Abstract
Magnetically separable Fe3O4 nanoparticles were used as an environmental friendly catalyst for the synthesis of mono- and bis-tetrahydro-4H-chromene and mono- and bis-1,4-dihydropyridine derivatives via three-component reactions of aromatic aldehydes and malononitrile with cyclic β-dicarbonyls or cyclic β-enaminoketones respectively. These reactions were carried out in EtOH and at reflux. Fe3O4 nanoparticles can be magnetically separated from the reaction mixture by a magnet and recycled without significant loss of catalytic activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions (MCRs) are useful to make complex molecules with reliable synthetic yield, frequently with high stereoselectivity, and easily accessible reactants [1–3]. Improvement of MCRs can result in useful synthetic methods to produce many organic compounds potentially applicable in current organic, bioorganic, and medicinal chemistry [4–6].
Due to their unique chemical and biological properties, bis-heterocyclic compounds have attracted much attention in recent years [7–9]. Particularly, bis-heterocyclic compounds, which exhibit various biological activities, including antibacterial, fungicidal, tuberculostatic, and antiamoebic properties [10–12], play important roles in the drug discovery processes and analysis of drugs in late development.
In this context, the three-component reaction of aromatic aldehydes and malononitrile with cyclic β-dicarbonyls or cyclic β-enaminoketones has been one of the most well-studied MCRs in recent years. The reaction affords formation of mono- and bis-tetrahydro-4H-chromene and mono- and bis-1,4-dihydropyridine derivatives, respectively.
Tetrahydro-4H-chromenes due to their lucrative biological and pharmacological properties are used as a momentous class of heterocyclic compounds in the pharmaceutical applications such as anticancer [13], antimalarial [14], antileishmanial [15], antibacterial [16], antifungal [17], antianaphylactic [18], antiallergenic [19], diuretic [20], and hypotensive [21] agents.
Also, 1,4-dihydropyridines are of an important class of compounds with a wide range of biological activities [22]. Due to being pharmacologically active and acting as antitumor [23], calcium channel blocker [24], antitubercular [25], analgesic [26], antithrombotic [27], anti-inflammatory [28], anticonvulsant agents [29], they are of many interests.
Applications of tetrahydro-4H-chromene and 1,4-dihydropyridine derivatives necessitates the advancement in the environmentally friendly procedures to synthesize these compounds by three-component reaction of aromatic aldehydes and malononitrile with cyclic β-dicarbonyls or cyclic β-enaminoketones, respectively. There are various catalytic systems for the synthesis of tetrahydro-4H-chromene [30–39] and 1,4-dihydropyridine [40, 41] derivatives by MCR, but these systems suffer from disadvantages like the use of toxic organic solvents, costly catalysts, existence of transition metals, difficult work up, time-consuming reaction, and low yield. Thus, a simple and green synthesis of tetrahydro-4H-chromenes and 1,4-dihydropyridines needs to be carried out without association of these catalytic systems.
The field of nanocatalysis is a rapidly growing field, which involves the use of nanoparticles as catalysts for a variety of homogeneous and heterogeneous catalysis applications, owing to their remarkably different and unique properties, which include a high surface area, easy recovery, and nanosize [42, 43]. Nanocatalysts are esteemed as materials of enormous surface areas and with new investigation, developments may offer expanding catalytic abilities [44–46]. Heterogeneous catalysis characterizes one of the oldest commercial practices of nanoscience, nanoparticles of metals and other compounds have been extensively used for important chemical reactions. Fe3O4 nanoparticles (Fe3O4 NPs) have been used as recyclable catalysts in the development of sustainable methodologies [47] for synthesis of compounds such as xanthenes [48], oximes [49], quinoxalines [50], fused azo-linked pyrazolo[4,3-e]pyridines [51], 1,8-dioxo-decahydroacridine [52], propargylamines [53] and many more, because of their significant characteristics such as the ease of magnetic separation from the reaction mixture, non-toxicity, and being widely accessible. But Fe3O4 NPs has never been used in the synthesis of tetrahydro-4H-chromene (4a–x) and 1,4-dihydropyridine (7a–t) derivatives. Hence, we set about to synthesize the aimed compounds in refluxing EtOH by using Fe3O4 NPs as a catalyst for the first time.
Results and discussion
To optimize the reaction conditions, a methodical study considering different variables affecting the reaction time and yield was carried out for the three-component reaction of benzaldehyde 1a, malononitrile 2, and dimedone 3a in the presence of Fe3O4 NPs as catalysts for preparing compound 2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 4a (Scheme 1). The results are shown in Table 1.
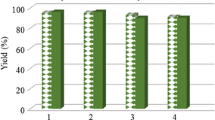
We used some polar and nonpolar solvents in this reaction for which the best results in terms of reaction time and yield of the desired product 4a was obtained when the reaction was conducted in ethanol (Table 1, entry 1–4). We also optimized the quantity of the catalysts. The best results were obtained when the reactions were carried out in the presence of 30 mol% Fe3O4 NPs (Table 1, entry 5–8). We also attempted to reuse the catalyst; the catalyst was easily separated from the reaction mixture by an external magnet and reused (after washing with ethanol and dried) for three consecutive runs, and no obvious diminishing activity was observed (Table 1, entry 9–11).
Relying on our collected data, we decided to apply this method for synthesis of tetrahydro-4H-chromene (4a–k, m–w) and 1,4-dihydropyridine (7a–d, f–i, k–n, p–s) derivatives by a three-component reaction of aromatic aldehydes (1a–k) and malononitrile (2) with cyclic β-dicarbonyls (3a, b) or cyclic β-enaminoketone (6a–d) respectively, in EtOH, under reflux, and in the presence of Fe3O4 NPs (30 mol%) (Scheme 2; Tables 2, 3).


We were stimulated by this success, so the versatility of the reaction was explored further by extending the procedure to the synthesis of bis-tetrahydro-4H-chromene (4l, x) and bis-1,4-dihydropyridine derivatives (7e, j, o, t) via reaction of para phthalaldehyde (1l) with two equiv. malononitrile (2) and β-dicarbonyls (3a, b) or cyclic β-enaminoketone (6a–d) under similar conditions (Scheme 3; Tables 2, 3).
A plausible mechanism for the nano Fe3O4 catalyzed is illustrated in Scheme 4. The nano Fe3O4 facilitates the Knoevenagel condensation and Michael addition through coordination of its Fe3+ with O of the carbonyl group and with N from CN.
Conclusions
In summary, we have reported the use of magnetically separable Fe3O4 nanoparticles for the synthesis of mono- and bis-tetrahydro-4H-chromene and mono- and bis-1,4-dihydropyridine derivatives via the three-component reaction of aromatic aldehydes and malononitrile with cyclic β-dicarbonyls or cyclic β-enaminoketones, respectively. These reactions were carried out in EtOH at reflux. Recycling of the catalyst, high yields, short reaction times, and clean reaction conditions are advantages of this procedure that make it a useful practical process for the synthesis of these compounds.
Experimental section
General
Melting points were measured on a Electrothermal-9100 apparatus and are uncorrected. IR spectra were recorded on a Brucker FT-IR Tensor 27 infrared spectrophotometer. 1H NMR and spectra were recorded on a Avance III 400 MHz Bruker spectrometer. The cyclic enaminoketones 6a–d [54] were prepared from according to procedures described in the literature.
Preparation of Fe3O4 nanoparticles
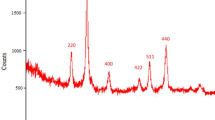
To a solution of FeCl2·4H2O (2.5 g) and FeCl3·6H2O (6 g) in 30 mL deionized water was added dropwise 1.0 mL of concentrated hydrochloric acid at room temperature. The solution was added into 300 mL of 1.5 mol/L NaOH, and then the solution was stirred vigorously at 70 °C until precipitation. Afterwards, the prepared magnetic nanoparticles were separated magnetically, washed with deionized water, and then dried at 70 °C for 8 h [48].
General procedure for the preparation of mono- and bis-tetrahydro-4H-chromene (4a–x) and mono- and bis-1,4-dihydropyridine (7a–t) derivatives
A mixture of aromatic aldehydes 1a–k (2 mmol) [if para phthalaldehyde 1l (1 mmol)], malononitrile 2 (2 mmol), cyclic β-dicarbonyls 3a, b or cyclic β-enaminoketones 6a–d (2 mmol) and Fe3O4 nanoparticles (30 mol%) in ethanol (10 mL) was refluxed for the time reported in Table 2, 3 (the progress of the reaction being monitored by TLC and was used hexane/ethyl acetate as an eluent). After completion of the reaction, the catalyst was separated magnetically from the reaction mixture. Then the reaction mixture was poured into ice-cold water; the crude product was filtered, dried, and recrystallized from ethanol.
4,4′-(1,4-Phenylene)bis(2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile) (4x)
Yellow powder; IR (KBr, ν max/cm−1): 3456, 3392 (NH2), 2192 (CN), 1680 (C=O), 1593 (C=C). 1H NMR (400 MHz, DMSO-d6): 7.38 (s, 4H, CH-Ar), 7.04 (s, 4H, 2NH2), 4.11 (s, 2H, 2CH), 3.31–1.32 (m, 12H, 6CH2). 13C NMR (100 MHz, DMSO-d6): 195.21 (C=O), 163.27 (C2), 157.12 (C8a), 146.47 (C–Ar), 128.63 (CH-Ar), 121.11 (C4a), 114.08 (CN), 62.25 (C3), 48.98 (CH2), 43.87 (CH2), 37.89 (C4), 26.14 (CH2). Anal. calcd. for C26H22N4O4: C, 68.71; H, 4.88; N, 12.33 %. Found: C, 68.49; H, 4.69; N, 12.14 %.
2-Amino-7,7-dimethyl-5-oxo-4-(pyridin-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (7d)
Yellow powder; IR (KBr, ν max/cm−1): 3472, 3360, 3312 (NH2, NH), 2192 (CN), 1699 (C=O), 1606 (C=C). 1H NMR (400 MHz, DMSO-d6): 9.07 (s, 1H, NH), 8.27 (s, 1H, CH-Ar), 7.78–7.25 (m, 3H, CH-Ar), 5.38 (s, 2H, NH2), 4.43 (s, 1H, CH), 2.27 (d, 1H, 2 J HH = 4 Hz, CH), 2.21 (d, 1H, 2 J HH = 4 Hz, CH), 2.07 (d, 1H, 2 J HH = 8 Hz, CH), 1.78 (d, 1H, 2 J HH = 8 Hz, CH), 1.07 (s, 3H, CH3), 0.91 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6): 192.21 (C=O), 164.78 (C2), 158.14 (C8a), 153.25, 137.02, 135.14, 130.54, 128.11, 124.32 (C4a), 114.21 (CN), 63.70 (C3), 49.74 (CH2), 41.89 (CH2), 38.25 (C4), 35.28 (CMe2), 32.21 (CH3), 28.98 (CH3). Anal. calcd. for C17H18N4O: C, 69.37; H, 6.16; N, 19.03 %. Found: C, 69.15; H, 5.98; N, 18.84 %.
4,4′-(1,4-Phenylene)bis(2-amino-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile) (7e)
Yellow powders; IR (KBr, ν max/cm−1): 3472, 3296, 3232 (NH2, NH), 2176 (CN), 1648 (C=O), 1590 (C=C). 1H NMR (400 MHz, DMSO-d6): 8.98 (s, 2H, 2NH), 7.71 (s, 4H, CH-Ar), 5.27 (s, 4H, 2NH2), 4.43 (s, 2H, 2CH), 2.51 (d, 2H, 2 J HH = 8 Hz, 2CH), 2.35 (d, 2H, 2 J HH = 8 Hz, 2CH), 2.18 (d, 2H, 2 J HH = 8 Hz, 2CH), 1.61 (d, 2H, 2 J HH = 8 Hz, 2CH), 1.01 (s, 6H, 2CH3), 0.92 (s, 6H, 2CH3). 13C NMR (100 MHz, DMSO-d6): 193.92 (C=O), 167.87 (C2), 160.34 (C8a), 144.70 (C–Ar), 128.82 (CH-Ar), 121.20 (C4a), 114.08 (CN), 61.28 (C3), 52.48 (CH2), 46.91 (CH2), 43.74 (C4), 32.04 (CMe2), 30.26 (CH3), 27.77 (CH3). Anal. calcd. for C30H32N6O2: C, 70.84; H, 6.34; N, 16.52 %. Found: C, 70.63; H, 6.16; N, 16.33 %.
2-Amino-7,7-dimethyl-5-oxo-1-phenyl-4-(pyridin-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (7i)
Pale orange powders; IR (KBr, ν max/cm−1): 3392, 3312 (NH2), 2192 (CN), 1670 (C=O), 1587 (C=C). 1H NMR (400 MHz, DMSO-d6): 8.12–7.07 (m, 9H, CH-Ar), 5.53 (s, 2H, NH2), 4.43 (s, 1H, CH), 2.23 (d, 1H, 2 J HH = 4 Hz, CH), 2.18 (d, 1H, 2 J HH = 4 Hz, CH), 2.01 (d, 1H, 2 J HH = 8 Hz, CH), 1.77 (d, 1H, 2 J HH = 8 Hz, CH), 0.93 (s, 3H, CH3), 0.85 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6): 192.65 (C=O), 166.05 (C2), 160.06 (C8a), 157.05, 152.25, 135.29, 133.15, 132.47, 131.62, 131.27, 126.25, 123.58, 121.20 (C4a), 114.32 (CN), 62.69 (C3), 49.78 (CH2), 43.58 (CH2), 39.02 (C4), 35.87 (CMe2), 31.76 (CH3), 29.54 (CH3). Anal. calcd. for C23H22N4O: C, 74.57; H, 5.99; N, 15.12 %. Found: C, 74.35; H, 5.80; N, 14.92 %.
4,4′-(1,4-Phenylene)bis(2-amino-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile) (7j)
Yellow powders; IR (KBr, ν max/cm−1): 3472, 3328 (NH2), 2192 (CN), 1638 (C=O), 1590 (C=C). 1H NMR (400 MHz, DMSO-d6): 7.63–7.58 (m, 8H, CH-Ar), 7.43 (d, 2H, 3 J HH = 4 Hz, CH-Ar), 7.24 (d, 4H, 3 J HH = 4 Hz, CH-Ar), 5.32 (s, 4H, 2NH2), 4.47 (s, 2H, 2CH), 2.20 (d, 2H, 2 J HH = 8 Hz, 2CH), 2.15 (d, 2H, 2 J HH = 8 Hz, 2CH), 2.07 (d, 2H, 2 J HH = 8 Hz, 2CH), 1.77 (d, 2H, 2 J HH = 8 Hz, 2CH), 0.89 (s, 6H, 2CH3), 0.77 (s, 6H, 2CH3). 13C NMR (100 MHz, DMSO-d6): 194.90 (C=O), 165.04 (C2), 151.08 (C8a), 150.19, 144.41, 136.24, 130.27, 129.71, 126.78, 121.01 (C4a), 113.78 (CN), 60.57 (C3), 49.28 (CH2), 46.56 (CH2), 40.93 (C4), 35.91 (CMe2), 31.98 (CH3), 28.85 (CH3). Anal. calcd. for C42H40N6O2: C, 76.34; H, 6.10; N, 12.72 %. Found: C, 76.13; H, 5.91; N, 12.54 %.
2-Amino-5-oxo-4-(pyridin-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (7n)
Pale yellow powders; IR (KBr, ν max/cm−1): 3456, 3392, 3232 (NH2, NH), 2192 (CN), 1680 (C=O), 1593 (C=C). 1H NMR (400 MHz, DMSO-d6): 8.81 (s, 1H, NH), 8.03 (s, 1H, CH-Ar), 7.68–7.33 (m, 3H, CH-Ar), 5.75 (s, 2H, NH2), 4.41 (s, 1H, CH), 2.31–1.74 (m, 6H, 3CH2). 13C NMR (100 MHz, DMSO-d6): 192.25 (C=O), 166.02 (C2), 160.14 (C8a), 156.22, 148.27, 141.59, 137.47, 131.22, 120.75 (C4a), 113.14 (CN), 62.02 (C3), 48.01 (CH2), 37.12 (CH2), 36.58 (C4), 27.03 (CH2). Anal. calcd. for C15H14N4O: C, 67.65; H, 5.30; N, 21.04 %. Found: C, 67.43; H, 5.12; N, 20.85 %.
4,4′-(1,4-Phenylene)bis(2-amino-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile) (7o)
Yellow powders; IR (KBr, ν max/cm−1): 3392, 3328, 3232 (NH2, NH), 2176 (CN), 1657 (C=O), 1600 (C=C). 1H NMR (400 MHz, DMSO-d6): 8.90 (s, 2H, 2NH), 6.97 (s, 4H, CH-Ar), 5.56 (s, 4H, 2NH2), 4.84 (s, 2H, 2CH), 2.77–1.28 (m, 12H, 6CH2). 13C NMR (100 MHz, DMSO-d6): 193.89 (C=O), 169.41 (C2), 159.96 (C8a), 149.80 (C–Ar), 128.67 (CH-Ar), 121.25 (C4a), 115.09 (CN), 62.21 (C3), 49.82 (CH2), 43.70 (CH2), 36.54 (C4), 25.82 (CH2). Anal. calcd. for C26H24N6O2: C, 69.01; H, 5.35; N, 18.57 %. Found: C, 68.80; H, 5.17; N, 18.38 %.
2-Amino-5-oxo-1-phenyl-4-(pyridin-3-yl)-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (7s)
Cream powders; IR (KBr, ν max/cm−1): 3392, 3328 (NH2), 2192 (CN), 1658 (C=O), 1593 (C=C). 1H NMR (400 MHz, DMSO-d6): 8.23 (s, 1H, CH-Ar), 7.99–7.35 (m, 8H, CH-Ar), 5.78 (s, 2H, NH2), 4.42 (s, 1H, CH), 3.13–1.87 (m, 6H, 3CH2). 13C NMR (100 MHz, DMSO-d6): 191.97 (C=O), 166.48 (C2), 160.15 (C8a), 150.12, 142.35, 138.72, 135.28, 134.34, 132.19, 131.79, 128.86, 125.68, 121.14 (C4a), 113.78 (CN), 62.61 (C3), 49.47 (CH2), 40.25 (CH2), 39.59 (C4), 28.26 (CH2). Anal. calcd. for C21H18N4O: C, 73.67; H, 5.30; N, 16.36 %. Found: C, 73.45; H, 5.12; N, 16.17 %.
4,4′-(1,4-Phenylene)bis(2-amino-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile) (7t)
Yellow powders; IR (KBr, ν max/cm−1): 3408, 3328 (NH2), 2176 (CN), 1638 (C=O), 1596 (C=C). 1H NMR (400 MHz, DMSO-d6): 7.36–7.32 (m, 6H, CH-Ar), 7.20 (d, 2H, 3 J HH = 4 Hz, CH-Ar), 7.99 (s, 4H, CH-Ar), 5.03 (s, 4H, 2NH2), 4.24 (s, 2H, 2CH), 2.26–1.41 (m, 12H, 6CH2). 13C NMR (100 MHz, DMSO-d6): 195.15 (C=O), 165.12 (C2), 152.34 (C8a), 150.91, 144.58, 136.23, 130.04, 129.66, 126.82, 121.25 (C4a), 114.05 (CN), 60.48 (C3), 49.11 (CH2), 42.74 (CH2), 35.95 (C4), 27.76 (CH2). Anal. calcd. for C38H32N6O2: C, 75.48; H, 5.33; N, 13.90 %. Found: C, 75.27; H, 5.15; N, 13.72 %.
References
J. Zhu, Multicomponent Reactions (Wiley-VCH, Weinheim, 2005)
L.F. Tietze, Chem. Rev. 96, 115 (1996)
L.F. Tietze, U. Beifuss, Angew. Chem. 105, 137 (1993)
D.A. Horton, G.T. Bourne, M.L. Smythe, Chem. Rev. 103, 893 (2003)
A.S. Al-bogami, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1171-7
R. Sandaroos, S. Damavandi, Res. Chem. Intermed. 39, 4167 (2013)
R.M. Shaker, Arkivoc ix, 59 (2006)
F. Bellina, R. Rossi, Tetrahedron 62, 7213 (2006)
M. Abaszadeh, M. Seifi, Org. Biomol. Chem. 12, 7859 (2014)
P.D. Mehta, N.P.S. Sengar, A.K. Pathak, Eur. J. Med. Chem. 45, 5541 (2010)
A.R. Bhat, F. Athar, A. Azam, Eur. J. Med. Chem. 44, 426 (2009)
P.F. Iqbal, H. Parveen, A.R. Bhat, F. Hayat, A. Azam, Eur. J. Med. Chem. 44, 4747 (2009)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
J. Davoll, J. Clarke, E.F. Eislager, J. Med. Chem. 15, 837 (1972)
T.N. Shwetaa, S. Gupta, Bioorg. Med. Chem. Lett. 14, 3913 (2004)
A.M. El-Agrody, M.H. El-Hakium, M.S. Abd El-Latif, A.H. Fekry, E.S.M. El-Sayed, K.A. El-Gareab, Acta Pharm. 50, 111 (2000)
V.K. Ahluwalia, R. Batla, A. Khurana, R. Kumar, Indian J. Chem. Sec. B. 29, 1141 (1990)
J.L. Wang, D. Liu, Z.J. Zhang, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S. Alnemri, Z. Huang, Proc. Natl. Acad. Sci. U.S.A. 97, 7124 (2000)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
M. Kidwai, S. Saxena, M.K.R. Khan, S.S. Thukral, Bioorg. Med. Chem. Lett. 15, 4295 (2005)
D. Heber, C. Heers, U. Ravens, Pharmazie 48, 537 (1993)
D.M. Stout, A. Meyers, Chem. Rev. 82, 223 (1982)
R. Boer, V. Gekeler, Drugs Fut. 20, 499 (1995)
M. Ramesh, W.V. Matowe, J. Med. Chem. 41, 509 (1998)
S.R. Pattan, A.N. Parate, Indian. J. Heterocycl. Chem. 12, 387 (2003)
Y.S. Sadanandam, M.M. Shetty, Eur. J. Med. Chem. 29, 975 (1994)
K. Cooper, M.J. Fray, J. Med. Chem. 35, 3115 (1992)
S.R. Agudoawu, E.E. Knaus, J. Heterocycl. Chem. 37, 303 (2000)
A. Shafiee, N. Rastakari, Daru 12, 81 (2004)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
H.R. Safaei, M. Shekouhy, A. Shirinfeshan, S. Rahmanpur, Mol. Divers. 16, 669 (2012)
M. Seifi, H. Sheibani, Catal. Lett. 126, 275 (2008)
T.S. Jin, J.C. Xiao, S.J. Wang, T.S. Li, X.R. Song, Synlett, 2001 (2003)
C. Lu, X.J. Huang, Y.Q. Li, M.Y. Zhou, W. Zheng, Monatsh. Chem. 140, 45 (2009)
S. Abdolmohammadi, S. Balalaie, Tetrahedron Lett. 48, 3299 (2007)
M.R. Naimi-jamal, S. Mashkouri, A. Sharifi, Mol. Divers. 14, 473 (2010)
M.M. Heravi, B. Baghernejad, H.A. Oskooie, J. Chin. Chem. Soc. 55, 659 (2008)
M. Abaszadeh, M. Seifi, Lett. Org. Chem. 12, 271 (2015)
F. You-Jiana, M. Chun-Baoa, G. Yuanb, T. Shu-Jiang, F. Fang, S. Da-Qing, Chin. J. Chem. 22, 622 (2004)
B.V. Lichitsky, A.A. Dudinov, M.M. Krayushkin, Arkivoc ix, 73 (2001)
M. Abaszadeh, M. Seifi, A. Asadipour, Res. Chem. Intermed. 41, 5129 (2014)
S. Sa, M.B. Gawande, A. Velhinho, J.P. Veiga, N. Bundaleski, J. Trigueiro, A. Tolstogouzov, O.M.N.D. Teodoro, R. Zboril, R.S. Varma, P.S. Branco, Green Chem. 16, 3494 (2014)
M.B. Gawande, A.K. Rathi, I.D. Nogueira, R.S. Varma, P.S. Branco, Green Chem. 15, 1895 (2013)
D. Wang, D. Astruc, Chem. Rev. 114, 6949 (2014)
M.B. Gawande, R. Luque, R. Zboril, Chem. Cat. Chem. 6, 3312 (2014)
M.B. Gawande, Y. Monga, R. Zboril, R.K. Sharma, Coord. Chem. Rev. 288, 118 (2015)
M.B. Gawande, P.S. Brancoa, R.S. Varma, Chem. Soc. Rev. 42, 3371 (2013)
M.A. Ghasemzadeh, J. Safaei-Ghomi, S. Zahedi, J. Serb. Chem. Soc. 78, 769 (2013)
B. Zeynizadeh, M. Karimkoshteh, J. Nanostruct. Chem. 3, 57 (2013)
H.Y. Lü, S.H. Yang, J. Deng, Z.H. Zhang, Aust. J. Chem. 63, 1290 (2010)
M. Nikpassand, L. Zare, T. Shafaati, S. Shariati, Chin. J. Chem. 30, 604 (2012)
M.A. Ghasemzadeh, J. Safaei-Ghomi, H. Molaei, C. R. Chim. 15, 969 (2012)
T. Zeng, W.W. Chen, C.M. Cirtiu, A. Moores, G. Song, C.J. Li, Green Chem. 12, 570 (2010)
Z. Karimi-Jaberi, Z. Takmilifard, Eur. Chem. Bull. 2, 211 (2013)
Acknowledgments
The authors express their great appreciation to the Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, for supporting this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amirheidari, B., Seifi, M. & Abaszadeh, M. Evaluation of magnetically recyclable nano-Fe3O4 as a green catalyst for the synthesis of mono- and bis-tetrahydro-4H-chromene and mono and bis 1,4-dihydropyridine derivatives. Res Chem Intermed 42, 3413–3423 (2016). https://doi.org/10.1007/s11164-015-2220-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2220-1