Abstract
A simple, clean, and environmentally benign three-component process to the synthesis of 2-amino-4H-chromenes using N,N-dimethylaminoethylbenzyldimethylammonium chloride, [PhCH2Me2N+CH2CH2NMe2]Cl−, as an efficient catalyst under solvent-free condition is described. A wide range of aromatic aldehydes easily undergo condensations with α-naphthol and malononitrile under solvent-free condition to afford the desired products of good purity in excellent yields. Taking into account environmental and economical considerations, the protocol presented here has the merits of environmentally benign, simple operation, convenient work-up and good yields. Furthermore, the catalyst can be easily recovered and reused for at least five cycles without losing its activities.
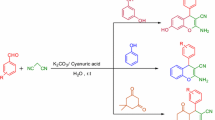
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2-Aminochromenes represent an important class of compounds being the main components of many naturally occurring products, and have been of interest in recent years due to their useful biological and pharmacological aspects, such as anticoagulant, spasmolytic, diuretic, insecticidal, anticancer, and antianaphylactin activity [1]. Some of them can also be employed as cosmetics and pigments [2], and utilized as potential biodegradable agrochemicals [3].
2-Aminochromenes are generally prepared by refluxing malononitrile, aldehyde, and activated phenol in the presence of hazardous organic bases like piperidine in organic solvents such as ethanol and acetonitrile for several hours [4]. A literature survey revealed several modified procedures using cetyltrimethylammonium chloride (CTAC) [5], tetrabutylammonium bromide (TBAB) [6], cetyltrimethylammonium bromide (CTAB) coupled with ultrasound [7], γ-alumina [8], K2CO3 [9], nanosize MgO [10], heteropolyacid [11], hexadecyltrimethylammonium bromide (HTMAB)[12], triethylbenzylammonium chloride (TEBA) [13] and TiCl4 [14]. However, some of the reported methods require prolonged reaction time, reagents in stoichiometric amounts, and toxic solvents, and generate moderate yields of the product.
The increasing attention during the last decades for environmental protection has led both modern academic and industrial groups to develop chemical processes with maximum yield and minimum cost while using non-toxic reagents, solvents, and catalysts or solvent-free condition. One of the tools used to combine economic aspects with the environmental ones is the multicomponent reaction (MCR) strategy; this process consists of two or more synthesis steps which are carried out without isolation of any intermediate thus reducing reaction time, and saving money, energy, and raw materials [15, 16].
As part of our program aimed at developing useful new selective and synthesis methods based on the use of functionalized ionic liquids as catalysts of fine chemicals preparation, we have studied using the MCR strategy for the synthesis of substituted 2-aminochromenes using the basic ionic liquid catalyst, N,N-dimethylaminoethylbenzyldimethylammonium chloride ([PhCH2Me2N+CH2CH2NMe2]Cl−), under solvent-free condition.
Results and discussion
The model reaction was carried out simply by mixing benzaldehyde, malononitrile (2), α-naphthol (3), and the ionic liquid catalyst at 80 °C for 35 min without any solvent to afford the desired product in good yield. The scope and the generality of the present method were then further demonstrated by the reaction of 1 with 2 and 3 (Scheme 1).
In all cases, good yields with good selectivity were obtained. All the results are shown in Table 1.
As can be seen from Table 1, electronic effects and the nature of substituents on the aromatic ring did show strong effects in terms of reaction time under the reaction conditions mentioned above. When aromatic aldehydes containing electron-donating groups (such as hydroxyl, alkoxyl, or methyl group) were employed (Table 1, entries 7–11), a longer reaction time was required than those of electron-withdrawing groups (such as nitro group, halide) on aromatic rings (Table 1, entries 2–6). It is worthy of note that the reaction proceeded without the protection of acidic hydroxyl substituents (Table 1, entries 9 and 11).
The ionic liquid catalyst is in favor of melting of the reaction mixture and plays a crucial role in the success of the reaction in terms of the rate and the yields. Reacting benzaldehyde with 2 and 3 as a reference, the reaction could not be carried out in the absence of the catalyst at 80 °C. When 1 mol% of the catalyst was used, the yield of the product reached 70% at 80 °C under solvent-free condition for 2 h. Increasing the amount of catalyst from 5 to 10 mol%, the reaction yields rose from 83 to 92%. Therefore, 10 mol% of the catalyst was enough to push the reaction to completion.
In view of the green chemistry, the catalyst was further explored for the reusability by a model reaction of 4-chlorobenzaldehyde and reactants 2 and 3 under similar conditions in the presence of 10 mol% catalyst. The catalyst was easily recovered by washing the reaction mixture with distilled water and directly reused for the next turn after evaporation of water under reduced pressure. The recycled catalyst has been reused five times to catalyze the model reaction affording the corresponding chromene in 91, 88, 90, 87, and 89% yields, and without appreciable decreases of yield.
In conclusion, we describe a practical and efficient procedure for the preparation of 2-amino-2-chromenes through the three-component reaction of aromatic aldehydes, malononitrile (2), and α-naphthol (3) using a catalytic amount of N,N-dimethylaminoethylbenzyldimethylammonium chloride as catalyst under solvent-free condition. This procedure offers several advantages including mild reaction conditions, cleaner reaction, and satisfactory yields of products, as well as a simple experimental and isolated procedure, which makes it a useful and attractive protocol for the synthesis of these compounds.
Experimental
Melting points were measured on an Electrothemal X6 microscopy digital melting point apparatus. IR spectra were recorded on a Bruker Equinox 55 spectrometer using KBr pellets. 1H NMR spectra were recorded in DMSO-d 6 on a Bruker AVANCE 300 (300 MHz) instrument with the TMS at δ 0.00 ppm as an internal standard. Chemicals used were of commercial grade without further purification. The ionic liquid was prepared according to the method reported in literature [17].
General procedure for the synthesis of 2-amino2-chromenes
An equimloar (4 mmol) mixture of an aromatic aldehyde (1), malononitrile (2), α-naphthol (3) and 10 mol% N,N-dimethylaminoethylbenzyldimethylammonium chloride was vigorously stirred at 80 °C for the specific time indicated in Table 1. The end of the reaction was monitored by TLC. Then, the crude product obtained was added the distilled water. The precipitated resulting solid was filtered out and purified by recrystallization from hot methanol to afford the pure products 4. The catalyst remained in water was reused another cycle after evaporation of water. All of the products are known and the data are found to be identical with those that reported in literature (Table 1).
References
Bonsignore L, Loy G, Secci D, Calignano A (1993) Eur J Med Chem 28:517
Ellis GP (1997) The Chemistry of heterocyclic compounds chromenes, chromanes and chromones, Chap. II. In: Weissberger A, Taylor EC (eds). Wiley, New York, p 11
Hafez EAA, Elnagdi MH, Elagamey AGA, Ei-Taweel FMAA (1987) Heterocycles 26:903
Elagamey AGA, El-Taweel FMAA (1990) Indian J Chem B 29B:885
Ballini R, Bosica G, Conforti ML, Maggi R, Mazzacanni A, Righi P, Sartori G (2001) Tetrahedron 57:1395
Jin TS, Xiao JC, Wang SJ, Li TS, Song XR (2003) Synlett 2001
Jin TS, Xiao JC, Wang SJ, Li TS (2004) Ultrason Sonochem 11:393
Maggi R, Ballini R, Sartori G, Sartorio R (2004) Tetrahedron Lett 45:2297
Kidwai M, Saxena S, Rahman Khan MK, Thukral SS (2005) Bioorg Med Chem Lett 15:4295
Kumar D, Reddy VB, Mishra GB, Rana RK, Nadagouda MN, Varma RS (2007) Tetrahedron 63:3093
Heravi MM, Bakhtiari K, Zadsirjan V, Bamoharram FF, Heravi OM (2007) Bioorg Med Chem Lett 17:4262
Jin TS, Zhang JS, Liu LB, Wang AQ, Li TS (2006) Synth Commun 36:2009
Shi DQ, Zhang S, Zhuang QY, Wang XS (2003) Chin J Org Chem 23:1419
Kumar BS, Srinivasulu N, Udupi RH, Rajitha B, Reddy YT, Reddy PN, Kumar PS (2006) J Heterocycl Chem 43:1691
Ugi I (2001) Pure Appl Chem 73:187
Dömling A, Herdtweck E, Ugi I (1998) Acta Chem Scand 52:107
Gokel GW, Garcia BJ (1978) Tetrahedron Lett 19:1743
Acknowledgments
We are grateful to the National Natural Science Foundation of China (No 20672046) and the Guangdong Natural Science Foundation (No 04010458) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Huang, XJ., Li, YQ. et al. A one-pot multicomponent reaction for the synthesis of 2-amino-2-chromenes promoted by N,N-dimethylamino-functionalized basic ionic liquid catalysis under solvent-free condition. Monatsh Chem 140, 45–47 (2009). https://doi.org/10.1007/s00706-008-0008-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-008-0008-3