Abstract
The South American silver croaker is a popular fish that has recently received substantial attention from scientists, mainly due to its importance as source of animal protein and as a key fisheries species. However, little is known about the conditions that explain its historical and current spatial distribution, both in its native habitat and where it is a successful invasive species. The aim of the present study was to explore the ecological information available for this species, to then critically examine ecological theories related to the conditions underpinning its success. To this end, an exhaustive literature search was conducted with the immediate aim of investigating whether the success of South American silver croaker was driven by species-climate or species–human interactions. The non-native populations were found to occupy climate niche spaces different from those observed in their native ranges. In addition, it was clear that humans played a role in facilitating the large-scale dispersion of silver croaker, and assisted as agents of impact driving the observed current and, probably, the future spatial distribution, which we can predict from our data and from the pattern of propagule pressure. Overall, the current biogeography of this species illustrates how the construction of dams, along with the introduction and stocking of non-native species, overfishing and other human activities can alter fish populations and assemblages. Such processes can reduce native species, increase the abundance and distribution of invasive species, as well as cause changes in life-history traits and genetic variability, all with long-term socioeconomic consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The South American silver croaker [Plagioscion squamosissimus (Heckel 1840), Sciaenidae—Fig. 1] is native to the Amazon Basin, and is considered an aggressive carnivore species, preferably eating other fish, including other piscivores (Hahn et al. 1997; Carolsfeld et al. 2003; Mèrona et al. 2010; Pereira et al. 2015; Bezerra et al. 2017). The sliver croaker undergoes short-distance migrations, has a reproductive cycle with peaks occurring during warmer months, and displays no parental care of the offspring (Suzuki et al. 2005; Graça and Pavanelli 2007). Currently, it is widely distributed throughout South America, and can be found in the river basins of the Amazon, Orinoco, Paraná, Paraguay, São Francisco and Guyana (Agostinho and Júlio Jr 1996; Hahn et al. 1997; Casatti 2003; Cella-Ribeiro et al. 2017). The silver croaker’s native distribution is the result of its natural historical biogeography, in which the colonization of freshwater habitats occurred during a marine transgression through western Venezuela that developed about 20 million years ago. After these initial events the members of the Plagioscion genus experienced a rapid diversification in freshwater ecosystems (Boeger and Kritsky 2003). Plagioscion squamosissimus emerged after the establishment of the modern Amazon River (Cooke et al. 2012), where the large-scale hydrochemical and ecological gradients appear to have acted as ecological barriers, maintaining population discontinuities even in the face of gene flow. Due to this adaptive divergence, P. squamosissimus can be abundant in habitats with wide variation in hydrology, sediment composition, geochemistry and optical characteristics (Cooke et al. 2012).
Specimens of Plagioscion squamosissimus recorded in some of the 20 freshwater ecoregions: one exemplar of the Madeira River, Amazonas, Brazil, FEOW-321 (a); artisanal fishing in Mamoré River, Amazon Bolivian, FEOW-318 (b–d); artisanal fishing in Tocantins River Basin, Brazil, FEOW-324 (e); exemplar from Upper Paraná River basin and deposited in fish collection (voucher number: LPB 3493), FEOW-344 (f); nocturnal fishing, Tocantins River Basin, Brazil, FEOW-324 (g); fishing on the banks of Rosana Reservoir, Paranapanema River, Southeastern Brazil, FEOW-344 (h)
Tolerance of distinct local conditions is a primary factor facilitating the expansion of species to new habitats and regions and, when associated with climate, life history, and human activity, can determine the global distribution patterns of the species (e.g. Moyle and Light 1996; Leibold et al. 2004; Holyoak et al. 2005; Moyle and Marchetti 2006; Zwiener et al. 2018). Non-native species that were translocated by human activities outside of their natural historical limits can occupy areas with climates different from those observed in their native ranges (Broennimann et al. 2007; Guo et al. 2013), but adaptations to their native range can affect their invasive potential in the new region. Thus, species are more likely to successfully invade locations with abiotic conditions similar to those in their native range (Zenni et al. 2014; Skóra et al. 2015).
It is widely accepted that characteristics such as reproductive strategy, growth rate, trophic level and migratory patterns are major aspects in the life-histories of fish (e.g. Winemiller 1991; Skóra et al. 2015; Winemiller et al. 2015; Vitule et al. 2017). Such characteristics may vary according to water temperature and to differences in climate (Stenseth and Mysterud 2002; Carim et al. 2017), and are usually related to success in invasion events (Vitule et al. 2016, 2017). For example, at low latitudes, the long photoperiods, high water temperature, and availability of food resources are reflected by faster growth, a continuous annual reproductive period, shorter length at first maturation, and more energy allocated to reproduction, thus allowing the existence of fish with smaller size classes (Vazzoler 1996; Schultz and Conover 1997; McBride et al. 2015). However, our knowledge of life-history traits and their relationship to environmental factors remain unknown for many Neotropical fishes, including the South American silver croaker. Such information is of utmost importance when considering fish invasions given that similarity in life-history traits drives naturalization patterns (Zenni et al. 2014; Skóra et al. 2015; Vitule et al. 2016). However, invasive range expansions can lead to a fish species occupying climate niche spaces very different from those observed in their native ranges (Broennimann et al. 2007; Guo et al. 2013). This is promoted by specific mechanisms as phenotypic plasticity, which results from trait evolution and phylogenetic conservatism (Agosta and Klemens 2008). As the evolutionary history can promote phenotypic plasticity, the organismal traits relevant can evolve to suit other locations with different environmental conditions (Janzen 1985). In other words, the invasions of silver croaker, in particular, have had a broad success in South America, primarily because of the inheritance of traits from its Amazonian evolutionary history.
In addition to the contribution of life-history traits to the naturalization process and invasion success, the number of individuals introduced, and the number of source populations may also be powerful predictors of successful invasions (Lockwood et al. 2009; Zenni and Simberloff 2013). Propagule pressure, the number of individuals released in the non-native environment, is the main and consistent correlate of species establishment success (Lockwood et al. 2007, 2009; Cassey et al. 2018). Human activity is a good proxy that has also served to facilitate invasions of non-native fish by increasing the translocation of species and the propagule pressure (Hampe and Petit 2005; Leprieur et al. 2008; Caplat et al. 2013). Therefore, we expect that the native and non-native distributions of South American silver croaker will provide evidence that human activity is responsible for the magnitude of the propagule pressure and will therefore affect the success of this species’ establishment.
The South American silver croaker is an important species in Brazilian freshwater commercial fisheries (Petrere Jr 1978; Hahn et al. 1997; Carolsfeld et al. 2003). For this reason, it was introduced into reservoirs in northeastern Brazil in the 1940s with the dual goals of filling the available lentic environments and improving the quality of the fish supply in the region (e.g. Fontenele and Peixoto 1978; Hahn et al. 1997). Because of the high levels of production recorded in the Northeastern region, the first juveniles were introduced into reservoirs in the Brazilian Southeast and South (Agostinho and Júlio Jr 1996; Hahn et al. 1997; Bialetzki et al. 2004; Bennemann et al. 2006; Hoeinghaus et al. 2009) during a restocking program funded by the Energy Company of São Paulo State in 1974 (Torloni et al. 1993a, b). This also included other fish species as Triphorteus angulatus, Hoplias lacerdea, Astronotus ocelatus, Cyprinus carpio, Oreochromis niloticus, as well as the macrocrustaceans Macrobrachium amazonicum and Macrobrachium jelskii that were introduced as prey for these fish. In reservoirs where the South American silver croaker was introduced and later became established, such as those populations found in the Paraná and Paranapanema rivers basins in South Brazil, populations have contributed significantly to commercial and sport fisheries, often to the detriment of many commercial and high value native species (Carolsfeld et al. 2003; Hoeinghaus et al. 2009). In the Itaipu Reservoir, the main reservoir for hydropower generation in South America, where it was probably introduced in 1972, silver croaker reached high abundances and became one of the main species in local fish production, with a yield of 243 tons by 1990 (e.g. Agostinho et al. 1994; Agostinho and Júlio Jr 1996; Hoeinghaus et al. 2009). In the Paranapanema River basin, the South American silver croaker was recorded for the first time in 1992. By 2001 it already experienced a considerable increase in abundance and achieved a broad distribution (Bennemann et al. 2006).
The present study provides an overview of the literature concerning the biology, ecology and biogeography of the South American silver croaker, an important Neotropical fish species in South America. The primary objective was to explore data acquired from an exhaustive literature review in order to: (1) identify prior studies concerning native and non-native populations in different ecoregions in South America; (2) identify the life-history traits related to its distribution; (3) identify the main factors related to the success of silver croaker as an invader, based on evidence of species-climate and species-human interactions; (4) assess the impacts of non-native populations on fishing management and conservation; (5) suggest potential studies and strategies for the management of non-native populations and, more generally, for the conservation of the native fish fauna in remotes areas in megadiverse countries hosting high species richness located in hotspots of biodiversity.
Methods
Literature search and study selection
The methodology for the systematic review included separate steps for the identification, screening, and eligibility of the literature used, and included the creation of a checklist summarizing the information found in each paper identified during the literature search (Moher et al. 2009). The review was organized through an adapted Prisma flow diagram (Fig. S1). The systematic review began with a literature search, which aimed to identify previous records of P. squamosissimus in South America (Table S1 in Supplementary Material), covering all studies, both published and unpublished, between 1905 and 2017. The studies included scientific articles, books, registers, museum documents, monographs, dissertations, theses, technical reports, and conference papers. The majority of the studies were acquired using “Plagioscion squamosissimus” as the keyword search term in the following electronic databases: Biodiversity Heritage Library (BHL), CrossRef, Encyclopedia of Life (EOL), Google Scholar, Global Biodiversity Information Facility (GBIF), ResearchGate, Scopus, Web of Science and ScienceDirect. Other studies were obtained directly from the authors. The studies were written in English, French, German, Portuguese and Spanish. According our exclusion criteria in the adapted Prisma flow diagram, 39 articles were excluded due to the lack of contribution for our empirical analyses, or they were papers on another species where silver croaker was just cited as example, and/or studies on market research in which this species was found in fish shops (Fig. S1).
A scientometric approach was used to identify prior studies concerning native and non-native populations in the different ecoregions of South America, and to determine whether the human population density of the region affects the number of studies conducted. For this purpose, studies describing native populations were defined as those studies for which the study area was located in the native region of the species, with the species occurring as a result of natural processes. Non-native populations were considered those occurring outside of the known native range of the South American silver croaker, as result of extralimital introductions, even within the original country (e.g. Vitule et al. 2014; Daga et al. 2015; Liu et al. 2017). To determine the distribution of P. squamosissimus in South America, the occurrence of native and non-native populations was compiled from the Freshwater Ecoregions of the World (FEOW http://www.feow.org/). The areas occupied by native and introduced populations of the South American silver croaker were quantified by determining the geographic locality of each scientific study. Studies that covered regions with both native and non-native populations were denoted as “general”. To obtain better information from these “general” papers, we counted each observation from within these papers separately, resulting in data number not equal to the number of papers (Frehse et al. 2016). Climate characteristics, e.g. precipitation, temperature and latitude, were obtained for each FEOW (Abell et al. 2008) where South American silver croaker was reported. The human population density was obtained from the Gridded Population of the World Version 3 platform (CIESIN—CIAT—SEDAC: http://sedac.ciesin.columbia.edu/gpw).
In addition, each study was classified according to its research focus (distribution, ecology, ethno-ichthyology, feeding, fishing census, genetic, ichthyo-archeology, larval biology, length–weight relationship, morphology, morphometry, parasitology, population biology, register, reproductive biology, taxonomy, toxicology, trophic ecology), status of the population as synonymous to type of origin (native and non-native) (Simberloff and Vitule 2014; Paolucci et al. 2013), and type of habitat (dike, estuary, lake, reservoir, river channel and tributary).
Some individual studies, for example, those related to the ethno-ichthyology, feeding, genetic, ichthyo-archeology, morphology, morphometry, parasitology, register taxonomy and toxicology, were excluded from the abundance estimation because of their limited sample size. The data from articles in which the keywords “abundance”, “number of individuals”, “specimens”, and “catch per unit effort (CPUE)” were found in the Materials and methods sections, were used to calculate the residuals of linear regressions from the relationship of abundance of individuals and number of samplings, in order to standardize non-random samples within the study area. The abundance of individuals, juveniles and adults, were used as proxies for invasion success, since all ages of life stages of the South American silver croakers increase the chances of establishment, persistence, naturalization, and invasion. For example, success in the process of colonization and invasion is probably due to the high dispersion of eggs, refuges for larvae, a short early life stage (Vazzoler 1996; Bialetzki et al. 2004), carnivory by juvenile and adult stages (Lasso-Alcalá et al. 1998; Bezerra et al. 2017), and certain reproductive strategies, such as partial spawning, small eggs and high fertility (Vazzoler 1996; Barbosa et al. 2012).
Our hypotheses to explain the distributions of South American silver croaker are that (1) the invasion success will be primarily affected by human activity (Leprieur et al. 2008; Lima Jr et al. 2018), and that, (2) climate changes and human activity together affect the success of species establishment shown from the plasticity of life history traits.
Influence of climate and humans on invasion success
Invasion success was used to test if climate and/or humans affect the range of South American silver croaker. We expect that invasion success will be primarily affected by human activity (Leprieur et al. 2008; Lima Jr et al. 2018). With respect to the influence of climate, we suggest that non-native species were able to occupy areas with climates different from those observed in their native ranges (Broennimann et al. 2007; Guo et al. 2013); the null hypothesis assumes that the invaded regions will have climate conditions similar to those in their native range (Zenni et al. 2014), and excludes the idea that invasion success is influenced solely by propagule pressure originating from the human translocations.
Influence of climate and humans on life history traits
Climate is an important component of a species ecological niche, determining conditions where the species occurs when it has reached equilibrium (Peterson et al. 2011). It can define potential areas of distribution for species and represents an important tool for assessments of the impacts of climate change and biological invasions (Peterson et al. 2011; Gallardo et al. 2017). At the same time, we expect that this is not the only factor determining the distribution pattern of invasive species, especially in the establishment stage. Propagule pressure is one of the major factors in the establishment of introduced species (Lockwood et al. 2005; Colautti et al. 2006; Vitule et al. 2009). For instance, Cassey et al. (2018) pointed out that propagule pressure is the strongest and generally most consistent determinant of non-native species establishment. Therefore, we need evidence to support a clear policy and management target aiming to curb invasions by reducing propagule pressure. As the propagule pressure is a result of human acts, we expect that human activity will affect the success of species establishment of South American silver croaker, as evidenced by variations in quantitative life history traits as they adapt to new regions outside of their native distributions. Native species displaced to new ecoregions and exposed to changes in climate, tend to have the increase in their range correlated with broad environmental niches and large population sizes, especially if the species is a generalist with high invasion potential (Lawler et al. 2013; Bellard et al. 2014; Bezerra et al. 2017; Zwiener et al. 2018).
Statistical analyses
Relationships between the values of response variables (abundance, allometric coefficient, size at first maturity SFM, sex ratio, somatic gonadal relationship) and precipitation, temperature, latitude and status were evaluated through a Linear Mixed Models (LMM) analysis, using the Gaussian distribution. The models were fitted using the function lmer (Bates 2007) in R software (R Development Core Team 2016).
The use of abundance as a response variable was based on extracted residuals of the linear relationships between the number of individuals and the number of samplings to control for differences in sampling effort. The allometric coefficient β (beta) is a constant obtained from the W–L function (Weight = β Lengthα) used to quantify the type of growth (Goldman et al. 1990). Size at first maturity (SFM) is a proxy for the mean size at first sexual maturation in males and females, determined as the size at which 50% of the individuals are sexually mature (Vazzoler 1981). Sex ratio represents the female/male ratio. Somatic gonadal relationship (SGR) represents the mean value of the relationship between gonad weight (Wg) and total weight (Wt) for each individual (SGR = Wg/Wt × 100). SFM, sex ratio, and the somatic gonadal relationship are considered important factors in the reproductive cycle of teleost fish (Vazzoler 1996), therefore they were considered in our analyses.
Maximum precipitation (mm) and minimum temperature (°C) were obtained from FEOW (2018) and used because of their greater standard deviations, to express the large variation in climate to which the populations of South American silver croaker were subjected. Latitude was transformed into a decimal coordinate to test the effect of latitudinal variation on population structure.
Status (native and non-native) assumes the role of the independent variable indicating the influence of human management in the distribution. The FEOW were used as a random effect. These were assigned a categorical variable with 11 levels, and were characterized by a lack of independence among sampling units. Because freshwater species composition, population dynamics, and environmental conditions within a given ecoregion are more similar to each other than to those of surrounding ecoregions, populations of the same species in the same freshwater ecoregion tend to have similar structure.
Different statistical models were evaluated using the Corrected Akaike Information Criterion (AICc); AICc is an estimator of the relative quality of statistical models of a data set in cases where the sample size is small (Ward 2008; Lee and Ghosh 2009). The model with the lowest value of AICc was considered to be the best (most parsimonious) predictive model and used in further analyses. Confidence intervals (95%) were calculated for the model averaged coefficients (Zuur et al. 2009). Variables were defined as contributing significantly to the predictive model if their confidence intervals did not overlap zero. The variation in the data explained by random variables was assessed as significant if 95% confidence intervals for the estimated intercept overlapped the mean intercept. If the variation accounted for by these random variables was low, the random effects model was simplified to a Linear Regression Model (LRM) (Zuur et al. 2009).
Results
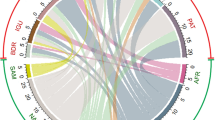
Studies of the South American silver croaker were spread over climatic niches (tropical and subtropical), and in different freshwater ecoregions (Fig. 2a). Native populations were found in fourteen ecoregions located in the northern and northwestern parts of South America, while non-native populations were found in six ecoregions located in the northeastern, southern and southeastern parts of Brazil. The most studied ecoregions were the Upper Paraná (34% of the studies), Amazonas Lowlands (12% of the studies) and Tocantins–Araguaia (11% of the studies) (Fig. 2a). The majority of studies of both native and non-native populations of South American silver croaker took place in ecoregions containing large numbers of reservoirs (including freshwater weirs) (Fig. 2b). Non-native populations only occupied river channels influenced by reservoirs (Fig. 2a).
Our results show that the human population density of a region affected the number of investigations of the South American silver croaker. A higher number of individual studies were recorded in regions with high concentrations of people (+ 1000 person per Km2) (Fig. 2a). Additional evidence of human influence is indicated by the chronological scale (Table 1). Although studies of native populations of South American silver croaker began in the 1950’s and the first studies assessing non-native populations were not published until much later in 1976 (Fig. 3 and Table 1), the number of publications concerned with non-native populations of South American silver croaker overtook the number of publications on native populations in the middle 1990’s (Fig. 3). From 1950 to the present, the research areas with higher publication numbers were population biology (23%), distribution patterns (21%), feeding habits (15%), and reproductive biology (9%) (Table 1). The increase in population biology studies was associated with studies of the non-native populations.
Coefficient confidence intervals that do not encompass zero suggest consistent and significant relationships among response variables across climates and humans. For example, abundance was inversely associated with temperature (Table 2). Abundance, allometric coefficient, SFM and sex ratio were inversely associated with status showing that introduction effort and life history traits varied with the human effect. SGR was positively correlated with latitude, precipitation and status, showing again that life history traits varied across climate and with degree of human activity (Table 2). Marginal R2 and conditional R2 values were low (always < 0.37; Table 1), except for SGR, which had a conditional R2 value of 0.75, indicating that this last variable varied the most across the FEOW (Table 2).
Analyses of biological attributes revealed a variation in growth parameters, including different types of allometry and isometry for the different ecoregions occupied by native and non-native populations (Table 3). The variation of SFM values in the native region is smaller than in non-native populations. With respect to the sex ratio, the proportion of females was higher than that of males, except for the non-native population in Upper Paraná ecoregion (Table 3). The SGR was higher for the non-native population in Northeastern Caatinga and Coastal Drainages (1.63), and lowest in the native range in the Amazon Estuary and Coastal Drainages (0.01) (Table 3). A continuous reproductive period was reported for the Orinoco Llanos ecoregion, while continuous reproductive periods with peaks were seen for native regions in the Amazonas Lowlands, Mamore-Madre de Dios Piedmont, Madeira Brazilian Shield, Xingu, Amazonas Estuary and Coastal Drainages and Tocantins–Araguaia ecoregions (Table 3). A seasonal reproductive period was seen in the non-native populations of the Upper Paraná. With respect to diet, the South American silver croaker was predominantly carnivorous in the adult phase, feeding primarily on fish and shrimp in most of the ecoregions (Table 3).
Discussion
With our complete set of results, the current human-altered biogeography and the degree of invasiveness of the South American silver croaker was revised, suggesting that climate, life history and human activity all facilitate the dispersion and establishment of this fish in the recipient regions, including many extralimital introductions into Brazil. This kind of introduction—into the same country—is ignored by many of the general papers and reviews about invasive species globally (e.g. Leprieur et al. 2008; Dawson et al. 2017). Extralimital introductions must also be treated as a paramount issue, particularly if they are more frequent than other alien introductions. The study of biological invasions is further neglected by the lack of recognition or distinction between translocated and alien species.
The climate, as a component of determination of the ecological niche distribution at the larger scale (Peterson et al. 2011), shows that this species is successful in tropical and subtropical climates. Humans, as facilitators of the dispersion process, have an indirect role by altering habitat and constructing reservoirs, and a direct role by promoting introductions and the pressure associated with massive numbers of propagules (Colautti et al. 2006; Lockwood et al. 2007; Vitule et al. 2009; Zwiener et al. 2018; Frehse et al. 2016; Cassey et al. 2018). Here, we highlight the fact that non-native populations of South American silver croaker occupied river channels only where reservoirs had an influence. The plasticity of life history characteristics facilitates the establishment, allows the species’ exploration of distant habitats, and underpins its ability to deal with climate changes (Peterson et al. 2011; Gallardo et al. 2017; Zwiener et al. 2018).
The distribution of the South American silver croaker in freshwater ecoregions
The patterns of distribution of the South American silver croaker were influenced by life-history (A), the climate (C), and human (D) drivers, as indicated by the black arrows in (Fig. 4). The current distribution in different ecoregions in South America confirms the idea that this species can also occupy subtropical climate niches, beyond the tropical climate niches in its native ranges (Box B in the Fig. 4). The local occupancy data showed that habitat conditions were similar for both native and non-native populations, indicating that they responded similarly to different climate conditions in equatorial and subtropical regions. This implies that using the climate variables in the native range to predict the potential invasive range of a non-native species may be misleading (e.g. Vellend et al. 2007; Zenni et al. 2014). It is clear that other parameters related to habitat alterations need to be considered when analyzing the potential establishment of non-native species in a new region, e.g. the propagule pressure, which is directly related to humans by translocation and stocking, or indirectly related by habitat modifications, e.g. pollution and dam construction (Colautti et al. 2006; Lockwood et al. 2007; Vitule et al. 2009; Daga et al. 2015; Zwiener et al. 2018).
Flow diagram to explain the patterns of distribution of the silver croaker. The factors that influence distribution are indicated by black arrows: life-history (Box A), climate (Box C), and human drivers (Box D). The types of distributions, native and non-native, are indicated in Box B. The implementation of management practices and population control (Box E) measures to each anthropic action (Box D) are indicated by the grey arrow. The dashed grey arrows show the process of introduction of silver croaker: the translocation of individuals to new localities that do not belong to their natural geographic distribution (1), form non-native populations (2), which are evident in areas of subtropical climate (3). In the new regions of geographic distribution, the introduced populations begin to compete with species in the local community, where they suffer biotic resistance by or cause the replacement of native freshwater fishes (4) that are the main examples of ecological changes (Box F)
Life-history strategies versus climate conditions
Life-history traits are fundamental to the rapid spread and successful colonization of South American silver croaker, especially in reservoirs, as demonstrated by the biological attributes of variation in growth, SFM, sex ratio, and reproductive period in the native and non-native ranges. The reproductive tactics of South American silver croaker underwent gradual changes during the first 6 years in the Itaipu Reservoir, as a result of the biotic and abiotic conditions in the new environment (e.g. Carnelós and Benedito-Cecilio 2002; Zwiener et al. 2018). This highlights the idea that reservoirs facilitate the establishment and spread of organisms pre-adapted to lentic conditions (Johnson et al. 2008; Lima Jr et al. 2018).
In accordance with this line of thinking, we added the property plasticity of growth (Box A in Fig. 4) as an attribute of successful adaptation. Isometry and different types of allometry were seen in ecoregions within the native and non-native ranges. The plasticity of fish growth can display considerable intraspecific variation in response to differences in such factors as temperature and food supply (Weatherley 1990). During long photoperiods, high water temperatures and the availability of food are reflected in a higher growth rate, continuous reproductive period, shorter length at first maturation, and more energy allocated to reproduction, promoting the prevalence of smaller size classes (Vazzoler 1996; Schultz and Conover 1997; McBride et al. 2015). Seasonality, especially winter, affects the structure of trophic chains. During winter, the growth of fishes is slower, but individuals reach the largest size classes, the reproductive period is seasonal, and the species reaches the length of first maturation later than in tropical ecosystems where the South American silver croaker is native (see also Conover 1990; Conover et al. 1997; McBride et al. 2015 for related rationales).
The differences in allometric growth among populations were more related to the size of energy reserves and the somatic growth rate than to reproductive investment. This was suggested by the non-significant correlations of fecundity with both the length and the weight of South American silver croakers (Box A in Fig. 4; see also Braga 1997). Weight relative to fecundity does not correlate with increases in total body mass acquired during growth (Braga 1997). This can be considered as an adaptation to conserve energetic investment for fecundity while there is an increase in body mass (Gennari Filho and Braga 1996).
Feeding patterns display ontogenetic variation between juveniles and adults. For example, juveniles showed a more diverse diet, whilst adults feed predominantly on fish and shrimp (Neves et al. 2015). Carnivorous diet patterns were conserved, despite climate differences in the ecoregions of South America (Table 2). South American silver croaker showed a preference for lentic sites with favorable conditions for piscivory (Chacon and Silva 1971; Hoeinghaus et al. 2009; Agostinho et al. 2016) in the adult phase (Hahn et al. 1997; Stefani and Rocha 2009; Neves et al. 2015; Froese and Pauly 2017). Its feeding tactics are different from that of other carnivores that are restricted to reservoirs where the transparency affects feeding tactics, because it is not visual predator and, therefore, can also occur in turbid waters (Cella-Ribeiro et al. 2017). This observation supports the idea that interactions and indirect effects across ecological levels could be influencing the ability to explore reservoirs (Braga et al. 2017). The reproduction of the South American silver croaker occurs preferentially in tributaries (Carnelós and Benedito-Cecílio 2002; Oliveira et al. 2005), because seasonal variations in water level have a direct influence on water characteristics (e.g. transparency), habitat availability (mainly in the riverine zone), and food resources (Santos et al. 2010), which favor the early life stages. Eggs, larvae, and juveniles of South American silver croakers find refuge against predators within turbid water and among the aquatic vegetation found in tributaries (Bialetzki et al. 2004; Bennemann et al. 2006).
Ecological changes affecting the fish community
The species distribution is influenced by the biotic interactions and the local abiotic conditions (Boucher 1985). With respect to the latter, the South American silver croaker is known to favor lentic habitats. However, little is known about how biotic interactions affect its distribution. In general, the intensity of interactions is related to how susceptible communities are to invasions (Bruno et al. 2003). Competition can serve as an example of biotic resistance by native communities to invasion (Box F in the Fig. 4). Diverse assemblages should use resources more completely and leave little niche space for potential new arrivals (Levine and D’Antonio 1999). Therefore, local communities with a greater diversity of native species are likely to be more resistant to invasion, especially communities with a higher number of native species functionally similar to the invader (Skóra et al. 2015). On other hand, the introduction of non-native species is considered to be one of the major agents of processes that cause changes in ecosystem structure, e.g. biotic homogenization (Rahel 2002; Clavero and García-Berthou 2006; Olden 2016; Bezerra et al. 2017). Some studies have shown that non-native species have massively disrupted local assemblages in the Upper Paraná ecoregion (e.g. Pelicice and Agostinho 2009; Pelicice et al. 2014; Vitule et al. 2012; Daga et al. 2015). Generally, the widespread colonization of introduced species changes the compositional dissimilarity of communities over time and space through their high capacity for dispersal, wide tolerance range and opportunism, and the replacement of native species (e.g. Hoeinghaus et al. 2009; Vitule et al. 2012, 2014; Agostinho et al. 2016). For example, it has been reported that the South American silver croaker replaced the native Pimellodus maculatus, because of the specific autoecology of the native species, and the loss of longitudinal connectivity promoted by the development of reservoirs (Petesse and Petrere Jr. 2012). There were still other indications that South American silver croaker preys intensively on young Hypophathalmus edentatus, the main species captured in the fishery of the Itaipu Reservoir (Agostinho et al. 1994; Agostinho and Júlio Jr. 1996; Hahn et al. 1997).
In addition, the introduction of South American silver croaker has a further important impact on subtropical hydrographic basins because of its piscivorous feeding habit (Hahn et al. 1997; Bennemann et al. 2006). More specifically, the piscivorous feeding habit of P. squamosissimus acts as a mechanism leading to its success in establishment and maintenance as an invasive species in new environments (Pereira et al. 2015). The invasion process depends directly on interactions with the recipient community and on opportunities for the occupation of niches and on indirect effects in the network of interactions according to the ecological levels (Braga et al. 2017). Thus, it is important to know what effects the invasive species have on other species. Invasions of piscivorous fish demonstrate how translocated species promote biotic homogenization (e.g. Clavero and García-Berthou 2006; Vitule et al. 2012; Daga et al. 2015; Bezerra et al. 2017; Liu et al. 2017), especially through the extirpation of functionally diverse freshwater fish species (Matsuzaki et al. 2013), and by reducing the abundance of native species, both of which exacerbate the loss of biodiversity (Barros et al. 2012). For example, Liu et al. (2017) found that unregulated translocations, primarily due to aquaculture practices, contributed more to the homogenization of China’s freshwater fish fauna than non-native species introduced from another country. This is a growing concern, particularly in regions considered to be hotspots of biodiversity, such as the Neotropics, a region with the highest taxonomic and functional richness of freshwater fishes (Toussaint et al. 2016), features which are often underestimated and already facing high risk (Vitule et al. 2009, 2016, 2017).
Anthropogenic action driving the South American silver croaker distribution
Humans have facilitated the dispersion of the South American silver croaker through actions such as: (1) facilitating the translocation of subpopulations from their native ecoregions to ecoregions where the species did not previously occur; (2) transforming pristine rivers in expansive lacustrine zones into reservoirs (e.g. Pelicice and Agostinho 2009; Hoeinghaus et al. 2009; Vitule et al. 2012; Gois et al. 2015; Agostinho et al. 2016); (3) expanding aquaculture based largely on the production of non-native species with high invasive potential, especially in hydroelectric reservoirs (Lima Jr. et al. 2018). We observed that South American silver croaker invasions occurred primarily in sites influenced by human activity, such as in ecoregions with numerous reservoirs, where this species dominates.
It is commonly agreed that the introduction of non-native species in aquatic ecosystems has been favored by proximity to large urban centers, with a high density of humans and a high level of economic activity (e.g. McKinney 2006; Leprieur et al. 2008; Frehse et al. 2016; Dawson et al. 2017). For example, the peacock-bass Cichla kelberi, another species native to the Amazon Basin, has been introduced into several reservoirs in the metropolitan regions of southeastern and southern Brazil, in order to enhance sport fishing (Espínola et al. 2010; Daga et al. 2016). In a study of C. kelberi’s ability to invade reservoirs of the Upper Paraná River basin, the authors observed that reservoirs located close to large urban centers had higher probability of invasion (Espínola et al. 2010). Our results were consistent with previous studies, and we reinforce the idea that the biogeography of fish invasions matches the geography of human impacts at large spatial scales (Leprieur et al. 2008).
The predominance of South American silver croaker in reservoirs (including freshwater weirs), can be related to the suitability of conditions in this type of aquatic environment for non-native species. Reservoirs provide new habitats with initially few colonizer species and with changes in environmental conditions, such as an increase in water temperature and transparency (Henry 2014). The large variation in productivity and water transparency serve as ecological filters promoting invasions across large geographic areas, through either predator abundance or propagule pressure (Bajer et al. 2015). Despite the fact that propagule pressure favors alien species establishment, it is still ignored as a hypothesis in studies on the importance of ecological filters (Cassey et al. 2018). Ecological filters promoting high propagule pressure must be considered as part of research studies and potential management actions, because they make it difficult to control intermittent colonization (sensus Lockwood et al. 2005, 2009; Frehse et al. 2016). This is an important example of how we need evidence to underpin clear policy and management targets for slowing invasion rates by reducing propagule pressures globally (Cassey et al. 2018).
Reservoir construction favors the extreme proliferation of some populations, which can then serve as food resources for colonizing piscivores (e.g. Havel et al. 2005; Agostinho et al. 2008; Johnson et al. 2008; Pelicice et al. 2014). The introduction of non-native species adapted to reservoir conditions has resulted in large fishery yields of species such as H. edentatus, Odontesthes bonariensis, P. squamosissimus and Pterodorus granulosus in reservoirs in the south of Brazil (e.g. Hoeinghaus et al. 2009; Agostinho et al. 2016; Santa-Fé and Gubiani 2016). Moreover, the disruption of the dendritic architecture of rivers and associated streams caused by dam construction may also facilitate colonization by non-native species (Havel et al. 2005; Johnson et al. 2008; Hoeinghaus et al. 2009; Agostinho et al. 2016), thereby promoting the process of biotic homogenization (Clavero and Hermoso 2011; Vitule et al. 2012; Daga et al. 2015).
Human activities favor the introduction of species into reservoirs, which are ideal environments for non-native fish species (Gido and Brown 1999; Havel et al. 2005; Pelicice and Agostinho 2009; Espínola et al. 2010; Gois et al. 2015; Liew et al. 2016). However, other human activities are not direct “facilitators,” and may be discussed separately. These include sport fishing (Ribeiro et al. 2017) and aquaculture (mainly fish farming in cages) (Vitule et al. 2014; Azevedo-Santos et al. 2015; Daga et al. 2016), as well as mercury contamination (Mendes et al. 2016) and pollution, which have direct impacts on native species.
Vectors of introduction—historical evidences
Panarari-Antunes et al. (2012) and Diamante et al. (2017) presented a brief history of the introduction of South American silver croaker in Brazil: “In 1949, specimens of the South American silver croaker were captured in lakes in Nazaré (Nazaré PI, Brazil) and in Feitoria (Oeiras PI, Brazil) and transported to the Pisciculture Station of Lima Campos, Ceará, Brazil (Fontenele and Peixoto 1978). In 1952, the first juveniles were distributed into the dams of the state of Ceará by the ‘Departamento Nacional de Obras Contra as Secas (DNOCS)’. Since results were satisfactory, the Electric Energy Company of São Paulo (CESP) brought specimens of the South American silver croaker from the northeastern dams to the state of São Paulo, between 1966 and 1973 (Torloni et al. 1993b), with the first successful introduction occurring in the Limoeiro Reservoir on the Pardo River, from where specimens of the South American silver croaker reached the Grande River and then colonized the Paraná River in 1972 (Machado 1974)”. By surveying gray literature, the only source of knowledge of the historical pathway of introductions, we offer important details to our understanding of how the subpopulations were displaced, how long ago this took place and where they were established.
It is clear that intentional introductions have been an important factor in the dispersion of this species. However, the consequences of segregated populations, caused by human facilitated dispersion, are poorly studied. However the tools of molecular biology may provide answers to questions of the genetic diversity of invasive and native populations. Genetic data indicate low genetic variability in the South American silver croaker populations introduced into the Upper Paraná River basin, suggesting that they probably originated from a common ancestor (Panarari-Antunes et al. 2012). This is in keeping with the historical records that show that the populations established in the Paraná River basin were derived from a native population from the Parnaíba River basin (Panarari-Antunes et al. 2012). It also provides evidence that introductions can affect the maintenance of genetic variability in recently established populations (Salmenkova 2008), and can lead to genetic homogenization (see Olden et al. 2004). In the meantime, life-history traits may overcome the effects of low genetic diversity (Schlaepfer et al. 2005), providing explanations for the success of South American silver croaker.
Fishing
The South American silver croaker is of great importance for commercial and recreational fisheries, depending on the region. For example, in northern Brazil, it accounts for approximately five per cent of the total inland fishery production (Cintra et al. 2014). In the Amazon region, where Brachyplatystoma vaillantii (Valenciennes 1840) is the target species, South American silver croaker is considered part of the bycatch (Pinheiro and Frédou 2004). In southeastern Brazil, on the other hand, South American silver croaker is one of the most important non-native species in the artisanal fishery (Torloni et al. 1993b; Agostinho et al. 1995, 2016; Hoeinghaus et al. 2009), even though it replaces commercially and culturally superior native species (higher trophic-position migratory, e.g. Pseudoplatystoma corruscans and Salminus brasiliensis); this has resulted in a severe decrease in the ecological efficiency of fisheries production, and has led to an increase in indirect energy costs and low market values (Hoeinghaus et al. 2009). Projects that seek to intensify the potential of sport fishing using extralimital release of target species could be considered examples of eco-vandalism, because they cause habitat degradation and promote multiple negative effects in the native ichthyofauna (Vitule et al. 2014; Ribeiro et al. 2017).
Cage net aquaculture
The increased use of fish for human consumption has led to a considerable increase in the introduction of commercial fish species (Azevedo-Santos et al. 2011, 2015) and, consequently, in the farming of fish in cages, an important vector for new introductions (Azevedo-Santos et al. 2011; Lima Jr. et al. 2018). Cage net aquaculture is thought to negatively affect South American silver croaker populations. There are reports of changes in abundance, in intensity of feeding, in size (length) and in parasitism of wild fauna associated with the presence of cages (Demetrio et al. 2012; Ramos et al. 2013). The consumption of decapods was higher in the stretch of river with cages. This increase was caused by the number of food pellets and amount detritus outside the cages, which attracted large numbers of organisms, including macrocrustaceans (Demetrio et al. 2012).
Farming in cages has increased the rate of infection with parasites by attracting several organisms that facilitate parasitism, including mollusks, fish and piscivorous birds, that are definitive and intermediate hosts in the life cycle such parasites as the metacercariae Austrodiplostomum compactum, an important eye parasite of South American silver croaker (Ramos et al. 2013). However, the use of South American silver croaker as a target species for farming in cages has not been the subject of detailed studies in the literature, although substantial records confirm that this species can adapt very well to life in cages, shows good growth and good feeding (Mojica 2011). The cultivation of South American silver croaker in cages can involve a high risk, especially when considering the possibility of escape as another vector for introduction and as a source of predation that could affect the trophic web, and, therefore, we believe should not be conducted outside of the South American silver croaker’s original environment. It presents social and economic consequences similar to the implications described for Arapaima gigas (Miranda-Chumacero et al. 2012), of invasive subpopulations modifying local fishery catches as they replace fish that are culturally typical targets of the local fishery.
Contaminants
A comparison of species exposed to contamination by dichlorodiphenyltrichloroethane (DDT), frequently used in the Amazon region for malaria control, indicates that South American silver croaker had the highest average concentrations of DDT; this is an important concern since the South American silver croaker is at the top of the food web and feeds on smaller fish (Mendes et al. 2016).
In addition, a few studies identified local situations that formed the basis for an evaluation of the level of mercury pollution in relation to South American silver croaker in Amazonas Estuary and Coastal Drainages (Porvari 1995), Amazonas Lowlands (Silva et al. 2009, 2013) and Upper Paraná (Wunderlich et al. 2015) ecoregions. Higher levels of mercury (Hg) contamination in predatory fish and in specific environmental compartments in the Amazonian region were directly related to human occupation, where riparian vegetation was replaced by agricultural and bare soil surfaces (Silva et al. 2009). Wunderlich et al. (2015), while studying toxic contaminants in fresh South American silver croaker, suggest that females contain higher concentrations than males, because the accumulation of lipids promotes a high accumulation potential for hydrophobic contaminants during spawning.
Studies related to the South American silver croaker in Neotropical region
The construction of dams and the subsequent invasion of South American silver croaker contributed to the advance in research into the biology and ecology of this species. The increase in the number of population biology studies on non-native populations from the middle 1990’s (Fig. 4 and Table 1) was probably a result of the increased interest in evaluating and sampling the reservoirs located near areas of greater human population density and research centers. This was the case in the Volta Grande and Itaipu reservoirs and adjacent regions within the Upper Paraná ecoregion. Although the South American silver croaker had been introduced before impoundments in the Upper Paraná Basin (Torloni et al. 1993b), many studies have since confirmed the successful establishment of South American silver croaker in this large basin (e.g. Benedito-Cecílio et al. 1997; Hoeinghaus et al. 2009; Agostinho et al. 2016).
Implications from invasive species management
From the dataset presented here, we verify that the distribution patterns of the South American silver croaker serve to illustrate the processes associated with human interference in freshwater ecosystems, as indicated by dashed gray arrows in Fig. 4. Non-native populations of the South American silver croaker were important indicators of biotic homogenization, in particular by causing the replacement of native fish (e.g. Petesse and Petrere Jr 2012; Vitule et al. 2012; Daga et al. 2015). Therefore, the presence and establishment of non-native populations can be considered as an important metric of impact, as a factor that can predict changes in the local fish community. Previously, biotic homogenization was considered to be a more useful metric for the evaluation of environmental quality when diversity is unaffected, or when diversity tends to increase as a consequence of the introduction of more environmentally tolerant species (Petesse and Petrere Jr. 2012).
Management practices and population control measures for non-native fishes are necessary and urgent (Box E in Fig. 4), and should be aimed at reducing the establishment rate, spread and impact of non-native species (e.g. Ribeiro et al. 2017; Daga et al. 2016). In Brazil, hydroelectric companies are responsible for biomanipulation in reservoirs, and are currently changing their activities because of constant failures in stocking programs. For example, a recent tendency has been to encourage stocking using native species (Agostinho et al. 2004, 2010). This is one way in which solutions can be found to help with the conservation of fishery resources affected by invasive fishes. Because the South American silver croaker is originally from northern Brazil, has self-sustaining populations along virtually the entire Upper Paraná basin, and has become an important fish species in all fisheries in the region (Petrere Jr et al. 2002), we recommend constant monitoring at reservoirs in order to better predict the need for controls on non-native populations.
The monitoring of dispersal must be implemented for invasive fishes and for their pathogens. An invasive fish species, when introduced into a new environment, may lose its parasites, leading to an attenuation of the parasite load in the invaded environment. This might be a factor determining the success of the invasion (Colautti et al. 2004; Lacerda et al. 2012; Heger and Jeschke 2014), depending on the ecological traits of the parasites. For example, generalist parasites are resistant to changes in the environment, and are, therefore, better competitors and have a greater chance of successful introduction than specialist parasites (Roy et al. 2011; Clavero 2013; Braga et al. 2017). Studies of the spread of infections between populations provide a strong basis for the development and application of management strategies, including heightened surveillance systems and early warning systems to prevent the occurrence of an outbreak of fish diseases (Al-Shorbaji et al. 2016).
With respect to fisheries, while non-native populations are at this point considered challengers to fishing in southeastern Brazil, the native Amazon populations of South American silver croaker are subject to overfishing as part of bycatch (Pinheiro and Frédou 2004). Studies on the decline of South American silver croaker in its natural range due to overfishing are scarce. Data of the effects of fishing intensity on abundance as estimated through data on fish landings are essential to quantify this impact.
The South American silver croaker is one of the species most affected by mercury and DDT pollution in the Amazon basin, since it is at the top of the food chain, it acquires greater concentrations of these substances. According to Wunderlich et al. (2015 this species can be used as a bioindicator and as pollution biomarkers in monitoring programs in tropical and subtropical freshwater reservoirs.
Conclusion and onward
We have shown that South American silver croaker can be a powerful model to help us understand how the construction of reservoirs, the introduction of non-native species, fishing pressure, and other human activities, have substantial effects on fish populations by reducing native species, promoting the abundance of invasive species and leading to changes in life-history traits and genetic variability.
Human actions facilitate the translocation of species and cause severe changes in the environment, but the final successful establishment of the invading species is determined, in part, by the characteristics of the species. We highlight the consensus opinion that knowledge of the biological attributes of a species is necessary to assess whether the conditions in the invaded environment are within the species’ limits. Individuals with higher propagule pressure should be more successful in their establishment; we show that reservoirs are the major source of propagules for the non-native South American silver croaker.
Future studies need to take into account the fact that basins and sub-basins with large numbers of reservoirs and other human infrastructure, may also have higher propagule pressure. However, non-native sites within physiological limits of the invaders can have fish with different attributes than the fish in the invader’s native environment, and interactions of the invaders and the native species can affect the success of native species. Local communities with higher native diversity are more resistant to invasion, explaining differences in the success of invasions between dams and reservoirs and other aquatic habitats.
References
Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N et al (2008) Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience 58:403–414
Agosta SJ, Klemens JA (2008) Ecological fitting by phenotypically flexible genotypes: implications for species associations, community assembly and evolution. Ecol Lett 11:1123–1134. https://doi.org/10.1111/j.1461-0248.2008.01237.x
Agostinho AA, Julio HF (1996) Ameaça ecológica: peixes de outras águas. Ciência hoje 21:36–45
Agostinho AA, Julio HF Jr, Petrere M Jr (1994) Itaipu reservoir (Brazil): impacts of the impoundment on the fish fauna and fisheries. In: Cowx IG (ed) Rehabilitation of freshwater fisheries. Fishing News Books, Oxford, pp 171–184
Agostinho AA, Matsuura Y, Okada EK, Nakatani K (1995) The catfish, Rhinelepis aspera (Teleostei; Loricariidae), in the Guaíra region of the Paraná River: an example of population estimation from catch-effort and tagging data when emigration and immigration are high. Fish Res 23:333–344. https://doi.org/10.1016/0165-7836(94)00347-Y
Agostinho AA, Gomes LC, Veríssimo S, Okada E (2004) Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Rev Fish Biol Fish 14:11–19
Agostinho AA, Pelicice FM, Gomes LC (2008) Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol 68:1119–1132. https://doi.org/10.1590/S1519-69842008000500019
Agostinho AA, Pelicice FM, Gomes LC, Júlio HF Jr (2010) Reservoir fish stocking: when one plus one may be less than two. Nat Conservação 8(2):103–111
Agostinho AA, Gomes LC, Santos NC, Ortega JC, Pelicice FM (2016) Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fish Res 173:26–36. https://doi.org/10.1016/j.fishres.2015.04.006
Al-Shorbaji F, Roche B, Gozlan R, Britton R, Andreou D (2016) The consequences of reservoir host eradication on disease epidemiology in animal communities. Emerg Microbes Infect 5:e46. https://doi.org/10.1038/emi.2016.46
Azevedo-Santos VM, Rigolin-Sá O, Pelicice FM (2011) Growing, losing or introducing? Cage aquaculture as a vector for the introduction of non-native fish in Furnas Reservoir, Minas Gerais, Brazil. Neotrop Ichthyol 9:915–919. https://doi.org/10.1590/S1679-62252011000400024
Azevedo-Santos VM, Pelicice FM, Lima-Junior DP, Magalhães AL, Orsi ML, Vitule JRS et al (2015) How to avoid fish introductions in Brazil: education and information as alternatives. Nat Conservação 13:123–132. https://doi.org/10.1016/j.ncon.2015.06.002
Bajer PG, Cross TK, Lechelt JD, Chizinski CJ, Weber MJ, Sorensen PW (2015) Across ecoregion analysis suggests a hierarchy of ecological filters that regulate recruitment of a globally invasive fish. Divers Distrib 21(5):500–510
Barbosa ND, Rocha RM, Frédou FL (2012) The reproductive biology of Plagioscion squamosissimus (Heckel, 1840) in the Pará River estuary (Amazon Estuary). J Appl Ichthyol 28(5):800–805
Barros LC, Santos U, Zanuncio JC, Dergam JA (2012) Plagioscion squamosissimus (Sciaenidae) and Parachromis managuensis (Cichlidae): a threat to native fishes of the Doce River in Minas Gerais, Brazil. PLoS ONE 7:e39138. https://doi.org/10.1371/journal.pone.0039138
Bates D (2007) lme4: linear mixed-effects models using S4 classes. R package version 0.9975-13
Bellard C, Leclerc C, Leroy B, Bakkenes M, Veloz S, Thuiller W, Courchamp F (2014) Vulnerability of biodiversity hotspots to global change. Glob Ecol Biogeogr 23:1376–1386
Benedito-Cecílio E, Agostinho AA, Gomes LC (1997) Estrutura das populações de peixes do reservatório de Segredo. In: Agostinho AA (ed) Reservatório de Segredo: bases ecológicas para o manejo. EDUEM, Maringá, pp 113–139
Bennemann ST, Casatti L, Oliveira DC (2006) Alimentação de peixes: proposta para análise de itens registrados em conteúdos gástricos. Biota Neotrop 6:1–8. https://doi.org/10.1590/S1676-06032006000200013
Bezerra LAV, Angelini R, Vitule JRS, Coll M, Sánchez-Botero JI (2017) Food web changes associated with drought and invasive species in a tropical semiarid reservoir. Hydrobiologia. https://doi.org/10.1007/s10750-017-3432-8
Bialetzki A, Nakatani K, Sanches PV, Baumgartner G (2004) Eggs and larvae of the ‘curvina’ Plagioscion squamosissimus (Heckel, 1840) (Osteichthyes, Sciaenidae) in the Baía River, Mato Grosso do Sul State, Brazil. J Plankton Res 26:1327–1336. https://doi.org/10.1093/plankt/fbh123
Boeger WA, Kritsky DC (2003) Parasites, fossils and geologic history: historical biogeography of the South American freshwater croakers, Plagioscion spp. (Teleostei, Sciaenidae). Zool Scr 32:3–11. https://doi.org/10.1046/j.1463-6409.2003.00109.x
Boucher DH (1985) The idea of mutualism, past and future. In: Boucher DH (ed) The biology of mutualism: ecology and evolution. Oxford University Press, New York, pp 1–27
Braga FDS (1997) Biologia reprodutiva de Plagioscion squamosissimus (Teleostei, Sciaenidae) na represa de Barra Bonita, rio Piracicaba (SP). Rev Unimar 19(2):447–460
Braga RR, Gómez-Aparicio L, Heger T, Vitule JRS, Jeschke JM (2017) Structuring evidence for invasional meltdown: broad support but with biases and gaps. Biol Invasions. https://doi.org/10.1007/s10530-017-1582-2
Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709. https://doi.org/10.1111/j.1461-0248.2007.01060.x
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125. https://doi.org/10.1016/S0169-5347(02)00045-9
Caplat P, Cheptou P-O, Diez J, Guisan A, Larson BM, Macdougall AS et al (2013) Movement, impacts and management of plant distributions in response to climate change: insights from invasions. Oikos 122:1265–1274. https://doi.org/10.1111/j.1600-0706.2013.00430.x
Carim KJ, Vindenes Y, Eby LA, Barfoot C, Vøllestad LA (2017) Life history, population viability, and the potential for local adaptation in isolated trout populations. Glob Ecol Conserv 10:93–102. https://doi.org/10.1016/j.gecco.2017.02.001
Carnelós RC, Benedito-Cecilio E (2002) Reproductive strategies of Plagioscion squamosissimus Heckel, 1840 (Osteichthyes Sciaenidae) in the Itaipu Reservoir, Brazil. Braz Arch Biol Technol 45(3):317–324
Carolsfeld J, Harvey B, Baer A, Ross C (2003) Migratory fishes of South America: biology, social importance and conservation status. World Fisheries Trust, Victoria
Casatti L (2003) Sciaenidae (Drums or croakers). In: Reis RE, Kullander SO, Ferraris CJ Jr (eds) Checklist of the freshwater fishes of South and Central America. EDIPUCRS, Porto Alegre, pp 599–602
Cassey P, Delean S, Lockwood JL, Sadowski JS, Blackburn TM (2018) Dissecting the null model for biological invasions: a meta-analysis of the propagule pressure effect. PLoS Biol 16(4):e2005987. https://doi.org/10.1371/journal.pbio.2005987
Cella-Ribeiro A, Torrente-Vilara G, Lima-Filho JA, Doria CRC (2017) Ecologia e Biologia de peixes do Rio Madeira. EDUFRO, Porto Velho
Center for International Earth Science Information Network (CIESIN), Centro Internacional de Agricultura Tropical (CIAT), and Socioeconomic Data and Applications Center (SEDAC) (2017) Gridded population of the world version 3 (GPWv3): population density grids. Columbia University. http://sedac.ciesin.columbia.edu/gpw. Accessed 15 Mar 2017
Chacon JD, Silva JWB (1971) Alimentação da pescada-do-piauí, Plagioscion squamosissimus (Heckel). Bol Soc Cear Agron 12:41–44
Cintra IHA, Flexa CE, da Silva MB, de Araújo MVLF, de Araújo Silva KC (2014) A pesca no reservatório da usina hidrelétrica de Tucuruí, Amazônia, Brasil. Acta Fish Aquat Res 1(1):48–57
Clavero M (2013) Biodiversity in heavily modified waterbodies: native and introduced fish in Iberian reservoirs. Freshw Biol 58:1190–1201. https://doi.org/10.1111/fwb.12120
Clavero M, García-Berthou E (2006) Homogenization dynamics and introduction routes of invasive freshwater fish in the Iberian Peninsula. Ecol Appl 16:2313–2324. https://doi.org/10.1890/1051-0761
Clavero M, Hermoso V (2011) Reservoirs promote the taxonomic homogenization of fish communities within river basins. Biodivers Conserv 20:41–57
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733. https://doi.org/10.1111/j.1461-0248.2004.00616.x
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037
Conover DO (1990) The relation between capacity for growth and length of growing season: evidence for and implications of countergradient variation. Trans Am Fish Soc 119:416–430. https://doi.org/10.1577/1548-8659
Conover DO, Brown JJ, Ehtisham A (1997) Countergradient variation in growth of young striped bass (Morone saxatilis) from different latitudes. Can J Fish Aquat Sci 54:2401–2409. https://doi.org/10.1139/f97-147
Cooke GM, Chao NL, Beheregaray LB (2012) Marine incursions, cryptic species and ecological diversification in Amazonia: the biogeographic history of the croaker genus Plagioscion (Sciaenidae). J Biogeogr 39:724–738. https://doi.org/10.1111/j.1365-2699.2011.02635.x
Daga VS, Skóra F, Padial AA, Abilhoa V, Gubiani ÉA, Vitule JRS (2015) Homogenization dynamics of the fish assemblages in Neotropical reservoirs: comparing the roles of introduced species and their vectors. Hydrobiologia 746:327–347
Daga VS, Debona T, Abilhoa V, Gubiani ÉA, Vitule JRS (2016) Non-native fish invasions of a Neotropical ecoregion with high endemism: a review of the Iguaçu River. Aqua Invasions 11:209–223
Dawson W, Moser D, van Kleunen M, Kreft H, Pergl J, Pyšek P et al (2017) Global hotspots and correlates of alien species richness across taxonomic groups. Nat Ecol Evol 1(7):0186
Demetrio JA, Gomes LC, Latini JD, Agostinho AA (2012) Influence of net cage farming on the diet of associated wild fish in a Neotropical reservoir. Aquaculture 330:172–178
Diamante NA, Prioli SMAP, Oliveira AV, Fabrin TMC, Prioli LM, Prioli AJ (2017) Genetic relationships of Plagioscion squamosissimus (Perciformes, Sciaenidae) from five Neotropical river basins evaluated using mitochondrial atpase6/8 gene sequences. J Fish Biol 91(1):375–384
Espínola LA, Minte-Vera CV, Júlio HF Jr (2010) Invasibility of reservoirs in the Paraná Basin, Brazil, to Cichla kelberi Kullander and Ferreira, 2006. Biol Invasions 12:1873–1888
FEOW - Freshwater Ecoregions of the World (2018) http://www.feow.org. Accessed 5 Jan 2018
Fontenele O, Peixoto JT (1978) Análise dos resultados de introdução da pescada do Piauí, Plagioscion squamosissimus (Heckel, 1840), nos açudes do Nordeste. Bol Téc DNOCS 36:85–112
Frehse FD, Braga RR, Nocera GA, Vitule JRS (2016) Non-native species and invasion biology in a megadiverse country: scientometric analysis and ecological interactions in Brazil. Biol Invasions 18:3713–3725. https://doi.org/10.1007/s10530-016-1260-9
Froese R, Pauly D (2017) FishBase. World Wide Web electronic publication. www.fishbase.org. Accessed 5 Jan 2018
Gallardo B, Aldridge DC, Gonzalez-Moreno P, Pergl J, Pizarro M, Pysek P et al (2017) Protected areas offer refuge from invasive species spreading under climate change. Glob Change Biol 23:5331–5343
Gennari Filho O, Braga FDS (1996) Fecundidade e desova de Astyanax bimaculatus e A. schubarti (Characidae, Tetragonopterinae) na represa de Barra Bonita, rio Piracicaba (SP). Rev UNIMAR Mar 18(2):241–254
Gido KB, Brown JH (1999) Invasion of North American drainages by alien fish species. Fresh Biol 42:387–399. https://doi.org/10.1046/j.1365-2427.1999.444490.x
Gois KS, Pelicice FM, Gomes LC, Agostinho AA (2015) Invasion of an Amazonian cichlid in the Upper Paraná River: facilitation by dams and decline of a phylogenetically related species. Hydrobiologia 746:401–413
Goldman CA, Snell RR, Thomason JJ, Brown DB (1990) Principles of allometry. In: Proceedings of the eleventh workshop/conference of the association for biology laboratory education
Graça WJ, Pavanelli CS (2007) Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. EDUEM, Maringá
Guo WY, Lambertini C, Li XZ, Meyerson LA, Brix H (2013) Invasion of Old World Phragmites australis in the New World: precipitation and temperature patterns combined with human influences redesign the invasive niche. Glob Change Biol 19(11):3406–3422. https://doi.org/10.1111/gcb.12295
Hahn NS, Agostinho AA, Goitein R (1997) Feeding ecology of curvina Plagioscion squamosissimus (Heckel, 1840) (Osteichthyes, Perciformes) in the Itaipu reservoir and Porto Rico floodplain. Acta Limnol Bras 9(1):11–22
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467. https://doi.org/10.1111/j.1461-0248.2005.00739.x
Havel JE, Lee CE, Zanden VA (2005) Do reservoirs facilitate invasions into landscapes? Bioscience 55:518–525. https://doi.org/10.1641/0006-3568
Heger T, Jeschke JM (2014) The enemy release hypothesis as a hierarchy of hypotheses. Oikos 123:741–750. https://doi.org/10.1111/j.1600-0706.2013.01263.x
Henry R (2014) Represa de Jurumirim: ecologia, modelagem e aspectos sociais. Holos Editora, Ribeirão Preto
Hoeinghaus DA, Agostinho AN, Gomes LU, Pelicice FE, Okada ED, Latini JO et al (2009) Effects of river impoundment on ecosystem services of large tropical rivers: embodied energy and market value of artisanal fisheries. Conserv Biol 23:1222–1231. https://doi.org/10.1111/j.1523-1739.2009.01248.x
Holyoak M, Leibold MA, Holt RD (2005) Metacommunities: spatial dynamics and ecological communities. University of Chicago Press, Chicago
Janzen DH (1985) Dan Janzen’s thoughts from the tropics 1: on ecological fitting. Oikos 45(3):308–310
Johnson PT, Olden JD, Zanden VA (2008) Dam invaders: impoundments facilitate biological invasions into freshwaters. Front Ecol Environ 6:357–363. https://doi.org/10.1890/070156
Lacerda ACF, Takemoto RM, Tavares-Dias M, Poulin R, Pavanelli GC (2012) Comparative parasitism of the fish Plagioscion squamosissimus in native and invaded river basins. J Parasitol 98(4):713–717
Lasso-Alcalá OM, Lasso CA, Señaris JC (1998) Aspectos de la biología y ecología de la Curvinata Plagioscion squamosissimus (Heckel, 1840) (Pisces: Sciaenidae) en los llanos inundables del Estado Apure, Venezuela. Memoria Sociedad de Ciencias Naturales La Salle 149:3–33
Lawler JJ, Spencer B, Olden JD, Kim S-H, Lowe C, Bolton S et al (2013) Mitigation and adaptation strategies to reduce climate vulnerabilities and maintain ecosystem services. In: Pielke RA, Suding K, Seastedt T (eds) Climate vulnerability: understanding and addressing threats to essential resources, 1st edn. Elsevier, Oxford, pp 315–335
Lee H, Ghosh SK (2009) Performance of information criteria for spatial models. J Stat Comput Simul 79(1):93–106
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7(7):601–613
Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S (2008) Fish invasions in the worldś river systems: when natural processes are blurred by human activities. PLoS Biol 6:28. https://doi.org/10.1371/journal.pbio.0060028
Levine JM, D’Ántonio CM (1999) Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87:15–26. https://doi.org/10.2307/3546992
Liew JH, Tan HH, Yeo DC (2016) Dammed rivers: impoundments facilitate fish invasions. Freshw Biol 61:1421–1429. https://doi.org/10.1111/fwb.12781
Lima DP Jr, Magalhães ALB, Pelicice FM, Vitule JRS, Azevedo-Santos VM, Orsi ML et al (2018) Aquaculture expansion in Brazilian freshwaters against the Aichi biodiversity targets. Ambio. https://doi.org/10.1007/s13280-017-1001-z
Liu C, He D, Chen Y, Olden JD (2017) Species invasions threaten the antiquity of Chinaś freshwater fish fauna. Divers Distrib 23:556–566. https://doi.org/10.1111/ddi.12541
Lockwood JL, Cassey P, Blackburn TM (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20(5):223–228
Lockwood JL, Hoopes MF, Marchetti MP (2007) Invasion Ecology. Blackwell, Malden
Lockwood JL, Cassey P, Blackburn TM (2009) The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib 15:904–910. https://doi.org/10.1111/j.1472-4642.2009.00594.x
Machado CEM (1974) Ação da CESP no meio ambiente. CESP, São Paulo
Matsuzaki S-IS, Sasaki T, Akasaka M (2013) Consequences of the introduction of exotic and translocated species and future extirpations on the functional diversity of freshwater fish assemblages. Glob Ecol Biogeogr 22:1071–1082. https://doi.org/10.1111/geb.12067
McBride RS, Somarakis S, Fitzhugh GR, Albert A, Yaragina NA, Wuenschel MJ et al (2015) Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish 16:23–57. https://doi.org/10.1111/faf.12043
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Mendes RA, da Costa Lopes AS, de Souza LC, de Oliveira Lima M, da Silva Santos L (2016) DDT concentration in fish from the Tapajós River in the Amazon region, Brazil. Chemosphere 153:340–345. https://doi.org/10.1016/j.chemosphere.2016.03.054
Mèrona B, Juras AA, Santos GM, Cintra IH (2010) Os peixes ea pesca no Baixo Tocantins: 20 anos depois da UHE Tucurui. Eletronorte, Brasília
Miranda-Chumacero G, Wallace R, Calderón H, Calderón G, Willink P, Guerrero M et al (2012) Distribution of arapaima (Arapaima gigas) (Pisces: Arapaimatidae) in Bolivia: implications in the control and management of a non-native population. Bioinvasions Rec 1(2):129–138
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed1000097
Mojica ALB (2011) Aspectos reprodutivos de Plagioscion squamosissimus (teleostei, sciaenidae) mantidos em tanque rede na comunidade do Lago do Catalão, Amazônia Central. Dissertation, PPG-CIPET, UFAM
Moyle PB, Light T (1996) Biological invasions of fresh water: empirical rules and assembly theory. Biol Conserv 78:149–161
Moyle PB, Marchetti MP (2006) Predicting invasion success: freshwater fishes in California as a model. Bioscience 56(6):515–524
Neves MP, Delariva RL, Guimarães ATB, Sanches PV (2015) Carnivory during ontogeny of the Plagioscion squamosissimus: a successful non-native fish in a lentic environment of the Upper Paraná River Basin. PLoS ONE 10(11):e0141651
Olden JD (2016) Challenges and opportunities for fish conservation in dam-impacted waters. Cambridge University Press, Cambridge
Olden JD, Poff NL, Douglas MR, Douglas ME, Fausch KD (2004) Ecological and evolutionary consequences of biotic homogenization. Trends Ecol Evol 19:18–24. https://doi.org/10.1016/j.tree.2003.09.010
Oliveira EF, Minte-Vera CV, Goulart E (2005) Structure of fish assemblages along spatial gradients in a deep subtropical reservoir (Itaipu Reservoir, Brazil-Paraguay border). Environ Biol Fishes 72(3):283–304
Panarari-Antunes RS, Prioli AJ, Prioli SM, Gomes VN, Júlio HF, Agostinho CS et al (2012) Genetic divergence among invasive and native populations of Plagioscion squamosissimus (Perciformes, Sciaenidae) in Neotropical regions. J Fish Biol 80:2434–2447. https://doi.org/10.1111/j.1095-8649.2012.03290.x
Paolucci EM, MacIsaac HJ, Ricciardi A (2013) Origin matters: alien consumers inflict greater damage on prey populations than do native consumers. Divers Distrib 19(8):988–995
Pelicice FM, Agostinho AA (2009) Fish fauna destruction after the introduction of a non-native predator (Cichla kelberi) in a Neotropical reservoir. Biol Invasions 11:1789–1801
Pelicice FM, Vitule JRS, Junior LA, Orsi ML, Agostinho AA (2014) A serious new threat to Brazilian freshwater ecosystems: the naturalization of nonnative fish by decree. Conserv Lett 7:55–60. https://doi.org/10.1111/conl.12029
Pereira LS, Agostinho AA, Gomes LC (2015) Eating the competitor—a mechanism of invasion. Hydrobiologia 746:223–231. https://doi.org/10.1007/s10750-014-2031-1
Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M, Araújo MB (2011) Ecological niches and geographic distributions (MPB-49). Princeton University Press, New Jersey
Petesse ML, Petrere M (2012) Tendency towards homogenization in fish assemblages in the cascade reservoir system of the Tietê river basin, Brazil. Ecol Eng 48:109–116. https://doi.org/10.1016/j.ecoleng.2011.06.033
Petrere M Jr (1978) Pesca e esforço de pesca no estado do Amazonas. I. Esforço e captura por unidade de esforço. Acta Amaz 8:439–454
Petrere M Jr, Agostinho AA, Okada EK, Júlio HF Jr (2002) Review of the fisheries in the Brazilian portion of the Paraná/Pantanal basin. In: Cowx IG (ed) Management and ecology of lake and reservoir fisheries. Fishing News Books, Oxford, pp 123–143
Pinheiro LA, Frédou FL (2004) General characteristics of industrial fishing landings in the state of Pará. Sci J UFPA 4:1–16
Porvari P (1995) Mercury levels of fish in Tucuruí hydroelectric reservoir and in River Mojú in Amazonia, in the state of Pará, Brazil. Sci Total Environ 175:109–117. https://doi.org/10.1016/0048-9697(95)04907-X
R Core Development Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahel FJ (2002) Homogenization of freshwater faunas. Annu Rev Ecol Evol Syst 33:291–315
Ramos IP, Franceschini L, Zago AC, Zica ÉOP, Wunderlich AC, Carvalho ED, Silva RJD (2013) New host records and a checklist of fishes infected with Austrodiplostomum compactum (Digenea: Diplostomidae) in Brazil. Rev Bras Parasitol Vet 22(4):511–518
Ribeiro VR, da Silva PR, Gubiani ÃA, Faria L, Daga VS, Vitule JRS (2017) Imminent threat of the predator fish invasion Salminus brasiliensis in a Neotropical ecoregion: eco-vandalism masked as an environmental project. Perspect Ecol Conserv. https://doi.org/10.1016/j.pecon.2017.03.004
Roy HE, Handley LJL, Schönrogge K et al (2011) Can the enemy release hypothesis explain the success of invasive alien predators and parasitoids? BioControl 56:451–468. https://doi.org/10.1007/s10526-011-9349-7
Salmenkova EA (2008) Population genetic processes in introduction of fish. Russ J Genet 44:758–766
Santa-Fé ÚMG, Gubiani EA (2016) Seletividade de redes de emalhar para uma espécie de peixe não-nativo em um reservatório neotropical, Paraná, Brasil. Bol Inst Pesca 42:167–179
Santos ABI, Terra BDF, Araújo FG (2010) Influence of the river flow on the structure of fish assemblage along the longitudinal gradient from river to reservoir. Zoologia 27(5):732–740
Schlaepfer MA, Hoover C, Dodd CK Jr (2005) Challenges in evaluating the impact of the trade in amphibians and reptiles on wild populations. Bioscience 55:256–264. https://doi.org/10.1641/0006-3568
Schultz ET, Conover DO (1997) Latitudinal differences in somatic energy storage: adaptive responses to seasonality in an estuarine fish (Atherinidae: Menidia menidia). Oecologia 109:516–529. https://doi.org/10.1007/s004420050112
Silva DS, Lucotte M, Paquet S, Davidson R (2009) Influence of ecological factors and of land use on mercury levels in fish in the Tapajós River basin, Amazon. Environ Res 109:432–446. https://doi.org/10.1016/j.envres.2009.02.011
Silva DS, Lucotte M, Paquet S, Brux G, Lemire M (2013) Inverse mercury and selenium concentration patterns between herbivorous and piscivorous fish in the Tapajos River, Brazilian Amazon. Ecotoxicol Environ Saf 97:17–25. https://doi.org/10.1016/j.ecoenv.2013.06.025
Simberloff D, Vitule JR (2014) A call for an end to calls for the end of invasion biology. Oikos 123(4):408–413
Skóra F, Abilhoa V, Padial AA, Vitule JRS (2015) Darwin’s hypotheses to explain colonization trends: evidence from a quasi-natural experiment and a new conceptual model. Divers Distrib 21:583–594. https://doi.org/10.1111/ddi.12308
Stefani PM, Rocha O (2009) Diet composition of Plagioscion squamosissimus (Heckel, 1840), a fish introduced into the Tietê River system. Braz J Biol 69(3):805–812
Stenseth NC, Mysterud A (2002) Climate, changing phenology, and other life history traits: nonlinearity and match-mismatch to the environment. Proc Natl Acad Sci 99:13379–13381
Suzuki HI, Bulla CK, Agostinho AA, Gomes LC (2005) Estratégias reprodutivas de assembléias de peixes em reservatórios. In: Rodrigues L, Thomaz SM, Agostinho AA, Gomes LC (org) Biocenoses em reservatórios: padrões espaciais e temporais. Rima, Londrina, pp 223–236
Torloni CEC, Corrêa ARA, Carvalho AA Jr, Santos JJ, Gonçalves JL, Gereto EJ et al (1993a) Produção pesqueira e composição das capturas em reservatórios sob concessão da CESP nos rios Tietê, Paraná e Grande, no período de 1986 a 1991. Série Pesquisa e Desenvolvimento, São Paulo
Torloni CEC, Santos JJ, Carvalho AA Jr, Corrêa ARA (1993b) A pescada-do-piauí Plagioscion squamosissimus (Heckel, 1840) (Osteichthyes, Perciformes), nos reservatórios da Companhia Energética de São Paulo—CESP. Série de Pesquisa e Desenvolvimento, São Paulo
Toussaint A, Charpin N, Brosse S, Villéger S (2016) Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci Rep 6:22125
Vazzoler AEAM (1981) Manual de métodos para estudos biológicos de populações de peixes: reprodução e crescimento. CNPq
Vazzoler AEAM (1996) Biologia da reprodução de peixes teleósteos: teoria e prática. EDUEM, Maringá
Vellend M, Harmon LJ, Lockwood JL, Mayfield MM, Hughes AR, Wares JP, Sax DF (2007) Effects of exotic species on evolutionary diversification. Trends Ecol Evol 22(9):481–488
Vitule JRS, Freire CA, Simberloff D (2009) Introduction of non-native freshwater fish can certainly be bad. Fish Fish 10:98–108. https://doi.org/10.1111/j.1467-2979.2008.00312.x
Vitule JRS, Skóra F, Abilhoa V (2012) Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in Neotropics. Divers Distrib 18:111–120. https://doi.org/10.1111/j.1472-4642.2011.00821.x
Vitule JRS, Bornatowski H, Freire CA, Abilhoa V (2014) Extralimital introductions of Salminus brasiliensis (Cuvier, 1816)(Teleostei, Characidae) for sport fishing purposes: a growing challenge for the conservation of biodiversity in neotropical aquatic ecosystems. Bioinvasions Rec 3:291–296
Vitule JRS, da Costa AP, Frehse FA, Bezerra LA, Occhi TV, Daga VS et al (2016) Comments on ‘Fish biodiversity and conservation in South America by Reis et al. (2016)’. J Fish Biol 90:1182–1190
Vitule JRS, Agostinho AA, Azevedo-Santos VM, Daga VS, Darwall WR, Fitzgerald DB et al (2017) We need better understanding about functional diversity and vulnerability of tropical freshwater fishes. Biodivers Conserv 267:57–762
Ward EJ (2008) A review and comparison of four commonly used Bayesian and maximum likelihood model selection tools. Ecol Model 211(1):1–10. https://doi.org/10.1016/j.ecolmodel.2007.10.030
Weatherley AH (1990) Approaches to understanding fish growth. Trans Am Fish Soc 119(4):662–672. https://doi.org/10.1577/1548-8659
Winemiller KO (1991) Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr 61:343–365. https://doi.org/10.2307/2937046
Winemiller KO, Fitzgerald DB, Bower LM, Pianka ER (2015) Functional traits, convergent evolution, and periodic tables of niches. Ecol Lett 18:737–751. https://doi.org/10.1111/ele.12462
Wunderlich AC, Silva RJ, Zica ÉO, Rebelo MF, Parente TE, Vidal-Martínez VM (2015) The influence of seasonality, fish size and reproductive status on EROD activity in Plagioscion squamosissimus: implications for biomonitoring of tropical/subtropical reservoirs. Ecol Indic 58:267–276. https://doi.org/10.1016/j.ecolind.2015.05.063
Zenni RD, Simberloff D (2013) Number of source populations as a potential driver of pine invasions in Brazil. Biol Invasions 15:1623–1639
Zenni RD, Bailey JK, Simberloff D (2014) Rapid evolution and range expansion of an invasive plant are driven by provenance environment interactions. Ecol Lett 17:727–735. https://doi.org/10.1111/ele.12278
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Zwiener VP, Lira-Noriega A, Grady CJ, Padial AA, Vitule JRS (2018) Climate change as a driver of biotic homogenization of woody plants in the Atlantic forest. Glob Ecol Biogeogr 27:298–309. https://doi.org/10.1111/geb.12695
Acknowledgements
We thank MSc. V. M. Azevedo-Santos and Dr. J. A. Nienow as reviewers for shrewd comments, T. H. S. Pires, B. S. Barros, A. Galuch for Fig. 1a, Prof. G. Loubens for providing documents and the photo Fig. 1b–d, and F. M. Pelicice for the photo Fig. 1e–h. The authors acknowledge Eletronorte, which provided the data from its environmental monitoring program to be used for scientific purposes. This work was supported by the Fish Ecology and Biology Laboratory, Department of Morphology, Bioscience Institute of Botucatu headed by Prof. Edmir Daniel Carvalho (in memoriam). We are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for constant financial support and research grants provided to JRSV (Process Numbers: 310850/2012-6, 303776/2015-3), and VSD (Process Number: 167382/2017-9).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11160_2018_9526_MOESM1_ESM.doc
Additional Supporting Information found in the online version: Figure S1—Flow diagram of the systematic review methodology (adapted of Prisma 2009). Table S1—List of the previous studies of the South American silver croaker in Neotropical region. (DOC 434 kb)
Rights and permissions
About this article
Cite this article
Queiroz-Sousa, J., Brambilla, E.M., Garcia-Ayala, J.R. et al. Biology, ecology and biogeography of the South American silver croaker, an important Neotropical fish species in South America. Rev Fish Biol Fisheries 28, 693–714 (2018). https://doi.org/10.1007/s11160-018-9526-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-018-9526-1