate in 1 % nitric acid solution, and diluting to 1,000 mL. A thorium stock solution containing 1,000 mg/L of Th(IV) was prepared by dissolving 2.46 g of thorium nitrate in 1,000 mL solution.
The concentration of Th(IV) and U(VI) solutions was estimated spectrophotometrically using thoron 1 and Arsenazo I method, respectively [7]. Metal ions concentration was determined using PC scanning spectrophotometer UV/VIS double beam of the type LABOMED, INC (USA).
Preparation of GMA/DVB/THBA resin
The GMA/DVB copolymers (containing 15 mol % DVB) were synthesized by suspension radical copolymerization of GMA and DVB with an initiator of radical polymerization, 2,2-azobis(isobutyronitrile) (AIBN) in the presence of 2-ethyl-1-hexanol (diluent). The continuous phase consisted of a 2 wt% aqueous solution of Poly vinyl alcohol 500 (PVA) (suspension stabilizer). The polymerization was carried out in a flask fitted with a mechanical stirrer, and a condenser at 60 °C for 4 h, 70 °C for 4 h and finally at 80 °C for 4 h, with a stirring rate of 300 rpm. After completion of the reaction, the beads formed were decanted and washed several times with excess of water and ethanol, respectively, and then dried in the drying oven at about 40 °C for approximately 3 h. Finally, the particle size distribution was determined by sieve analysis. The data indicate that the particles size is in the range of 75–500 mm. The particles with diameter in the range of 150–250 mm were used for further investigation.
The GMA/DVB/THBA chelating resin was prepared by adding 5 g of the obtained GMA/DVB copolymers to the hot stirred solution of 50 mL dimethylformamide containing 10 g of 3,4,5-trihydroxybenzoic acid in a proper flask. The mixture was placed in an oil bath at 110 °C for 72 h, with stirring speed of 300 rpm. After completion of the reaction, the beads formed were decanted simply and washed several times with excess of water and ethanol, respectively and then dried in the drying oven at about 40 °C for approximately 3 h.
Adsorption experiments
In order to obtain maximum recovery of Th(IV) and U(VI) from aqueous solution, it was necessary to optimize the experimental parameters such as pH, concentration … etc. In order to evaluate the effect of pH on the recovery, the pH was investigated in the range of 1–5 by adding nitric acid or sodium hydroxide solutions, by placing portions of 50 mg resin in a series of flasks, 100 mL solution of 100 ppm of the studied metal ions were added. The flasks were shaken on a shaking water bath model-1083 (Labortechnik mbH-Germany) at 300 rpm for 2 h at 20 °C. After equilibration, the residual concentration of the metal ion was determined spectrophotometrically and the uptake was calculated [9] using the Eq. (1):
where q is the uptake (mg/g), C i and C f are the initial and final concentrations of the studied metal ion (mg/L), V is the volume (mL) and w is the weight of the resin (g).
The uptakes of the studied metal ions at different initial concentrations were obtained by placing portions of 50 mg resin in a series of flasks. 100 mL of metal ions solution, at pH 4.5 for U(VI) and at pH 3.5 for Th(IV) with different initial concentrations were added to each flasks. The contents of the flasks were equilibrated on the shaker at 300 rpm for 2 h and at a definite temperature. After equilibration the residual concentration of Th(IV) and U(VI) was determined and the uptake value was calculated.
The uptake of Th(IV) and U(VI) at different time intervals was obtained by placing 50 mg resin in a series of flasks. 100 mL of metal ion solution at pH 4.5 for U(VI) and at pH 3.5 for Th(IV) was added. The contents of the flasks were shaken on a shaker at 300 rpm at 20 °C for the required time period. After equilibration, the residual concentration of the investigated metal ion was measured to calculate the metal ion uptake. All experiments were performed in duplicates, and ±4 % was the experimental error of each duplicates.
Results and discussions
Suspension polymerization of the mixture containing GMA (0.85 mol) and DVB (0.15 mol) yields cross linked polymer resin in bead form as shown in Scheme 1. The chemical modification of GMA/DVB resin through epoxide ring which can be cured through the reaction of epoxide groups with trihydroxybenzoic acid as shown in Scheme 2. To confirm the formation of GMA/DVB/TBA resin from the interaction of GMA/DVB with THBA, the infrared spectra of the reactant and product were recorded (Fig. 1). The infrared spectrum of the synthesized resin GMA/DVB/TBA exhibits a very strong band at 1,721 cm−1 due to the stretching vibration motion of (C=O) characteristic for carboxylic group. Also, the infrared spectrum of the synthesis resin showed a shift for the bards characteristic of (O–H), (C=C) and (C–C) to a lower or high values compared with the spectrum of the GMA/DVB resin.
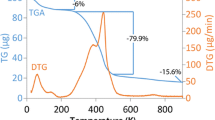
The chemical stability of the resin in acid and alkaline media was tested by shaking a 0.5 g portion of the resin in turn with 100 mL of 2 M HCl and NaOH for 24 h. The resin was then filtered off and washed with water. The adsorption capacity after the treatments was reduced by only 6 %, which was denoted as desirable stability of the resin.
Effect of pH
To find the optimal conditions, the separation of Th(IV) and U(VI) from aqueous solutions the chelating resin were determined under non-competitive conditions at different pHs as shown in Fig. 2. The pH affects the resin chemistry as well as Th(IV) and U(VI) ion chemistry in solution. The pH of the solution has a significant effect on the recovery of Th(IV) and U(VI). The recovery of Th(IV) and U(VI) increased as the pH increased from 1 to 3.5 and 4.5, respectively.
After pH 3.5 Th(IV) begin to participate as Th(IV) hydroxide, On the other hand, at pH above 4.5, various oligomeric and monomeric hydrolysis species of U(VI) are formed such as At pH > 4.5 uranium begin to precipitate and various hydrolyzed species are exist including [UO2OH]+, [(UO2)3(OH)4]2+, [(UO2)3(OH)5]+, [(UO2)2(OH)2]2+, [(UO2)2OH]3+, [(UO2)3(OH)]5+, [(UO2)4(OH)]7+, [UO2(OH)4]2− and [(UO2)3(OH)7]− are occur in varying concentrations, depending on pH and the total U(VI) concentration [9, 15, 16], which cannot attached the resin active sites also the resin favor diffusion of smaller ions; therefore, larger species like polymeric hydroxo complexes of U(VI) stand a poorer chance to be effectively absorbed.
Adsorption kinetics
The effect of equilibrium time in the range of 5–180 min. on the adsorption of Th(IV) and U(VI) on the studied resin at pH 3.5 and 4.5, respectively, and 20 °C is shown in Fig. 3. The adsorption of Th(IV) and U(VI) on the adsorbents increase with increase time. The equilibrium was attained within 90 and 180 min for Th(IV) and U(VI),respectively.
The adsorption data were treated according to different kinetic models to verify the order of the adsorption. The pseudo first order kinetics model [9] was tested according to the Eq. (2)
where q e and q t (mg/g) refer the amount of investigated metal ions adsorbed at equilibrium and at time (t), respectively, k 1 is the rate constant of pseudo first order (min−1). Values of k 1 and q e were calculated from the slope and intercept values of the straight line by plotting log (q e−q t) versus (t) as shown in Fig. 4 and are reported in Table 1. The first order mechanism suffered from inadequacies when applied to the investigated metal ion sorption on the sorbents. One of the major discrepancies was observed when q e values obtained from straight line of plotting were compared with the experimental q e values, Table 1. The experimental q e values differed from the corresponding theoretical values for both Th(IV) and U(VI). Thus, good linearity of the plots is not guarantee that the interactions of Th(IV) and U(VI) with the synthesized sorbents will follow first order kinetics.
The adsorption data were also treated according to the pseudo second order kinetics [9, 17] using the Eq. (3):
where k 2 is the overall rate constant of the pseudo second order adsorption. The plot of t/q t versus (t) gives a straight line with slope and intercept equal to 1/q e and 1/k 2 q 2e , respectively. The calculated values of q e and k 2 were obtained from Fig. 5 and are reported in Table 1. The fitness of the straight lines in Fig. 5 is better compared to that in Fig. 4. Moreover, the calculated correlation coefficient is extremely high and closer to unity for pseudo second order kinetic model than for pseudo first order kinetic model. The calculated equilibrium sorption capacity (q e) is consistent with the experimental data (Table 1). Therefore, the sorption reaction can be approximated more favorably by the pseudo second order kinetic model. These results suggest that a pseudo second order sorption is the predominant mechanism and the over all rate constant of Th(IV) and U(VI) ions appears to be controlled by the chemisorptions process. The rate of adsorption is less controlled by intra-particle diffusion due to the bulky size of Th(IV) and U(VI) ions. This implies that the adsorption of Th(IV) and U(VI) is dependent on concentration of both the metal ions and active sites concentrations.
Adsorption isotherms
One of the most important parameters on adsorption system, which can influence the adsorption behavior of thorium and uranium, is the initial concentration. The adsorption is increasing with increase initial concentration until it the concentration reaches optimum value after which the adsorption is constant with no further increases. The maximum adsorption s of Th(IV) and U(VI) were 56 and 83.6 mg/g, respectively.
The effect of sorption temperature on uptake of Th(IV) and U(VI) ions by sorbents was carried out in temperature range from 393 to 323 K. Figure 6 shows the adsorption isotherms of Th(IV) and U(VI) on resin at different temperatures. An increase in temperature resulted in increase the amount of Th(IV) and U(VI) sorbed per unit mass of the resin showing an endothermic nature of the sorption process. The extent to which the metal ion sorption capacity increase with increasing temperature might be attributed to the change in surface properties of the sorbent, solubility of the solute species and endothermic nature of the sorption process. The increase in temperature may be result in increased the number of active sites available for interaction with metal ion along with the decreased dehydration of the metal ions and active sites.
The adsorption equilibrium isotherm data were evaluated by Langmuir and Freundlich adsorption isotherm equations. Langmuir isotherm model represented by the Eq. 4 assumes that the adsorbent surface is homogeneous and the adsorption sites are energetically identical indicating that the adsorbed molecules don’t react with each other. The linear form of Langmuir equation [18, 19] can be depicted as the following equation:
where C e is the equilibrium concentration of ions in solution (mg/L), q e is the amount adsorbed at C e (mg/g), Q max is the maximum adsorption capacity (mg/g), and K L is the binding constant which is related to the energy of adsorption (L/mg). A plot of C e/q e against C e was drown. 1/Q max calculated from the slope and 1/K L Q max calculated from the intercept. The values of K L and Q max at different temperatures were obtained from Fig. 7, and are reported in Table 2. The value of K L and Q max increase as the temperature increases. The observed increase in the value of K L with temperature increase implies a stronger binding between Th(IV) and U(VI) ions and the active sites of the resin at elevated temperatures.
The Freundlich isotherm model is an empirical relationship describing the sorption of solutes from a liquid to a solid surface [18] and assumes that different sites with several sorption energies are involved (the surface of adsorbent is heterogeneous), and the linear equation is given by Eq. (5):
where q e (mg/g) and C e (mg/L) are the equilibrium concentrations of metal ion in the solid and liquid phase, respectively, and. K F (mg/g) and n are characteristic constants related to the relative sorption capacity of the sorbent and the intensity of sorption, respectively. The plot of logq e against logC e was drown and found to be linear from the slop of which 1/n was calculated and the intercept equal to logK F , respectively. Figure 8 shows the adsorption isotherms for Th(IV) and U(VI) ions, which conforms the Freundlich equation. The Freundlich plot gave a slope less than 1, indicating nonlinear sorption behaviour with the concentration of Th(IV) and U(VI) in the concentration range studied. The observed values of K F of Th(IV) and U(VI) were found to 13.46 and 9.97 (mg/g), respectively.
It can be seen that the values of the correlation coefficients of Langmuir equation were higher than the Freundlich isotherm values, which indicated the Langmuir isotherm correctly fitted the equilibrium data, confirming the monolayer coverage of uranium onto the resin. Also, the value of equilibrium sorption capacity of Langmuir equation (Q max) is more consistent with the experimental data than Freundlich isotherm model. Therefore, the sorption reaction can be approximated more favorably by Langmuir equation model, confirming the monolayer coverage of Th(IV) and U(VI) onto the resin.
The Langmuir parameter K L can be used to predict the affinity between the adsobate and sorbent using the separation factor (R L ) value. R L > 1 unsuitable; R L = 1 linear; 0 < R L < 1 suitable; R L = 0 irreversible [18–20]. The value of R L could be calculated from the following Eq. (6):
where C o is the initial concentration of Th(IV) and U(VI) ions (mM). The values of R L lie between 0.08 and 0.16 indicating the suitability of the resin as adsorbents for Th(IV) and U(VI) from aqueous solution.
Therefore, thermodynamic parameters were evaluated to assess the thermodynamic feasibility and to confirm the nature of the sorption process [20]. The thermodynamic parameters corresponding to Th(IV) and U(VI) adsorption on the studied resin were assessed using van't Hoff equation [21], Eq. (7):
where R is the universal gas constant (8.314 J/mol K), T is the absolute temperature (Kelvin). Plotting ln K L against 1/T gives a straight line with intercept and slope equal ∆S°/R and −∆H°/R, respectively. The values of ∆S° and ∆H° were calculated from Fig. 9 and are also reported in Table 3. The Gibbs free energy of adsorption reaction (∆G°) was calculated using the following relation [9].
The values of ∆G° obtained at different temperatures are given in Table 3. The negative values of ∆G° values confirm the spontaneous nature and feasibility of the sorption process. With the increase of temperature, negative values of ∆G° increasing from −22.85 to −27.27. This indicates that favorable Th(IV) and U(VI) sorption takes place with increasing temperature. The positive values of ∆H° indicated an endothermic nature of adsorption process. The positive value of ∆S° may be explained by the increased degree of randomness at the resin/solution interface during the progress of sorption process. This is due to liberation of free water molecules as a result of the substitution reaction between chelating active sites and hydrated Th(IV) and U(VI) ion. Also the data showed that ∣∆H°∣ < ∣T∆S°∣ in the studied temperature range. This indicated that the adsorption process was dominated by entropic rather than enthalpic changes. In our study the numerical value of mean adsorption energy ∆G° values obtained was >20 kJ/mol. So adsorption process is chemisorptions.
Resin regeneration
In order to inspect any probable activity lost in the following steps, loading and desorbing processes were repeated several times. The resin can be regenerated by washing with dilute HNO3. Also acid contaminants are simply washed with distilled water after which the resin becomes regenerated and ready for the next use. Elution efficiency was found to be 90 % over 3 cycles with a standard deviation of ±3 %.
Conclusion
Chelating resin derived from glycidyl methacrylate and cross linked with divinylbenzene was prepared. The obtained resin was anchored with THBA as active moieties to give GMA/DVB/THBA. The resulting chelating resin was investigated for separation of Th(IV) and U(VI) from their aqueous solution and they show a high capacity for Th(IV) and U(VI). The maximum adsorption of Th(IV) and U(IV) were 56 and 83.6 mg/g, respectively. The adsorption of Th(IV) and U(VI) was found to proceed according to pseudo second order kinetics indicating the influence of textural properties of resin on the rate of adsorption. The thermodynamic parameters obtained showed that the adsorption process is endothermic, spontaneous process and dominated by entropy rather than enthalpy change.
References
Loveland WD, Morrissey DJ, Seaborg GT (2006) Modern nuclear chemistry. Wiley, London
Kumar A, Singhal RK, Rout S, Narayanan U, Karpe R, Ravi PM (2013) Adsorption and kinetic behavior of uranium and thorium in seawater-sediment system. J Radioanal Nucl Chem 295:649–656
Kadam RB, Mali GG, Mohite BS (2013) Analytical application of poly [dibenzo-18-crown-6] for chromatographic separation of thorium(IV) from uranium(VI) and other elements in glycine medium. J Radioanal Nucl Chem 295:501–511
Dauner J, Workman S (2012) Comparison of TEVA® resin beads, PAN fibers, and ePTFE membranes as a solid support for Aliquat-336 in immobilized liquid extraction chromatography for separation of actinides. J Radioanal Nucl Chem 292:967–972
Rao TP, Metilda P, Gladis JM (2006) Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination. Talanta 68:1047–1064
Meinrath G (1998) Aquatic chemistry of uranium. Geoscience 1:1–101
Marczenko Z (1986) Separation and spectrophotometric determination of elements. Ellis Harwood, Chichester
Cotton S (2006) Lanthanide and actinide chemistry. Wiley, London
Donia AM, Atia AA, Moussa EMM, El-Sherif AM, Abd El-Magied MO (2009) Removal of uranium(VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95:183–189
Merdivan M, Buchmeiser MR, Bonn G (1999) Phosphonate-based resins for the selective enrichment of uranium(VI). Anal Chim Acta 402:91–97
Lee W, Lee SE, Lee CH, Kim YS, Lee YI (2001) A chelating resin containing 1-(2-thiazolyazo)-2-naphtol as the functional group; synthesis and sorption behavior for trace metal ions. Microchem J 70:195–203
Metilda P, Sanghamitra K, Gladis JM, Naidu GRK, Rao TP (2005) Amberlite XAD-4 functionalized with succinic acid for the solid phase extraction preconcentration and separation of uranium(VI). Talanta 65:192–200
Jain VK, Pandya RA, Pillai SG, Shrivastav PS (2006) Simultaneous preconcentration of uranium(VI) and thorium(IV) from aqueous solutions using a chelating calyx[4]arene anchored chloromethylated polystyrene solid phase. Talanta 70:257–266
Aydin FA, Soylak M (2007) Solid phase extraction and preconcentration of uranium(VI) and thorium(IV) on Duolite XAD761 prior their inductively coupled plasma mass spectrometric determination. Talanta 72:187–192
Trivedi UV, Menon SK, Agrawal YK (2002) Polymer supported calix[6]arene hydroxamic acid, a novel chelating resin. React Funct Polym 50:205–216
Pathak R, Rao GN (1996) Synthesis and metal sorption studies of p-tert- butylcalix[8]arene chemically bound to polymeric support. Anal Chim Acta 335:283–290
Shriver DF, Atkins PW, Langford CH (1994) Inorganic chemistry, 2nd edn. Oxford University Press, Oxford
Tian G, Geng J, Jin Y, Wang C, Li S, Chen Z, Wang H, Zhao Y, Li S (2011) Sorption of uranium(VI) using oxime-grafted ordered mesoporous carbon CMK-5. J Hazard Mater. doi:10.1016/j.jhazmat.2011.03.066
Zhang L, Huang T, Zhang M, Guo X, Yuan Z (2008) Studies on the capability and behavior of adsorption of thallium on nano-Al2O3. J Hazard Mater 157:352–357
Sharma P, Tomar R (2008) Synthesis and application of an analogue of mesolite for the removal of uranium(VI), thorium(IV), and europium(III) from aqueous waste. Microporous Mesoporous Mater 116:641–652
Mpofu VP, Mensah JA, Ralston J (2004) Temperature influence of nonionic polyethylene oxide and anionic polyacrylamide on flocculation and dewatering behavior of kaolinite dispersions. J Colloid Interface Sci 271:145–156
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadeek, S.A., El-Sayed, M.A., Amine, M.M. et al. A chelating resin containing trihydroxybenzoic acid as the functional group: synthesis and adsorption behavior for Th(IV) and U(VI) ions. J Radioanal Nucl Chem 299, 1299–1306 (2014). https://doi.org/10.1007/s10967-013-2847-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2847-6